Published online Jan 15, 2003. doi: 10.3748/wjg.v9.i1.169
Revised: June 24, 2002
Accepted: July 6, 2002
Published online: January 15, 2003
AIM: To investigate experimentally the effects of methionine enkephalin on signal transduction of mouse myeloma NS-1 cells.
METHODS: The antigen determinate of delta opioid receptor was designed in this lab and the polypeptide fragment of antigen determinate with 12 amino acids residues was synthesized. Monoclonal antibody against this peptide fragment was prepared. Proliferation of Mouse NS-1 cells treated with methionine enkephalin of 1 × 10-6 mol·L-1 was observed. The activities of protein kinase A (PKA) and protein kinase C (PKC) were measured and thereby the mechanism of effect of methionine enkephalin was postulated.
RESULTS: The results demonstrated that methionine enkephalin could enhance the proliferation of NS-1 cells and the effect of methionine enkephalin could be particularly blocked by monoclonal antibody. The activity of PKA was increased in both cytosol and cell membrane. With reference to PKC, the intracellular activity of PKC in NS-1 cells was elevated at 1 × 10-7 mol·L-1 and then declined gradually as the concentration of methionine enkephalin was raised. The effects of methionine enkephalin might be reversed by both naloxone and monoclonal antibody.
CONCLUSION: Coupled with the findings, it in-dicates that the signal transduction systems via PKA and PKC are involved in the effects of methionine enkephalin by binding with the traditional opioid receptors, and therefore resulting in different biological effects.
- Citation: Liu XH, Huang DA, Yang FY, Hao YS, Du GG, Li PF, Li G. A new cytokine: the possible effect pathway of methionine enkephalin. World J Gastroenterol 2003; 9(1): 169-173
- URL: https://www.wjgnet.com/1007-9327/full/v9/i1/169.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i1.169
A number of studies have documented the involvement of endogenous opioid peptide on the cellular functions. It has been known that opioid receptors exist on the surface of cells pertinent to immune function, and that the activation or inhibition of these receptors may enhance or down regulate some cell activities. Methionine enkephalin, the native opioid peptide, has been identified and defined as a cytokine because of its non-neurotransmitter function and sharing all of the major properties of cytokines[1-4]. Although numerous studies have shown that opioid-induced alteration of cellular function can be mediated indirectly via the central nervous system (CNS) or through direct interaction with cells, the precise cellular mechanisms underlying the immunomodulatory effects of opioids are largely unknown.
It is especially true that the opioid receptors contain consensus sites for phosphorylation by numerous protein kinases. Protein kinase C (PKC) has been shown to catalyze the in vitro phosphorylation of delta-opioid receptors and to potentiate agonist-induced receptor desensitization[5,6]. On the other hand, studies suggest that acute and chronic opioid can regulate the cAMP-dependent protein kinase (PKA) signaling pathway and the changes in this pathway may be involved in opioid tolerance[7-9]. It has been documented that increased PKA activity can maintain cellular tolerance to opioid receptor agonist by chronic opioid treatment[10].
Although there is mounting evidence supporting the concept that opioids are members of the cytokine-like family, the relative contribution of the opioids to immunoregulation remains unclear. Furthermore, little has been studied how methionine enkephalin acts as binding with the receptor of cell surface and trigger the intracellular biological events via some kinases. In this series of experiments, we analyzed the effect of methionine enkephalin, the endogenoue opioid, on the activities of PKA and PKC at various dosages. The effects of monoclonal antibody and naloxone were also determined so that the mechanism of effect of methionine enkephalin on the signal transduction will be further clarified.
Methionine enkephalin, naloxone, dithiothreitol (DTT), leupeptin, histone, phosphatidyl serine (PS), diacylglycerol (DG) and ATP were purchased from SIGMA; DMEM medium, bovine serum albumin (BSA) and 1-eth-3-3-dim-ethylaminopioply carbodimde-HCl (EDC) were purchased from GIBCO; phenylmethyl sulfonyl fluoride (PMSF) and egtazic acid (EGTA) were from SERVAL.
Design of polypeptide fragment of delta opioid receptor Delta opioid receptor is composed of 372 amino acids residues. Hydrophilic analysis and structure prediction were performed in our laboratory by typing the sequence of opioid receptor into a special computer program designed according to the principle of prediction of antigen determinants, and by which fragment of antigen determinant was selected[11]. The amino acids residues sequence of antigen determinant was selected from the 3rd hydrophilic peak based on the feature of possibility of antigen determinant and listed as follows: NH2-Gly-Ser-Leu-Arg-Arg-Pro-Arg-Gln-Ala-Thr-Thr-Arg-COOH.
Synthesis of polypeptide fragment and preparation of monoclonal antibody Polypeptide fragment with 12 amino acids residues of delta opioid receptor was synthesized in North West University, USA. Monoclonal antibody against this polypeptide fragment was prepared according to the routine procedure. In short, BABL/C mice was immunized with synthesized polypeptide fragment conjugated with bovine serum albumin in complete Freunds adjuvant at 2- to 3-week intervals. The splenic cells separated from mice were fused with myeloma cells to form a stable antibody-producing hybridoma cell line. Positive clones were screened by the method of ELISA and inoculated into BALB/C mice. The antibody was harvested from ascitic fluid and purified with affinity chromatography. The titers of monoclonal antibodies were higher than 3000. The specificity and effects of monoclonal antibody were verified in this experiment.
Effect of methionine enkephalin on proliferation of NS-1 cell lines NS-1, Mouse myeloma cell line, was cultured in DMEM medium containing 10% fetal calf serum at 37 °C in a humidified atmosphere of 5% CO2. After the cell growth occupied full of the bottom of the flasks, the cells were washed once and resuspended in medium at a density of 5 × 104 cells per ml. 1 ml of the cells per well was liquated in a 24 wells plate. When the cells were grown about 70% full of wells, the supernatant was taken out. Then the cells were resuspended in 1 ml of medium without serum and cultured for one more day. After 1 d, supernatants in all wells were removed and the cells were resuspended in 1 ml of medium (with 10% fetal calf serum). The cells were administrated with 1 × 10-6 mol·L-1 of methionine enkephalin. Different concentrations of monoclonal antibody (0.1-10 × 10-9 mol·L-1) were used to block the effect of methionine enkephalin. The culture was continued for 2 d and then pulsed with 18.5 × 103 Bq of 3H-TdR in each well. 4 h later, the cells were harvested onto glass microfiber filter using a multiple sample harvester. The incorporation of 3H-TdR was measured by using LKB 1209 Rackbeta liquid scintillation counter.
Determination of protein kinase A activity NS-1 cells were adjusted to 5 × 104 cells per ml with DEME medium (containing 10% fetal calf serum) and aliquot into 24 well plate at 1 ml cell suspension per well. When the cells were grown about 70% full of the wells, the supernatant was taken out. Then the cells were resuspended in 1 ml of medium without serum, cultured for one more day and added 1 × 10-6 mol·L-1 of methionine enkephalin. For the blocking assay, the different concentration of monoclonal antibody (0.1-10 × 10-6 mol·L-1) and naloxone (0.1-10 × 10-6 mol·L-1) were added at the same time. After 24 h, the cells were collected, resuspended in 500 μl of buffer A (containing 200 mmol·L-1 Tris-HCl pH7.5, 0.25 mol·L-1 sucrose, 2 mmol·L-1 edetic acid, 2 mmol·L-1 dithiothreitol, 10 mg·L-1 leupeptin and 0.5 mmol·L-1 PMSF) and destroyed by supersonic instrument for 2 min in ice bath. The supernatants were collected following a spin at 10000 g for 45 min and defined as the cytosol fraction. The pellet was resuspended in 400 μl of buffer A containing 0.5% Triton X-100, supersonically destroyed for 2 min and defined as the membrane fraction. The measurement of PKA activity was carried out as described with modifications[12]. In short, 40 μl of extracted enzyme fractions were mixed with 160 μl of the solution at the final concentration of 20 mmol·L-1 Tris-HCl pH7.5, 5 mmol·L-1 MgCl2, 0.25 g·L-1 BSA, 0.5 g·L-1 histone, 2 × 10-7 mol·L-1 ATP (γ-32P ATP, 3.7 × 104 Bq) and 8.0 μmol·L-1 of cAMP at 37 °C for 10 min. After followed by incubation in ice bath for 5 min to terminate the reaction, 150 μl of the solution from each sample was collected onto Whatmen GF/C filter paper. After washing 2 × with 10% TCA-2% phosphoric acid for 30 min at room temperature followed by 2 × wash with 5% TCA for 30 min, the activities of PKA were measured by using liquid scintillation counter and expressed as pmol value of 32P in histone catalyzed by per mg protein per min.
Determination of protein kinase C activity The procedures of cell treatment and enzyme extraction were similar to that described in the determination of PKA instead of cells resuspended in 500 μl of buffer B (buffer A + 10 mmol·L-1 egtazic acid). The final volume was 200 μl with final concentration of 20 mmol·L-1 Tris-HCl pH7.5, 5 mmol·L-1 MgCl2, 0.25 g·L-1 BSA, 0.5 g·L-1 histone, 2 × 10-5 mol·L-1 ATP ([γ-32P] ATP, 3.7 × 104 Bq), and 40 μl of extracted enzyme fraction. The measurement of activity of PKC was performed as described by Choi et al[13] with modification. Briefly, 5 mmol·L-1 CaCl2, 80 mg·L-1 PS and 3 mg·L-1 DG were added into reaction system. The collection and assay of samples and the addition of methionine enkephalin, also monoclonal antibody and naloxone, were according to the measurement of PKA. The activities of PKC were expressed with pmol value of 32P in histone catalyzed by per mg protein per min (pmol·mg-1·min-1).
The data were expressed as -χ±s obtained from at least 3 independent experiments and analyzed by t test.
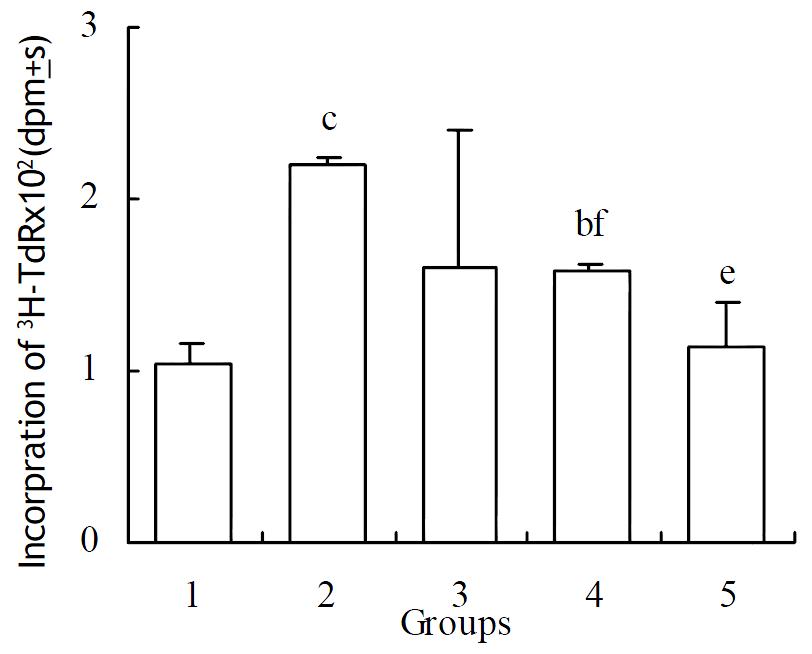
Methionine enkephalin could stimulate the proliferation of NS-1 cells. When 1 × 10-6 mol·L-1 of methionine enkephalin was added into the cultured cells, the cells could proliferate up to 109%. Monoclonal antibody at a lower concentration of 0.1 × 10-9 mol·L-1 could not block the effect of methionine enkephalin. Whereas 1 and 10 × 10-9 mol·L-1 of monoclonal antibody could reverse the enhancing effect of methionine enkephalin on the cell proliferation that showed significantly the differences as compared with treatment group of methionine enkephalin alone (Figure 1).
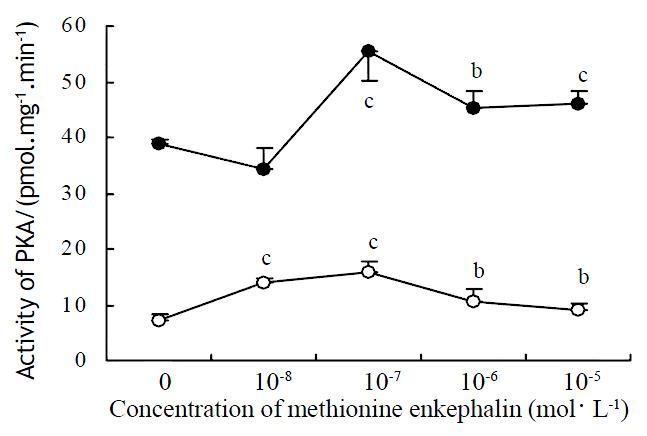
Methionine enkephalin at various concentrations could enhance the level of activity of PKA in cytosol and cell membrane. The effects could be observed at the concentration of 0.1-10 × 10-6 mol·L-1 in cytosol and 0.01-10 × 10-6 mol·L-1 in cell membrane. The effects of methionine enkephalin on the activity of PKA were consistent in the cytosol and the cell membrane (Figure 2).
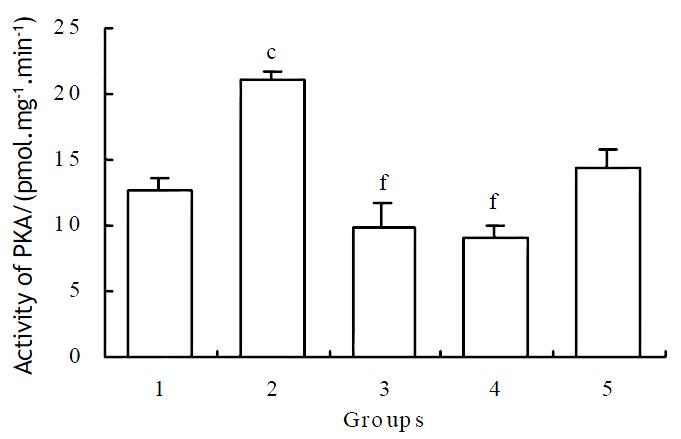
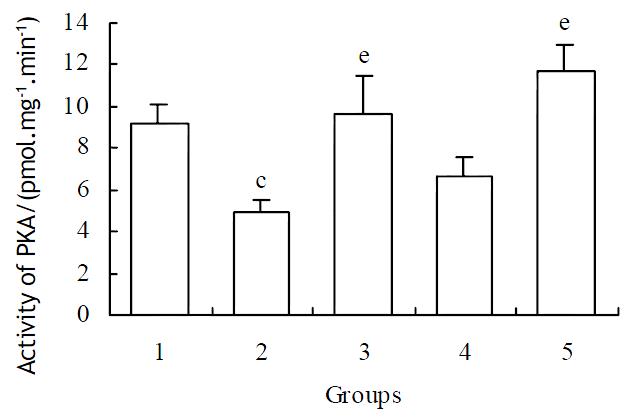
1 × 10-6 mol·L-1 of methionine enkephalin was used for the blocking assay of monoclonal antibody on the activity of PKA. The reversed effects could be observed at the concentration of 1 × 10-10 and 1 × 10-9 mol·L-1 of antibody (Table 1). After administration of different concentrations of naloxone in the case of cytosol, the reversed effects were also obvious (Figure 3).
| MENK+MAb | Activity of PKA in cytosol/pmol·mg-1·min-1 | Activity of PKA in membrane/pmol·mg-1·min-1 |
| Control | 21.10 ± 1.09 | 6.81 ± 1.63 |
| MENK | 24.69 ± 0.49c | 10.42 ± 0.71b |
| MENK+10-11 mol·L-1 MAb | 29.98 ± 0.59cf | 13.73 ± 1.84ce |
| MENK+10-10 mol·L-1 MAb | 20.03 ± 1.47 | 7.59 ± 0.35e |
| MENK+10-9 mol·L-1 MAb | 12.99 ± 0.95cf | 7.11 ± 2.04e |
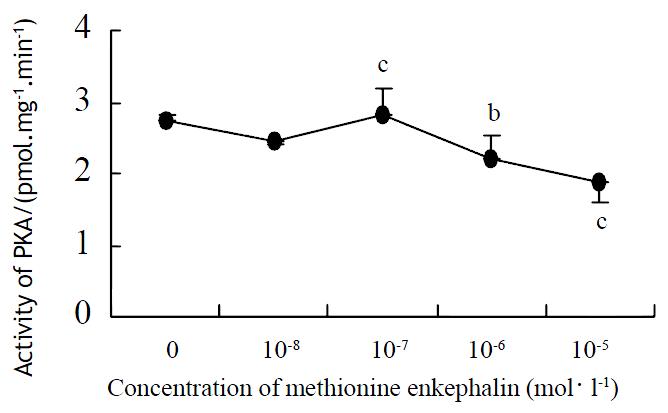
In Figure 4, a narrow effective range of methionine enkephalin was displayed. At the concentration of 1 × 10-7 mol·L-1, methionine enkephalin could enhance the intracellular activity of PKC (Figure 4), but the higher concentration (10-6-10-5 mol·L-1) of methionine enkephalin showed a suppressive effect compared with control (0 mol·L-1).
Based on the data in Figure 4, 1 × 10-6 mol·L-1 of methionine enkephalin was used to inhibit the PKC. Like observed in the case of PKA, the effect of methionine enkephalin could be blocked by different concentrations of monoclonal antibody (Table 2). Naloxone at concentrations of 1 × 10-6 and 1 × 10-5 mol·L-1 could also reverse the suppressed effect of methionine enkephalin in the cytosol (Figure 5).
| MENK+MAb | Activity of PKC (pmol·mg-1·min-1) |
| Control | 3.06 ± 0.19 |
| MENK | 2.26 ± 0.03b |
| MENK+10-11 mol·L-1 MAb | 2.92 ± 0.02e |
| MENK+10-10 mol·L-1 MAb | 2.80 ± 0.4e |
| MENK+10-9 mol·L-1 MAb | 3.13 ± 0.45ff |
The biological and clinical effects of opiate interaction with immune cells are well appreciated. In recent years, investigations from several laboratories have indicated that opioids can operate as cytokines, the principal communication signals of the immune system[1,2]. Our previous studies have also proved the cellular modulation of methionine enkephalin[3,4]. In this experiment, the results that methionine enkephalin could enhance the proliferation of NS-1 cells and perform the effect of growth factor-like, were consistent with the conclusion.
One possible component in the receptor signal cascade that can be responsible for these differences is the ligand-receptor interaction site. Receptor chimera studies followed by mutational analysis have revealed that functions of receptor domains were different for various opioid alkaloids and opioid peptides[14-19]. Based on the principle of antigen determinant, we prepared the monoclonal antibody against delta opioid receptor. Our data showed that the effects of methionine enkephalin were reversible in the presence of different concentration of monoclonal antibody, which indicating the existence of a functional domain at the peptides segment.
Although some laboratories have provided evidences that supporting agonist-induced down-regulation of opioid receptors appear to require the phosphorylation of the receptor protein[20-24], the identities of the specific protein kinases that perform this task remain uncertain. Moreover, it is unknown whether the change of protein kinase activation was dependent on the effect of methionine enkephalin. Our data showed that the activities of PKA were up-regulated in both cytosol and membrane of NS-1 cells in a variety of concentrations of methionine enkephalin. The elevation of PKA activity showed dose-independent and the most efficient concentration of methionine enkephalin was at 10-7 mol·L-1. It had been shown that increased PKA activity related to the maintenance of cellular tolerance to opioid receptor agonists[10,25,26].
However, in the case of PKC, the enzyme activity was elevated when methionine enkephalin at the concentration of 1 × 10-7 mol·L-1 and declined gradually at 1 × 10-6 to 10-5 mol·L-1. The coincident results have also been observed in other laboratory. It had been reported that a biphasic response of opioid on expression of some cytokines had been demonstrated that nanomolar concentration of opioid augmented the secretion of both IL-6 and TNF-alpha, whereas micromolar concentration inhibited their synthesis[27]. It had also been reported that opioid-induced PKC translocation followed a time-dependent and biphasic pattern beginning 2 h after opioid addition, when a pronounced translocation of PKC to the plasma membrane occurred. When exposure to opioids was lengthened to > 12 h, both cytosolic and particulate PKC levels dropped significantly below those of control-treated cells because of the decrease of membrane-bound PKC density[5,28].
From our data, the effect of methionine enkephalin could be reversed by a lower concentration of monoclonal antibody. Although little previous information was available to compare the usage of antibody, it was postulated that a complex ligand-receptor interaction was involved. The results that an antagonism of monoclonal antibody to the effect of methionine enkephalin on the activity of PKA or PKC indicated that the antigen determinant of the receptor fragment was also the functional domain of the receptor. The postulate was reinforced by studies involving μ/δ receptor chimeras that investigated the function of each domain[14,15]. Likewise, the same results could be observed in the assay of naloxone, the antagonist of opioid receptor. The effect of naloxone abolishing the effect of opioid on the activity of PKA had been reported[29,30]. Thus, a traditional opioid mechanism on signaling pathway of PKA and PKC was thereby involved.
| 1. | Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Plotnikoff NP, Faith RE, Murgo AJ, Herberman RB, Good RA. Methionine enkephalin: a new cytokine--human studies. Clin Immunol Immunopathol. 1997;82:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Li G, Fraker PJ. Methionine-enkephalin alteration of mitogenic and mixed lymphocyte culture responses in zinc-deficient mice. Zhongguo Yaoli Xuebao. 1989;10:216-221. [PubMed] |
| 4. | Li G, Yu J. Inhibition of bone marrow immature B lymphocytes from zinc deficient mice by methionine enkephalin. Zhongguo Yaoli Xuebao. 1991;12:500-503. [PubMed] |
| 5. | Kramer HK, Simon EJ. Role of protein kinase C (PKC) in agonist-induced mu-opioid receptor down-regulation: II. Activation and involvement of the alpha, epsilon, and zeta isoforms of PKC. J Neurochem. 1999;72:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Narita M, Mizoguchi H, Kampine JP, Tseng LF. Role of protein kinase C in desensitization of spinal delta-opioid-mediated antinociception in the mouse. Br J Pharmacol. 1996;118:1829-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Shen J, Benedict Gomes A, Gallagher A, Stafford K, Yoburn BC. Role of cAMP-dependent protein kinase (PKA) in opioid agonist-induced mu-opioid receptor downregulation and tolerance in mice. Synapse. 2000;38:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Avidor-Reiss T, Bayewitch M, Levy R, Matus-Leibovitch N, Nevo I, Vogel Z. Adenylylcyclase supersensitization in mu-opioid receptor-transfected Chinese hamster ovary cells following chronic opioid treatment. J Biol Chem. 1995;270:29732-29738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Liu JG, Anand KJ. Protein kinases modulate the cellular adaptations associated with opioid tolerance and dependence. Brain Res Brain Res Rev. 2001;38:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Wagner EJ, Rønnekleiv OK, Kelly MJ. Protein kinase A maintains cellular tolerance to mu opioid receptor agonists in hypothalamic neurosecretory cells with chronic morphine treatment: convergence on a common pathway with estrogen in modulating mu opioid receptor/effector coupling. J Pharmacol Exp Ther. 1998;285:1266-1273. [PubMed] |
| 11. | Hopp TP, Woods KR. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci USA. 1981;78:3824-3828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2408] [Cited by in RCA: 2515] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 12. | Li MS, Li PF, He SP, Du GG, Li G. The promoting molecular mechanism of alpha-fetoprotein on the growth of human hepatoma Bel7402 cell line. World J Gastroenterol. 2002;8:469-475. [PubMed] |
| 13. | Choi SW, Park HY, Rubeiz NG, Sachs D, Gilchrest BA. Protein kinase C-alpha levels are inversely associated with growth rate in cultured human dermal fibroblasts. J Dermatol Sci. 1998;18:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Fukuda K, Kato S, Mori K. Location of regions of the opioid receptor involved in selective agonist binding. J Biol Chem. 1995;270:6702-6709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Pepin MC, Yue SY, Roberts E, Wahlestedt C, Walker P. Novel "restoration of function" mutagenesis strategy to identify amino acids of the delta-opioid receptor involved in ligand binding. J Biol Chem. 1997;272:9260-9267. [PubMed] |
| 16. | Meng F, Ueda Y, Hoversten MT, Thompson RC, Taylor L, Watson SJ, Akil H. Mapping the receptor domains critical for the binding selectivity of delta-opioid receptor ligands. Eur J Pharmacol. 1996;311:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Metzger TG, Paterlini MG, Ferguson DM, Portoghese PS. Investigation of the selectivity of oxymorphone- and naltrexone-derived ligands via site-directed mutagenesis of opioid receptors: exploring the "address" recognition locus. J Med Chem. 2001;44:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Ide S, Sakano K, Seki T, Awamura S, Minami M, Satoh M. Endomorphin-1 discriminates the mu-opioid receptor from the delta- and kappa-opioid receptors by recognizing the difference in multiple regions. Jpn J Pharmacol. 2000;83:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Bonner G, Meng F, Akil H. Selectivity of mu-opioid receptor determined by interfacial residues near third extracellular loop. Eur J Pharmacol. 2000;403:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 771] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 21. | Whistler JL, Tsao P, von Zastrow M. A phosphorylation-regulated brake mechanism controls the initial endocytosis of opioid receptors but is not required for post-endocytic sorting to lysosomes. J Biol Chem. 2001;276:34331-34338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Chaturvedi K, Bandari P, Chinen N, Howells RD. Proteasome involvement in agonist-induced down-regulation of mu and delta opioid receptors. J Biol Chem. 2001;276:12345-12355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Law PY, Kouhen OM, Solberg J, Wang W, Erickson LJ, Loh HH. Deltorphin II-induced rapid desensitization of delta-opioid receptor requires both phosphorylation and internalization of the receptor. J Biol Chem. 2000;275:32057-32065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Kramer HK, Andria ML, Esposito DH, Simon EJ. Tyrosine phosphorylation of the delta-opioid receptor. Evidence for its role in mitogen-activated protein kinase activation and receptor internalization*. Biochem Pharmacol. 2000;60:781-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Yoshikawa M, Nakayama H, Ueno S, Hirano M, Hatanaka H, Furuya H. Chronic fentanyl treatments induce the up-regulation of mu opioid receptor mRNA in rat pheochromocytoma cells. Brain Res. 2000;859:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Wang Z, Sadée W. Tolerance to morphine at the mu-opioid receptor differentially induced by cAMP-dependent protein kinase activation and morphine. Eur J Pharmacol. 2000;389:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Roy S, Cain KJ, Chapin RB, Charboneau RG, Barke RA. Morphine modulates NF kappa B activation in macrophages. Biochem Biophys Res Commun. 1998;245:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Kramer HK, Simon EJ. Role of protein kinase C (PKC) in agonist-induced mu-opioid receptor down-regulation: I. PKC translocation to the membrane of SH-SY5Y neuroblastoma cells is induced by mu-opioid agonists. J Neurochem. 1999;72:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Chakrabarti S, Law PY, Loh HH. Distinct differences between morphine- and [D-Ala2,N-MePhe4,Gly-ol5]-enkephalin-mu-opioid receptor complexes demonstrated by cyclic AMP-dependent protein kinase phosphorylation. J Neurochem. 1998;71:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Sharma P, Kumar Bhardwaj S, Kaur Sandhu S, Kaur G. Opioid regulation of gonadotropin release: role of signal transduction cascade. Brain Res Bull. 2000;52:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Edited by Wu XN