INTRODUCTION
Early in 1948, Grossman first proposed that intragastric neurones could regulate gastric neuroendocrine response to gastric distention independent of the extrinsic nervous system[1], and later Schubert and Makhlouf reported that distention of the distal part of the isolated rat stomach activates the intrinsic VIP neurones and the intrinsic cholinergic mechanism, thereby regulating gastrin and somatostatin release[2]. However, the intrinsic pathways, independent of extrinsic nerves, which are activated by gastric distention and which modify gastrin release, have remained largely obscure until now.
After finding that distention of isolated rat stomach results in an inhibition of gastrin release and that distention of the extrinsically innervated stomach stimulates gastrin release in vivo[3], we performed this study to examine the intragastric mechanisms involved in regulation of gastrin and somatostatin response to gastric distention. For the study, gastric distention was performed in vitro in isolated vascularly perfused rat stomach, an extrinsically denervated preparation that retains the integrity of intramural neurones as well as intragastric paracrine pathways[4-7]. In this model the effect of graded gastric distention on the release of gastrin and somatostatin was determined. The importance of the intrinsic nervous system and, in particular, its cholinergic part was investigated by performing distention in presence of the axonal blocker tetrodotoxin (TTX) and the cholinergic antagonist atropine. The putative somatostatin-antagonist cyclo [7-aminoheptanoyl-Phe-D-Trp-Lys-Thr(Bzl)] was employed in order to evaluate the role of endogenous somatostatin on regulation of gastrin release during gastric distention.
MATERIALS AND METHODS
Materials
Male Wistar rats (n = 101), body weight 250-300 g (Charles River WigaGmbH, Sulzfelden, Germany); dextran T-70 (Pharmacia, Uppsala, Sweden); bovine serum albumine (Serva, Heidelberg, Germany); cyclo [7-aminoheptanoyl-Phe-D-Trp-Lys-Thr(Bzl)] (Bachem, Hannover, Germany); commercial gastrin-kit (Becton and Dickinson, Heidelberg, Germany); tetrodotoxin (TTX), atropine, somatostatin-14 and porcine gastrin-releasing peptide (GRP) (Sigma Chemie, Munich, Germany).
Experimental design
We first prepared the rat stomach. The stomach was isolated from rats fasted overnight using a procedure described by McIntosh et al[8,9]. The isolated stomach was perfused through the celiac artery in a single pass perfusion system at a rate of 1.5 mL/min with a modified Krebs-Ringer buffer solution. The perfusion medium contained 4% dextranT-70, 0.2% bovine serum albumin and 5.5 mM glucose and was gassed with 95% O2 and 5% CO2. The gastric venous effluent was collected via a catheter in the portal vein at two-minute intervals and frozen immediately for subsequent radioimmunoassay. A further catheter was placed in the stomach via the esophagus with the tip at the cardia. A distal catheter was placed in the stomach at the ligated pylorus to drain gastric contents.
After insertion of the gastric catheters, the lumen of the stomach was gently rinsed with isotonic pH7 saline until clear. Thereafter gastric lumen was continuously perfused with saline (1.5 mL/min) during an equilibration period of 25 min and a basal period of 10 min and the perfusate was allowed to flow off via a distal catheter. Subsequently, graded gastric distention was initiated by instillation of 5, 10 or 15 mL saline through the esophageal catheter at a rate of 2 mL per 10 sec while the distal catheter was blocked. After a distention period of 20 min the distal catheter was reopened and the gastric lumen was again perfused for a period of 20 min with saline. After instillation of the saline load, a fluid filled pressure transducer was connected to the esophageal catheter for recording of the intragastric pressure. During distention, intragastric pressure increased to 5.0 ± 0.4 cm water at 5 mL saline load, to 10.4 ± 0.9 cm water at 10 mL saline load and to 15.0 ± 1.1 cm water at 15 mL saline load. The increase in intragastric pressure induced by the tested distention volumes caused no visible mucosal damage and no impairment of vascular perfusion.
For neural blockade, either the neurotoxin TTX (10-6 M) or the cholinergic blocker atropine (10-7 M) was added to the vascular perfusate. In further experiments the putative somatostatin-antagonist cyclo [7-aminoheptanoyl-Phe-D-Trp-Lys-Thr(Bzl)] (10-6 M) was added to the perfusate alone or in combination with atropine (10-7 M). Since the somatostatin-antagonist did not completely abolish distention-induced inhibition of gastrin release, its effectiveness was tested against the inhibitory action of somatostatin-14 (10-8 M) on gastrin release prestimulated by mammalia bombsin peptide GRP (10-8 M), which is well know in stimulating gastrin secretion[10-12]. In each stomach only one experiment was performed.
Radioimmunoassay
Gastrin levels in the venous effluent were measured by radioimmunoassay as described elsewhere[13] employing a commercial gastrin-kit. Somatostatin was determined as described in detail previously[14], by employing antibody 80C which was generously provided by Dr. R.H. Unger (Dallas, TX, USA).
Data analysis
All data are expressed as mean ± SE. Integrated peptide secretion was calculated as the sum of the differences between the value of each time point during gastric distention and the mean value of the preceding baseline period. For statistical evaluation of the point-to-point variations, the Friedman two-way analysis of variance was used, followed by the Wilcoxon matched-pairs signed rank test if the former allowed rejection of the null hypothesis. The difference in the values between the treatment groups was statistically evaluated by analysis of variance for multiple determinations. Differences resulting in P-values of 0.05 or less were considered significant.
RESULTS
Effect of graded gastric distention on release of gastrin and somatostatin
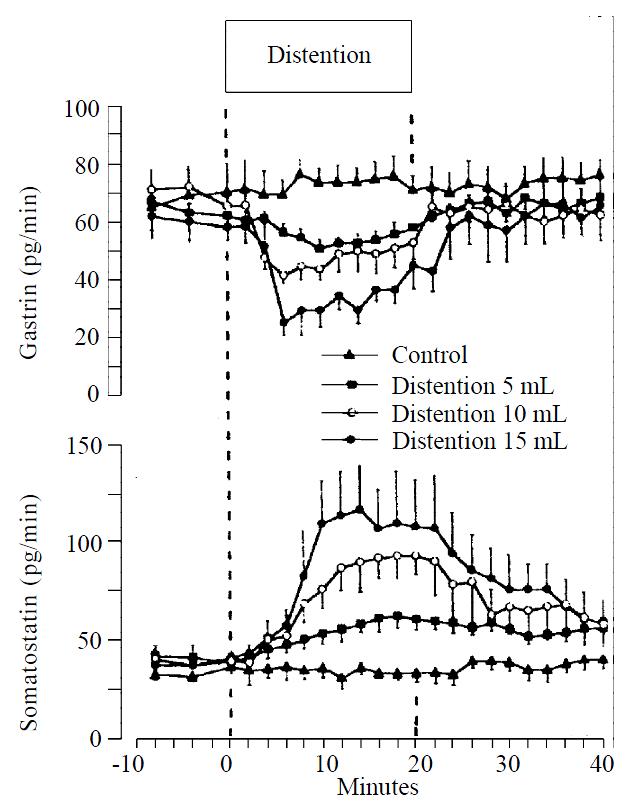
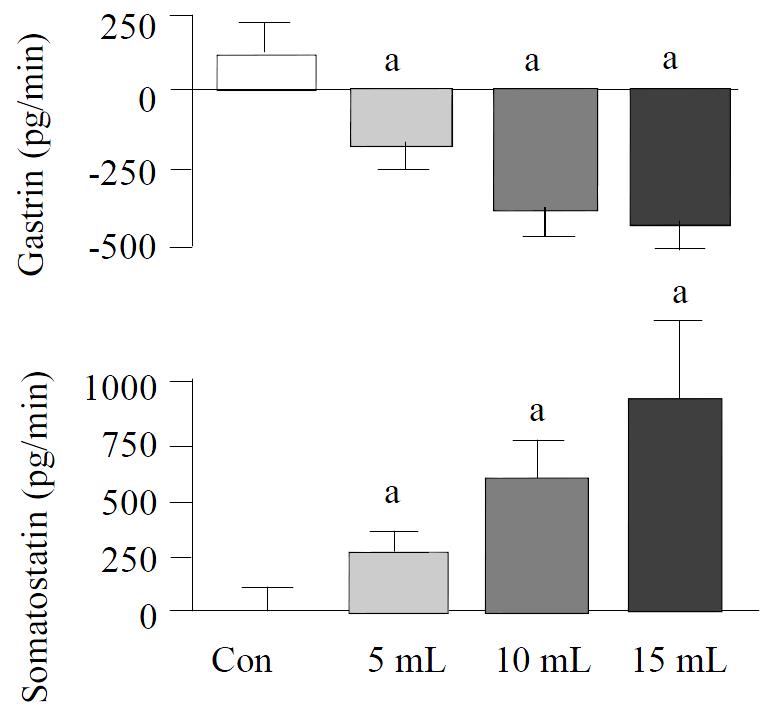
Graded distention of isolated rat stomach elicited a significant and volume dependent decrease in gastrin release from a mean baseline of 63 ± 4 to a minimum of 50 ± 3 pg/min (P < 0.05; n = 8), during distention with 5 mL, from 69 ± 6 to 41 ± 3 pg/min (P < 0.01; n = 15), with 10 mL and from 60 ± 7 to 25 ± 5 pg/min, and with 15 mL saline (P < 0.01; n = 8) (Figure 1). Gastrin secretion decreased throughout the distention period and returned promptly to baseline values after removal of the intragastric saline load. Integrated gastrin release decreased during distention by-183 ± 75 pg/20 min (P < 0.05) at 5 mL, by -385 ± 86 pg/20 min (P < 0.01) at 10 mL, and by -440 ± 85 pg/20 min (P < 0.01) at 15 mL saline distention (Figure 2).
Figure 1 Release of gastrin and somatostatin from perfused rat stomachs in control (n = 8) and during gastric distention with 5 mL (n = 8), 10 mL (n = 15) and 15 mL (n = 8) saline (mean ± SE)
Figure 2 Integrated release of gastrin, somatostatin from per-fused rat stomachs in control (n = 8) and during gastric distention with 5 mL (n = 8), 10 mL (n = 15) and 15 mL (n = 8) saline.
(mean ± SE) aP < 0.05 vs 10 mL distention in control
Somatostatin levels rose volume-dependently during gastric distention from a mean baseline of 41 ± 5 to a maximum level of 61 ± 6 pg/min (P < 0.05) at 5 mL, from 40 ± 5 to 92 ± 12 pg/min (P < 0.01) at 10 mL and from 39 ± 6 to 116 ± 22 pg/min (P < 0.01) at 15 mL saline load (Figure 1). After distention, somatostatin secretion decreased to its baseline level. The integrated incremental somatostatin release was 260 ± 102 pg/20 min (P < 0.05) during distention with 5 mL, 608 ± 148 pg/20 min (P < 0.01) during distention with 10 mL and 943 ± 316 pg/20 min (P < 0.05) during distention with 15 mL saline (Figure 2).
Effect of TTX and atropine on release of gastrin and somatostatin during gastric distention
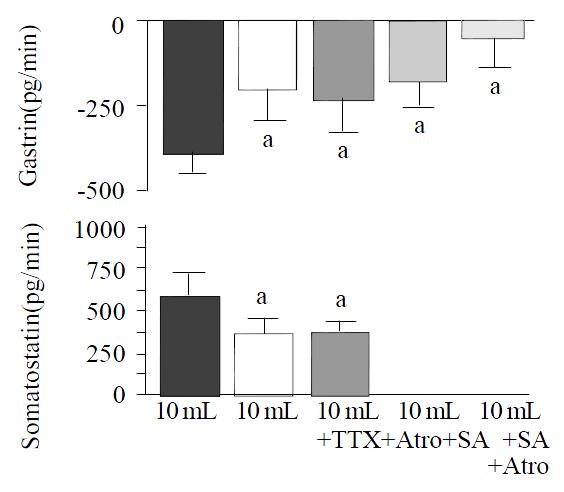
The axonal blocker TTX (10-6 M) and the cholinergic antagonist atropine (10-7 M) elicited similar effects on distention-induced inhibition of gastrin. The decrease of gastrin release in response to distention with 10 mL saline was attenuated by approximately 50% [from -385 ± 86 pg/20 min to -189 ± 89 pg/20 min (P < 0.05 vs control) during infusion of TTX (n = 10), and to -224 ± 95 pg/20 min (P < 0.05 vs control) during infusion of atropine (n = 10)] (Figure 3). The effects of TTX and atropine on distention-stimulated somatostatin release were also nearly identical, and the incremental somatostatin response to a 10 mL intragastric saline load was reduced about 40% [from 608 ± 148 pg/20 min to 370 ± 100 pg/20 min (P < 0.05 vs control) in presence of TTX, and to 404 ± 77 pg/20 min (P < 0.05 vs control) during infusion of atropine] (Figure 3).
Figure 3 Integrated release of gastrin and somatostatin from perfused rat stomachs during gastric distention with 10 mL in control (n = 15) and in presence of tetrodotoxin (TTX, 10-6 M; n = 10), atropine (Atro, 10-7 M; n = 10), somatostatin-antagonist (SA, 10-6 M; n = 15) and a combination of somatostatin-antagonist and atropine (n = 15), (mean ± SE).
aP < 0.05 vs 10 mL distention in control.
Effect of the somatostatin-antagonist on release of gastrin during gastric distention
In presence of the somatostatin-antagonist (10-6 M), the decrease in gastrin release induced by gastric distention with 10 mL saline was significantly attenuated (-167 ± 73 pg/20 min with somatostatin-antagonist vs -385 ± 86 pg/20 min without somatostatin-antagonist; P < 0.05, n = 15). Combined administration of the somatostatin-antagonist and atropine (n = 15) nearly completely abolished distention-induced inhibition of gastrin release (Figure 3).
Effect of the somatostatin-antagonist on the inhibitory action of exogenous somatostatin-14 on GRP-prestimulated gastrin release
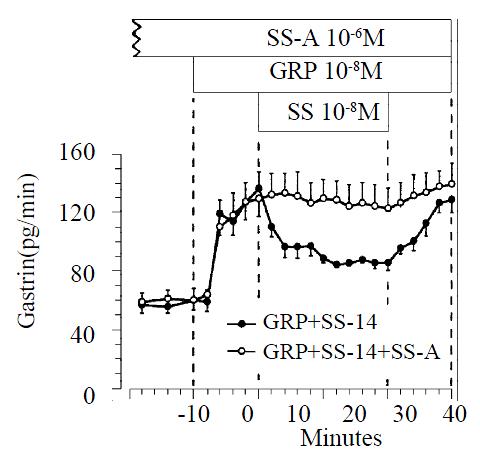
Infusion of GRP (10-8 M) produced a significant increase in gastrin release, from a baseline value of 58 ± 6 to a maximum of 136 ± 11 pg/min. GRP-prestimulated gastrin release was reduced significantly from 136 ± 11 pg/min to 84 ± 4 pg/min by exogenous somatostatin-14 (10-8 M; n = 11) (P < 0.05). When somatostatin-14 was removed from the perfusate, gastrin release promptly returned to prestimulated levels. At a dose of 10-6 M the somatostatin-antagonist did not change the baseline gastrin release, but completely blocked the inhibitory action of exogenous somatostatin-14 on GRP-prestimulated gastrin release (n = 9). (Figure 4).
Figure 4 Effect of somatostatin-14 (10-6 M) on gastrin release from perfused rat stomachs prestimulated by GRP (10-8 M) in control (n = 11) and during perfusion with somatostatin-antago-nist (10-6 M; n = 9).
(mean ± SE)
DISCUSSION
It is well established that gastric distention in vivo activates vagal mechanoreceptors within the gastric wall. The afferent vagal nerve fibers activate brainstem neurones which regulate vagal efferent fibers and thereby exocrine and endocrine functions of the stomach[15-19]. Apart from this extrinsic nervous system, the stomach itself contains regulatory systems within the gastric wall such as intrinsic neurons and intrinsic paracrine pathways[20-22]. The importance of these intragastric mechanisms on regulation of gastrin in response to gastric distention is largely unknown. Isolated perfused stomach allows examination of the remaining intragastric regulatory mechanisms since this model is separated from the extrinsic innervation and the systemic humoral signals, whereas the integrity of intrinsic neurocrine and paracrine pathways is maintained.
As previously reported, distention of the isolated stomach causes a decrease of gastrin release in proportion to the applied intragastric volume and intragastric pressure[3]. To examine the functional role of the intragastric nervous system we employed the neurotoxin tetrodotoxin (TTX) which blocks all neural elements that are activated by an influx of Na+. This comprises all the adrenergic and cholinergic neurons and, presumably, the majority of peptidergic neurons as well. The effect was observed while using a very low concentration of TTX (10-6-10-8 M) which had no noticeable effect on other membrane parameters[23]. The present data suggest that the inhibition of gastrin release during gastric distention is mediated in part by neural, particularly cholinergic, mechanisms.
However, the classical cholinergic neurotransmitter acetylcholine and its stable analogue carbachol stimulate gastrin release in vivo in the isolated stomach preparation and in cultured antral G-cells[24-27]. Therefore the inhibitory effect of the cholinergic system on gastrin release in our experiment seems to be indirect, perhaps via peptidergic neurotransmitters. Furthermore, the present data suggest that gastric distention activates endogenous somatostatin through cholinergic and non-cholinergic TTX insensitive pathways, and endogenously released somatostatin can cause distention-induced inhibition of gastrin release. Several studies have shown a reciprocal relationship between stimulation of somatostatin and inhibition of gastrin, suggesting a functional linkage between somatostatin and gastrin release[28-32]. Accordingly, the importance of endogenous somatostatin for regulation of gastrin release is supported by studies with antisomatostatin serum, demonstrating an augmented gastrin release after neutralization of endogenously released somatostatin in isolated rat stomach[33]. These findings imply that endogenous somatostatin exerts its inhibitory effect on gastrin release via intragastric mechanisms. The secretion and expression of gastrin are under the paracrine control of somatostatin, produced by D cells situated in close contact with gastrin-producing G cells, and gastric D-cells extend the long cytoplasmatic processes that terminate close to gastrin-secreting G-cells in antral mucosa of both humans and rats[34,35]. This morphological evidence for a paracrine mode of action is consistent with the functional study results.
The putative somatostatin-antagonist cyclo [7-aminoheptanoyl-Phe-D-Trp-Lys-Thr(Bzl)] has been described as an antagonist of somatostatin in some peripheral tissues such as endocrine rat pancreas, the papillary muscle of the guinea pig heart and the ferret trachea, as well as in neural tissues such as avian choroid, rat cortex, rat hippocampus and rat pituitary[36]. In the present study the somatostatin antagonist completely blocked the inhibitory effect of a high infusion rate of somatostatin-14 on gastrin release. During infusion of somatostatin-14 (10-8 M), somatostatin measured in the portal venous effluent of the isolated rat stomach rose to levels between 1000 and 1600 pg/min, whereas somatostatin secretion during gastric distention was only between 60 and 120 pg/min. Since the inhibitory effect of exogenous somatostatin was sufficiently antagonized by the dose of the somatostatin-antagonist employed, it seems most likely that a sufficient amount of antagonist was administered to block all the effects of endogenously released somatostatin during gastric distention. Therefore the residual inhibition of gastrin during gastric distention in presence of the somatostatin-antagonist seems to be independent of endogenous somatostatin and may be mediated by inhibitory cholinergic pathways.
Schubert and Makhlouf have previously shown that low distention of the antral part of the isolated rat stomach stimulated somatostatin and inhibited gastrin release probably via VIP-dependent mechanisms, whereas high distention caused an increase in gastrin and a decrease in somatostatin secretion via cholinergic pathways[2]. Distention of the whole stomach seems to activate other regulatory mechanisms than selective distention of the distal part of the stomach, since Debas et al[37] have shown that distention of the oxyntic gland area of the stomach can modulate antral gastrin release. Furthermore the mechanoreceptors in the antrum have been reported to respond mainly to gastric contractions, while those located in the corpus and fundus respond primarily to distention[38].
In conclusion, the present data suggest that distention of the isolated rat stomach inhibits gastrin release via intrinsic neurocrine and paracrine pathways. Both somatostatin and intrinsic cholinergic pathways are responsible for distention-induced inhibition of gastrin release. Somatostatin release is activated by gastric distention through cholinergic and non-cholinergic TTX-insensitive pathways.