Published online Dec 15, 2002. doi: 10.3748/wjg.v8.i6.982
Revised: April 30, 2002
Accepted: May 26, 2002
Published online: December 15, 2002
AIM: To investigate the roles of Fas signaling pathway in vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells.
METHODS: Human gastric cancer SGC-7901 cells were treated with VES at 5, 10, 20 mg·L-1, succinic acid and vitamin E as vehicle control and condition media only as untreated (UT) control. Apoptotic morphology was observed by DAPI staining. Western blot analysis was applied to measure the expression of Fas, FADD and caspase-8 proteins. After the cells were transiently transfected with Fas and FADD antisense oligonucleotides, respectively, caspase-8 activity was determined by flurometric method.
RESULTS: The morphologically apoptotic changes were observed after VES treatment by DAPI staining. 23.7% and 89.6% apoptosis occurred after 24 h and 48 h of 20 mg·L-1 VES treatment, respectively. The protein levels of Fas, FADD and caspase-8 were evidently increased in a dose-dependent manner after 24 h of VES treatment. The blockage of Fas by transfection with Fas antisense oligonucleotides obviously inhibited the expression of FADD protein. After SGC-7901 cells were transfected with Fas and FADD antisense oligonucleotides, caspase-8 activity was obviously decreased (P < 0.01), whereas Fas blocked more than FADD.
CONCLUSION: VES-induced apoptosis in human gastric cancer SGC-7901 cells involves Fas signaling pathway including the interaction of Fas, FADD and caspase-8.
- Citation: Wu K, Li Y, Zhao Y, Shan YJ, Xia W, Yu WP, Zhao L. Roles of Fas signaling pathway in vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells. World J Gastroenterol 2002; 8(6): 982-986
- URL: https://www.wjgnet.com/1007-9327/full/v8/i6/982.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i6.982
RRR-α-tocopheryl succinate (vitamin E succinate, VES), a derivative of natural vitamin E, has been shown to be a potent growth inhibitor of many kinds of cancer cell types[1-5]. Antiproliferative effects of VES on tumor cells are diverse, including G1 cell cycle blockage[6-8], DNA synthesis arrest[9-11], induction of differentiation[12-14] and apoptosis[15-17]. Meanwhile, tumor cell growth inhibition by VES has also been demonstrated in vivo[18,19]. VES is noteworthy for its non-toxic and non-inhibitory effects on normal cell types, indicating that VES can be used as a chemopreventive/chemotherapeutic agent against tumors.
Up to date, the precise mechanisms of VES-induced inhibition of tumor cell growth are not well understood, but some studies show that VES can increase the secretion and activation of transforming growth factor-βs (TGF-βs) and enhance the expression of TGF-β receptor II[10,20]. Yu et al[21] reported that VES-triggered apoptosis in human breast cancer cell lines is inhibited by 50% with antibody neutralization of TGF-β ligand and transient transfection of TGF-β antisense oligonucleotides, implicating that TGF-β plays a crucial role in VES-induced apoptosis and VES may induce cancer cells to undergo apoptosis through other pathways as well. Turley et al[22] observed that the expression of Fas, a cell surface receptor, is increased after treatment of breast cancer cells with VES and VES-induced apoptosis in breast cancer cells is inhibited when Fas neutralized antibody or transfection of Fas antisense oligonucleotides are applied to cancer cells, showing that Fas-mediated apoptosis may be another important pathway by which VES inhibits tumor cell growth.
Gastric cancer is one of the most common malignant tumors in China[23-32]. Our previous studies found that VES can block cell cycle, arrest DNA synthesis and induce apoptosis in human gastric cancer SGC-7901 cells, therefore inhibiting cell growth[33-36]. In addition, our in vivo research demonstrated that VES inhibits benzo(a)pyrene (B(a)P)-induced forestomach carcinogenesis in female mice[37]. The exact mechanisms of apoptosis are not clearly known, but we found that VES can secrete and activate biologically active TGF-β and then TGF-β increases the kinase activity of c-Jun N-terminal kinase(JNK) followed by phosphorylation of c-Jun, and finally activated c-Jun triggers apoptosis in human gastric cancer SGC-7901 cells[38]. In this study, signaling pathway of Fas-induced apoptosis in VES-treated SGC-7901 cells is determined to further investigate the mechanisms of VES-induced growth inhibition.
VES was purchased from Sigama Co. Ltd. RPMI 1640 media and LIPOFECTAMINE PLUSTM Reagent were obtained from Gibco BRL, ApoAlertTM Caspase-8 Fluorescent Assay kit from Clontech, Inc. DAPI (4’,6-diamidine-2’-phenylindole dihydrochloride), FADD and caspase-8 rabbit polyclonal antibodies, Fas antisense (GAGGGTCCAGATGCCCAGCAT) and FADD antisense (CAGCACCAGGAACGGGTCCAT) oligonucleotides were gifts from Dr. Sanders BG (University of Texas, Austin, USA). Fas rabbit polyclonal antibody was from Santa Cruz Biotechnology, Inc.
Cell culture Human gastric cancer cell lines SGC-7901 were maintained in RPMI 1640 medium supplemented with 100 mL·L-1 fetal calf serum (FCS), 100 kU·L-1 penicillin, 100 mg·L-1 streptomycin and 2 mmol·L-1 L-glutamine under 50 mL·L-1 CO2 in a humidified incubator at 37 °C. SGC-7901 cells were incubated for different time periods in the presence of VES at 5, 10 and 20 mg·L-1 (VES was dissolved in absolute ethanol and diluted in RPMI 1640 complete condition media correspondingly to a final concentration of VES and 1 mL·L-1 ethanol), succinic acid, vitamin E and ethanol equivalents as vehicle (VEH) control and condition media only as untreated (UT) control.
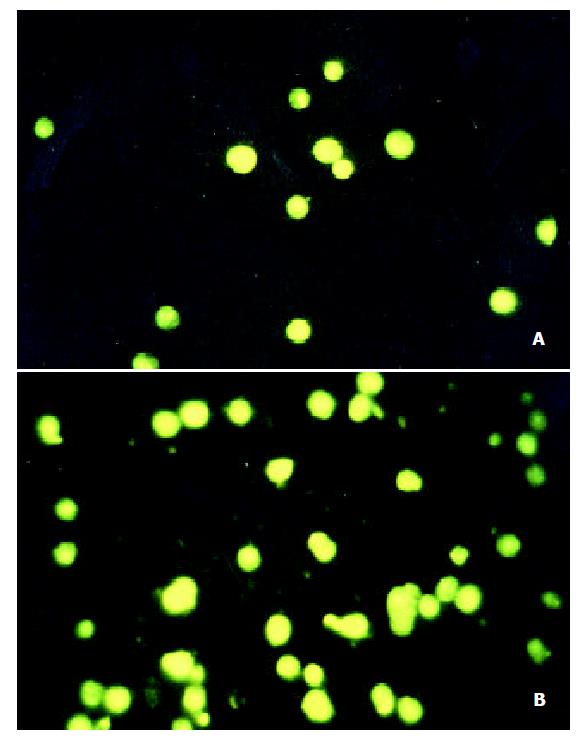
Determination of apoptosis Cells were treated with VES at 20 mg/L-1 for 48 h, then harvested, washed with PBS and stained with 2 mg·L-1 DAPI in 100% methanol for 30 min at 37 °C. Cells were viewed using a fluorescence microscope with ultraviolet (UV) excitation at 300-500 nm. Cells with nuclei that contained clearly condensed chromatin or cells with fragmented nuclei were scored as apoptotic.
Western blot analysis SGC-7901 cells treated with VES were harvested, washed in PBS and lyzed in lysis buffer containing 150 mmol·L-1 NaCl, 1 mL·L-1 NP-40, 5 mg·L-1 sodium deoxycholate, 1 g·L-1 SDS, 50 mmol·L-1 Tris (pH 7.4), 1 mmol·L-1 DTT, 0.5 mmol·L-1 Na3VO4, 10 mmol·L-1 phenylmethylsulfonyl fluoride (PMSF), 10 mg·L-1 trypsin, 10 mg·L-1 aprotinin and 5 mg·L -1 leupeptin. Following the centrifugation of 12000 × g for 30 min at 4 °C, the amount of protein in the supernatant was determined using Biorad DC protein assay. Equal amount of protein was separated on 10% SDS-PAGE and transferred to nitrocellulose filter (Gibco BRL, USA) overnight. Blocked with 50 g·L-1 defatty milk, the filter was incubated with Fas, FADD and caspase-8 rabbit polyclonal antibodies, respectively, and horseradish peroxidase-conjugated IgG, finally developed with DAB.
Transient transfection The cells were washed twice with serum-free medium without antibiotics and incubated for 3 h in 2 mL of serum-free medium containing 30 μL of LIPOFECTAMINE Reagent and 2 ug of Fas or FADD antisense oligonucleotides. After 3 h, the cells were treated with VES.
Caspase-8 activity assay Caspase-8 activity was determined according to the manufacurer’s instructions. Briefly, 50 μL of supertanant from VES-treated cell extracts were mixed with 50 μL of a mixture of 2 × reacion buffer and DTT, then 1 μL of 1 mmol·L-1 IETD-fmk and incubated for 30 min at 37 °C in water bath. Next, 5 μL of 1 mmol·L-1 IETD-AFC was added, followed by incubation for 1 h at 37 °C in water bath. The fluorescent absorbance (A) was measured at 400nm for emission and at 505 nm for excitaion.
The data were expressed as -x± s. Statistical analysis was performed using student’s t-test. P < 0.05 was considered significant.
SGC-7901 cells in untreated control group and 20 mg·L-1 VES group were cultured for 48 h, collected and stained with DAPI. The morphological changes were observed with fluorescent apoptosis occurred after 24 h and 48 h of 20 mg·L-1 VES treatment, respectively.microscope at 300-500 nm (Figure 1). The nuclei in UT control group exhibited circular-like shape, clear edge, and homogeneous staining; while those treated with VES showed uneven edge, chromatin condensation, pyknosis and formation of apoptotic body. 23.7% and 89.6%.
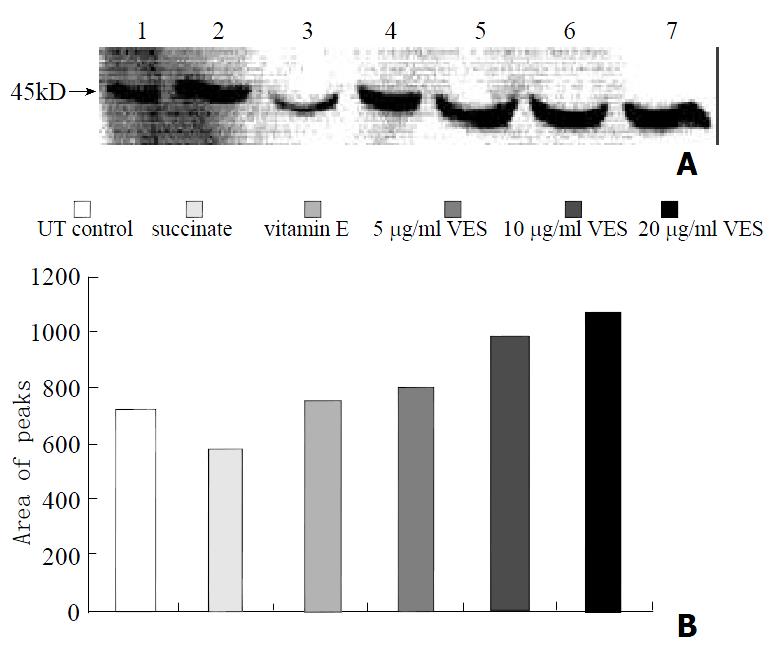
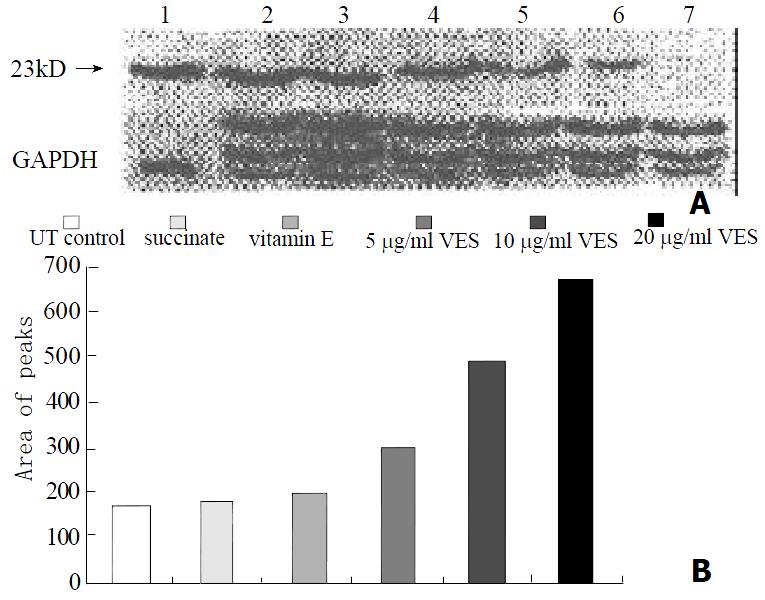
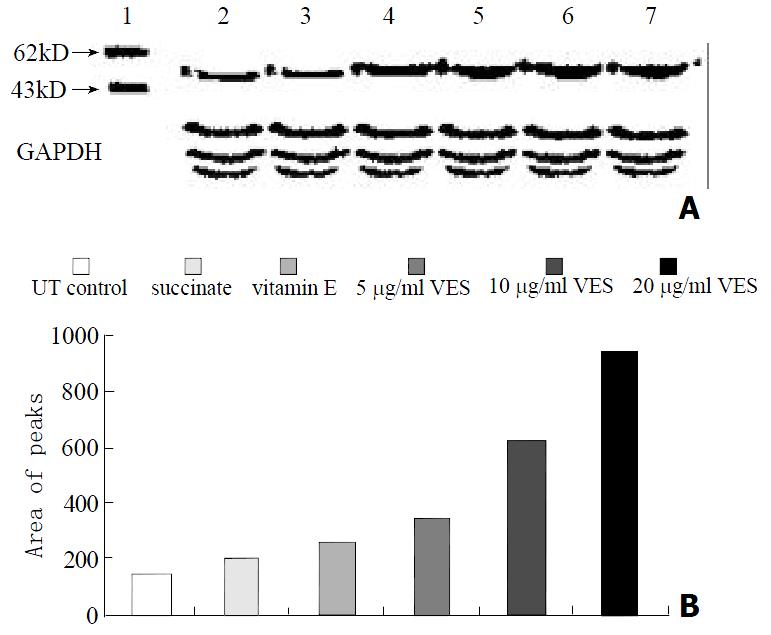
The protein levels of Fas, FADD and caspase-8, as determined by western blot analysis of cells extracts obtained from VES-treated SGC-7901 cells, were evidently increased in a dose-dependent manner after 24 h of VES treatment (Figures 2, 3 and 4).
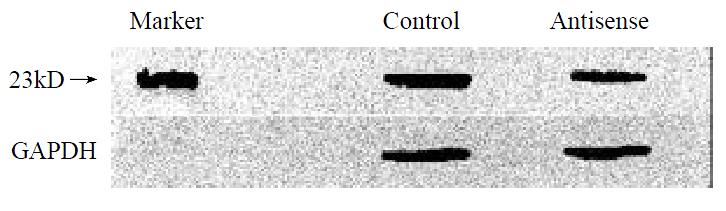
SGC-7901 cells were transiently transfected with antisense oligonucleotides to Fas, followed by 20 mg·L-1 VES treatment for 24 h. The expression of FADD protein was decreased by 77% compared with that in control group (Figure 5), indicating that the blockage of Fas signal obviously inhibited the expression of FADD protein.
SGC-7901 cells were transfected with Fas and FADD antisense oligonucleotides, respectively, followed by VES treatment for 24 h at different doses. Caspase-8 activity in both cases was decreased with significant differences compared with the activity in the same dose of VES-treated cells untransfected in an obviously dose-dependent manner (Table 1). Meanwhile, caspase-8 activity in Fas antisense-transfected cells was reduced more than that in FADD antisense-transfected cells and the differences were significant.
Fas (also called CD95/APO-1), a 45 kDa type I transmembrane protein, belongs to the nerve growrth factor(NGF)/tumor necrosis factor (TNF) receptor superfamily. As a member of five death domain-containing receptors, Fas intiates a signal-transduction cascade leading to programmed cell death[39-42]. In this study, we determined the expression of Fas protein in VES-treated SGC-7901 cells. The data showed that after 24 h of VES treatment, the expression of Fas protein was evidently increased with a marked dose-dependent relationship in comparison with control, indicating that Fas signal pathway is initiated in the course of VES-triggered apoptosis. After VES treatment following transfection of Fas antisense oligonuceotides into SGC-7901 cells, the expression of Fas protein and caspase-8 activity were obviously reduced and VES-induced apoptosis was inhibited by 50%, implicating that Fas may play an essential role in VES-mediated apoptosis in human gastric cancer SGC-7901 cells.
All the death receptors’ cytoplasmic regions contain a death domain (DD) reqired for cytotoxic signal transduction. Engagement of death receptors by their ligands can lead death receptors to oligomerization; then an adaptor protein is required to recruit death receptors to their effectors. Engagement of Fas by Fas ligand or agonistic anti-Fas antibodies can induce apoptosis in Fas-bearing cells[43-46]. The main death pathway initiated from Fas activation involves a series of death associated molecules including FAP-1 (Fas-associated protein 1), RIP (receptor interaction protein) and FADD (Fas-associated death domain-containing protein)[47]. Therefore, the roles of FADD in VES-treated SGC-7901 cells were also investigated in the present study.
FADD (also known as MORT1), a cytoplasmic protein, contains 208 amimo acids and N-terminal amino acids of FADD constitute a death effector domain (DED) essential to death signal transduction[47-50]. We treated SGC-7901 cells with VES and found that the level of FADD protein was obviously elevated compared with control in an evident dose-dependent manner, showing that FADD was also involved in the signaling pathway of VES-mediated apoptosis in SGC-7901 cells. In addition, we transfected SGC-7901 cells with FADD antisense oligonucleotides followed by treating them with VES. The results showed that the expression of FADD protein and caspase-8 activity was obviously inhibited, further suggesting that FADD was associated with VES-triggered apoptosis in human gastric cancer SGC-7901 cells.
Caspase-8 (MACH1/FLICE), a member of interleukin 1β-converting enzyme family of proapoptotic proteases, contains two N-terminal stretches that are apparently homologous to death effector domain (DED) of FADD through which FADD recruits to caspase-8 leading to the activation of the proteolytic cascade of caspases[51-54]. Kim et al[55] found that Fas-mediated apoptosis was completely blocked in caspase-8-deficient Jurkat T lymphocytes and restored in the case of recruitment of wild-type caspase-8, indicating that caspase-8 is an important mediator of Fas-induced apoptosis. We show here that the expression and activity of caspase-8 in SGC-7901 cells were apparently elevated, demonstrating that caspase-8 is associated with VES-induced apoptosis. In order to explore the relationship among Fas, FADD and caspase-8 in VES-induced apoptosis in SGC-7901 cells, we determined the activity of caspase-8 following transfection of SGC-7901 cells with Fas and FADD antisense oligonucleotides. The data showed that the blockage of Fas and FADD obviously reduced the activity of caspase-8 in VES-treated SGC-7901 cells, while the blockage of Fas did more than that of FADD.
In summary, the adaptor protein FADD is recruited to Fas receptor via mutual interaction of their DDs. FADD in turn recruits procaspase-8 through interaction between DEDs of FADD and procaspase-8. Upon formation of this death-inducing signaling complex, procaspase-8 is activated leading to the activation of the proteolytic cascade of caspases, so Fas may play a crucial role in VES-mediated apoptosis in human gastric cancer SGC-7901 cells. VES-mediated apoptosis is very complex. It is reported that mitochondria permeability transition (MPT) participates in apoptosis[56-61]. Therefore, additional studies should provide insight into the role of the biological significance of mitochondria in the mechanisms of tumor cell growth inhibition by VES in future.
| 1. | Fariss MW, Fortuna MB, Everett CK, Smith JD, Trent DF, Djuric Z. The selective antiproliferative effects of alpha-tocopheryl hemisuccinate and cholesteryl hemisuccinate on murine leukemia cells result from the action of the intact compounds. Cancer Res. 1994;54:3346-3351. [PubMed] |
| 2. | Ottino P, Duncan JR. Effect of alpha-tocopherol succinate on free radical and lipid peroxidation levels in BL6 melanoma cells. Free Radic Biol Med. 1997;22:1145-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Kogure K, Morita M, Nakashima S, Hama S, Tokumura A, Fukuzawa K. Superoxide is responsible for apoptosis in rat vascular smooth muscle cells induced by alpha-tocopheryl hemisuccinate. Biochim Biophys Acta. 2001;1528:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Turley JM, Ruscetti FW, Kim SJ, Fu T, Gou FV, Birchenall-Roberts MC. Vitamin E succinate inhibits proliferation of BT-20 human breast cancer cells: increased binding of cyclin A negatively regulates E2F transactivation activity. Cancer Res. 1997;57:2668-2675. [PubMed] |
| 5. | Neuzil J, Weber T, Gellert N, Weber C. Selective cancer cell killing by alpha-tocopheryl succinate. Br J Cancer. 2001;84:87-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 190] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Pussinen PJ, Lindner H, Glatter O, Reicher H, Kostner GM, Wintersperger A, Malle E, Sattler W. Lipoprotein-associated alpha-tocopheryl-succinate inhibits cell growth and induces apoptosis in human MCF-7 and HBL-100 breast cancer cells. Biochim Biophys Acta. 2000;1485:129-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Kline K, Yu W, Zhao B, Turley JM, Sanders BG. Vitamin E Succinate: Mechanisms of action as tumor cell growth inhibitor. In: Nutrients in Cancer Prevention and Treatment. Prasad KN. Santamaria L and Williams RM (eds). Totowa. NY:Humana. 1995;39-56. |
| 8. | Israel K, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits the proliferation of human prostatic tumor cells with defective cell cycle/differentiation pathways. Nutr Cancer. 1995;24:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits EL4 thymic lymphoma cell growth by inducing apoptosis and DNA synthesis arrest. Nutr Cancer. 1997;27:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Simmons-Menchaca M, Qian M, Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate inhibits DNA synthesis and enhances the production and secretion of biologically active transforming growth factor-beta by avian retrovirus-transformed lymphoid cells. Nutr Cancer. 1995;24:171-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Wu K, Zhao Y, Liu BH, Li Y, Liu F, Guo J, Yu WP. RRR-alpha-tocopheryl succinate inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2002;8:26-30. [PubMed] |
| 12. | Kim SJ, Bang OS, Lee YS, Kang SS. Production of inducible nitric oxide is required for monocytic differentiation of U937 cells induced by vitamin E-succinate. J Cell Sci. 1998;111:435-441. [PubMed] |
| 13. | You H, Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate induces MDA-MB-435 and MCF-7 human breast cancer cells to undergo differentiation. Cell Growth Differ. 2001;12:471-480. [PubMed] |
| 14. | Yu W, Sanders BG, Kline K. Modulation of murine EL-4 thymic lymphoma cell proliferation and cytokine production by vitamin E succinate. Nutr Cancer. 1996;25:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Yu W, Israel K, Liao QY, Aldaz CM, Sanders BG, Kline K. Vitamin E succinate (VES) induces Fas sensitivity in human breast cancer cells: role for Mr 43,000 Fas in VES-triggered apoptosis. Cancer Res. 1999;59:953-961. [PubMed] |
| 16. | Yu W, Liao QY, Hantash FM, Sanders BG, Kline K. Activation of extracellular signal-regulated kinase and c-Jun-NH(2)-terminal kinase but not p38 mitogen-activated protein kinases is required for RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells. Cancer Res. 2001;61:6569-6576. [PubMed] |
| 17. | Neuzil J, Weber T, Schröder A, Lu M, Ostermann G, Gellert N, Mayne GC, Olejnicka B, Nègre-Salvayre A, Stícha M. Induction of cancer cell apoptosis by alpha-tocopheryl succinate: molecular pathways and structural requirements. FASEB J. 2001;15:403-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 220] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Schwartz J, Shklar G. The selective cytotoxic effect of carotenoids and alpha-tocopherol on human cancer cell lines in vitro. J Oral Maxillofac Surg. 1992;50:367-73; discussion 373-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Malafa MP, Neitzel LT. Vitamin E succinate promotes breast cancer tumor dormancy. J Surg Res. 2000;93:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Ariazi EA, Satomi Y, Ellis MJ, Haag JD, Shi W, Sattler CA, Gould MN. Activation of the transforming growth factor beta signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res. 1999;59:1917-1928. [PubMed] |
| 21. | Kline K, Yu W, Sanders BG. Vitamin E: Mechanisms of Action as Tumor Cell Growth Inhibitors. Cancer and Nutrition. K.N. Prasad and W.C. Cole(Eds). IOS Press. 1998;37-53. |
| 22. | Turley JM, Fu T, Ruscetti FW, Mikovits JA, Bertolette DC, Birchenall-Roberts MC. Vitamin E succinate induces Fas-mediated apoptosis in estrogen receptor-negative human breast cancer cells. Cancer Res. 1997;57:881-890. [PubMed] |
| 23. | Liu HF, Liu WW, Fang DC. Study of the relationship between apoptosis and proliferation in gastric carcinoma and its precan-cerous lesion. Shijie Huaren Xiaohua Zazhi. 1999;7:649-651. |
| 24. | Zhuang XQ, Lin SR. Progress in research on the relationsh be-tween Hp and stomach cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:206-207. |
| 25. | Zhu ZH, Xia ZS, He SG. The effects of ATRA and 5Fu on telomerase activity and cell growth of gastric cancer cells in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:669-673. |
| 26. | Xia JZ, Zhu ZG, Liu BY, Yan M, Yin HR. Significance of immunohistoche mically demonstrated micrometastases to lymph nodes in gastric carcinomas. Shijie Huaren Xiaohua Zazhi. 2000;8:1113-1116. |
| 27. | Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532-539. [PubMed] |
| 28. | Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County,Fujian Province,China. World J Gastroenterol. 2000;6:374-376. [PubMed] |
| 29. | Yao XX, Yin L, Zhang JY, Bai WY, Li YM, Sun ZC. hTERT ex-pression and cellular immunity in gastric cancer and precancerosis. Shijie Huaren Xiaohua Zazhi. 2001;9:508-512. |
| 30. | Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403-406. [PubMed] |
| 31. | Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54-59. [PubMed] |
| 32. | Liu JR, Li BX, Chen BQ, Han XH, Xue YB, Yang YM, Zheng YM, Liu RH. Effect of cis-9, trans-11-conjugated linoleic acid on cell cycle of gastric adenocarcinoma cell line (SGC-7901). World J Gastroenterol. 2002;8:224-229. [PubMed] |
| 33. | Wu K, Ren Y, Guo J. The effects of vitamin E succinate on the cyclic regulation protein of human gastric cancer cells. Weisheng Dulixue Zazhi. 1998;12:203-207. |
| 34. | Liu B, Wu K, Zhao D. [Inhibition of human gastric carcinoma cell growth by vitamin E succinate]. Weisheng Yanjiu. 2000;29:172-174. [PubMed] |
| 35. | Wu K, Guo J, Shan YJ, Liu BH. The effects of vitamin E succinate on apoptosis in human gastric cancer. Weisheng Dulixue Zazhi. 1999;13:84-90. |
| 36. | Liu BH, Wu K. Study on the growth inhibition of Vitamin E Suc-cinate in human gastric cancer cell. Aibian Jibian Tubian. 2000;12:79-81. |
| 37. | Wu K, Shan YJ, Zhao Y, Yu JW, Liu BH. Inhibitory effects of RRR-alpha-tocopheryl succinate on benzo(a)pyrene (B(a)P)-induced forestomach carcinogenesis in female mice. World J Gastroenterol. 2001;7:60-65. [PubMed] |
| 38. | Wu K, Liu BH, Zhao DY, Zhao Y. Effect of vitamin E succinate on expression of TGF-beta1, c-Jun and JNK1 in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2001;7:83-87. [PubMed] |
| 39. | Zhao Y, Wu K. Cell death molecule Fas/CD95 and apoptosis. Aibian Jibian Tubian. 2001;13:55-58. |
| 40. | Droin N, Bichat F, Rébé C, Wotawa A, Sordet O, Hammann A, Bertrand R, Solary E. Involvement of caspase-2 long isoform in Fas-mediated cell death of human leukemic cells. Blood. 2001;97:1835-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Wei HS, Li DG, Lu HM. Hepatic cell apoptosis and fas gene. Shijie Huaren Xiaohua Zazhi. 1999;7:531-532. |
| 42. | Chatterjee D, Schmitz I, Krueger A, Yeung K, Kirchhoff S, Krammer PH, Peter ME, Wyche JH, Pantazis P. Induction of apoptosis in 9-nitrocamptothecin-treated DU145 human prostate carcinoma cells correlates with de novo synthesis of CD95 and CD95 ligand and down-regulation of c-FLIP(short). Cancer Res. 2001;61:7148-7154. [PubMed] |
| 43. | Liu HF, Liu WW, Fang DC. Effect of combined anti Fas mAb and IFN-γ on the induction of apoptosis in human gastric carcinoma cell line SGC-7901. Shijie Huaren Xiaohua Zazhi. 2000;8:1361-1364. |
| 44. | Tan LJ, Jiang W, Zhang N, Zhang XR, Qiu DH. Fas/FasL expres-sion of esophageal squamous cell carcinoma, dysplasia tissues and normal mucosa. Shijie Huaren Xiaohua Zazhi. 2001;9:15-19. |
| 45. | Peng ZH, Xing TH, Qiu GQ, Tang HM. Relationship between Fas/FasL expression and apoptosis of colon adenocarcinoma cell lines. World J Gastroenterol. 2001;7:88-92. [PubMed] |
| 46. | Boldrini L, Faviana P, Pistolesi F, Gisfredi S, Di Quirico D, Lucchi M, Mussi A, Angeletti CA, Baldinotti F, Fogli A. Alterations of Fas (APO-1/CD 95) gene and its relationship with p53 in non small cell lung cancer. Oncogene. 2001;20:6632-6637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Hu WH, Johnson H, Shu HB. Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem. 2000;275:10838-10844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 239] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Sun BH, Zhao XP, Wang BJ, Yang DL, Hao LJ. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World J Gastroenterol. 2000;6:223-227. [PubMed] |
| 49. | Strasser A, Newton K. FADD/MORT1, a signal transducer that can promote cell death or cell growth. Int J Biochem Cell Biol. 1999;31:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Kabra NH, Kang C, Hsing LC, Zhang J, Winoto A. T cell-specific FADD-deficient mice: FADD is required for early T cell development. Proc Natl Acad Sci USA. 2001;98:6307-6312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Engels IH, Stepczynska A, Stroh C, Lauber K, Berg C, Schwenzer R, Wajant H, Jänicke RU, Porter AG, Belka C. Caspase-8/FLICE functions as an executioner caspase in anticancer drug-induced apoptosis. Oncogene. 2000;19:4563-4573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 52. | Gómez-Angelats M, Cidlowski JA. Protein kinase C regulates FADD recruitment and death-inducing signaling complex formation in Fas/CD95-induced apoptosis. J Biol Chem. 2001;276:44944-44952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633-20640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 430] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 54. | Packard BZ, Komoriya A, Brotz TM, Henkart PA. Caspase 8 activity in membrane blebs after anti-Fas ligation. J Immunol. 2001;167:5061-5066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Kim IK, Chung CW, Woo HN, Hong GS, Nagata S, Jung YK. Reconstitution of caspase-8 sensitizes JB6 cells to TRAIL. Biochem Biophys Res Commun. 2000;277:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Halestrap AP, Doran E, Gillespie JP, O'Toole A. Mitochondria and cell death. Biochem Soc Trans. 2000;28:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 224] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 57. | Tafani M, Schneider TG, Pastorino JG, Farber JL. Cytochrome c-dependent activation of caspase-3 by tumor necrosis factor requires induction of the mitochondrial permeability transition. Am J Pathol. 2000;156:2111-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Costantini P, Jacotot E, Decaudin D, Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst. 2000;92:1042-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 399] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 59. | Zamzami N, El Hamel C, Maisse C, Brenner C, Muñoz-Pinedo C, Belzacq AS, Costantini P, Vieira H, Loeffler M, Molle G. Bid acts on the permeability transition pore complex to induce apoptosis. Oncogene. 2000;19:6342-6350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 60. | Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030-12034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 379] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 61. | Li HL, Chen DD, Li XH, Zhang HW, Lü JH, Ren XD, Wang CC. JTE-522-induced apoptosis in human gastric adenocarcinoma [correction of adenocarcinoma] cell line AGS cells by caspase activation accompanying cytochrome C release, membrane translocation of Bax and loss of mitochondrial membrane potential. World J Gastroenterol. 2002;8:217-223. [PubMed] |
Edited by Pang LH