Published online Dec 15, 2002. doi: 10.3748/wjg.v8.i6.1023
Revised: April 12, 2002
Accepted: April 20, 2002
Published online: December 15, 2002
AIM: To assess the safety and efficacy of different intravenous chemotherapeutic regimens in patients with gastric carcinomas who had undergone gastrectomy.
METHODS: A meta-analysis of all the relevant randomized controlled trials (RCTs) was performed. Language was restricted to Chinese and English. RCTs were identified from Medline and Embase (1980-2001/4), and Chinese Bio-medicine Database (1990-2001/1). Literature references were checked at the same time. We included randomized and quasi-randomized trials comparing the efficacy of intravenous chemotherapy after gastrectomy with that of surgery alone in patients with confirmed gastric carcinomas who had undergone gastrectomy. Selection criteria were: randomized or quasi-randomized trials with following-up results; Trials could be double-blind, single-blind or not blind; Chemotherapy groups were given intravenous chemotherapy after gastrectomy without neo-adjuvant chemotherapy, intraperitoneal hyperthermic perfusion, radiotherapy or chemoimmunotherapy; Controlled group included those receiving gastrectomy alone. The following data were extracted: the number of survival and death by the end of the follow-up; the different agents and doses of the intravenous chemotherapy; the baseline of the chemotherapy group and the controlled arm; the serious adverse events; the statistical consideration; cost-effectiveness analysis. The statistical analysis was performed by RevMan4.1 software which was provided by the Cochrane Collaboration. A P value of < 0.05 was considered statistically significant. Meta-analysis was done with random effects model. Heterogeneity was checked by chi-square test. Sensitivity analysis was performed by excluding the trials in which Jadad-scale was only 1 score. The result was expressed with odds ratio (OR) for the categorical variable.
RESULTS: Fourteen trials involving 4543 patients were included. Meta-analysis was done with random effects model Heterogeneity and sensitivity analysis were performed also. The effect of intravenous chemotherapy after gastrectomy was better than surgery alone (odds ratio 0.56, 95%CI 0.40-0.79). There was a significant difference between the two groups by u-test (P = 0.0008). Sensitivity analysis revealed the same difference (odds ratio 0.81, 95%CI 0.70-0.94). Of fourteen trials, only three studies were of high quality according to the Jadad-scale (with three score). There was one meta-analysis trial and the others, about ten trials, were of low quality. There was no trial which mentioned sample-size calculation, allocation concealment, intention-to-treat analysis. Most of the trials didn’t describe the blind-procedure. There were five trials which detailed the side-effects according to the toxicity grade by WHO standard. The side-effects halting treatment were haematologic and biochemical toxicity, debilitating nausea and vomiting. There were two patients died of chemotherapy toxicity.
CONCLUSION: Based on the review, intravenous chemotherapy after gastrectomy may have positive treatment effect on gastric cancer. However, the evidence is not strong because of the general low methodologic quality of the RCTs. Therefore, we can’t make the conclusion that intravenous chemotherapy after gastrectomy may have better treatment effect on gastric cancer than that of surgery alone. Rigorously designed, randomised, double-blind, placebo-controlled trials are required.
- Citation: Hu JK, Chen ZX, Zhou ZG, Zhang B, Tian J, Chen JP, Wang L, Wang CH, Chen HY, Li YP. Intravenous chemotherapy for resected gastric cancer: meta-analysis of randomized controlled trials. World J Gastroenterol 2002; 8(6): 1023-1028
- URL: https://www.wjgnet.com/1007-9327/full/v8/i6/1023.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i6.1023
Gastric cancer is one of the most common cancers worldwide. The outcome of patients with gastric carcinoma has recently been significantly improved with advances in experimental researches, early diagnosis and surgical techniques[1-44].Although chemotherapy and radiation therapy have been tried as either an adjuvant or palliative treatment, their values are limited by toxicity or the lack of efficacy[45].While surgery remains the mainstay of potentially curative treatment, survival rates for patients able to undergo complete resection are poor[46].The five year survival rate for resected gastric cancer is about 30%-60% which has been disappointing. A number of studies have investigated whether intravenous chemotherapy after a resection improves the survival rate or not, but the results are different and disputed. Hermans et al[47] reviewed the randomized controlled trials by meta-analysis, the results indicated that postoperative chemotherapy in general offered no additional survival benefit for patients with curatively resected gastric cancer. Janunger et al[48]performed a systematic overview of chemotherapy effects in gastric cancer by the Swedish Council of Technology Assessment in Health Care(SBU). A meta-analysis of 21 randomised adjuvant studies revealed a statistically significant survival benefit (OR = 0.84, 95%CI 0.74-0.96).
The aim of meta-analysis is to summarize the results of randomized trials performed to evaluate the effect of intravenous chemotherapy for gastric cancer[47]. The analysis is restricted to trials published since 1980. Surgical resection without any adjuvant therapy is considered standard treatment. Only intravenous chemotherapy trials with gastrectomy control arm were taken into consideration in this meta-analysis.
Randomized or quasi-randomized trials comparing the efficacy of intravenous chemotherapy after gastrectomy with that of surgery alone in patients with confirmed gastric carcinomas who had received gastrectomy were included in this meta-analysis. Language was restricted to Chinese and English.
Selection criteria were: randomized or quasi-randomized trials with following-up results; Trials could be double-blind, single-blind or not blind; Chemotherapy groups were given intravenous chemotherapy after gastrectomy without neo-adjuvant chemotherapy, intraperitoneal hyperthermic perfusion, radiotherapy or chemoimmunotherapy; Controlled group included those receiving gastrectomy alone.
Exclusion criteria were prior malignancy; neo-adjuvant chemotherapy, intraperitoneal hyperthermic perfusion, radiotherapy or chemoimmunotherapy; patients who didn’t receive gastrectomy; the controlled studies also included those without gastrectomy.
Search strategy Search was applied to the following electronic databases: the Cochrane Library, MEDLINE (1980-2001.4), EMBASE (1980-2001.4) and Chinese Bio-medicine Database (1990-2001/1). Literature reference proceedings were handsearched at the same time. The searching words were chemotherapy, stomach neoplasms and surgery.
Data collection and analysis Data were extracted independently by two reviewers. The methodological quality of trials was evaluated using the Jadad-scale plus allocation concealment. Intention-to-treat analyses were performed.
The following data were extracted: the number of survival and death by the end of the follow-up; the different agents and doses of the intravenous chemotherapy; the baseline of the chemotherapy group and the controlled arm; the serious adverse events; the statistical consideration; cost-effectiveness analysis.
The statistical analysis was performed by RevMan4.1 software which was provided by the Cochrane Collaboration. A P value of < 0.05 was considered statistically significant. Meta-analysis was done with random effects model. Heterogeneity was checked by chi-square test. If the results of the trials had heterogeneity, random effects model was used for meta-analysis. Sensitivity analyses was performed by excluding the trials which Jadad-scale was only 1 score. The result was expressed with odds ratio(OR) for the categorical variable.
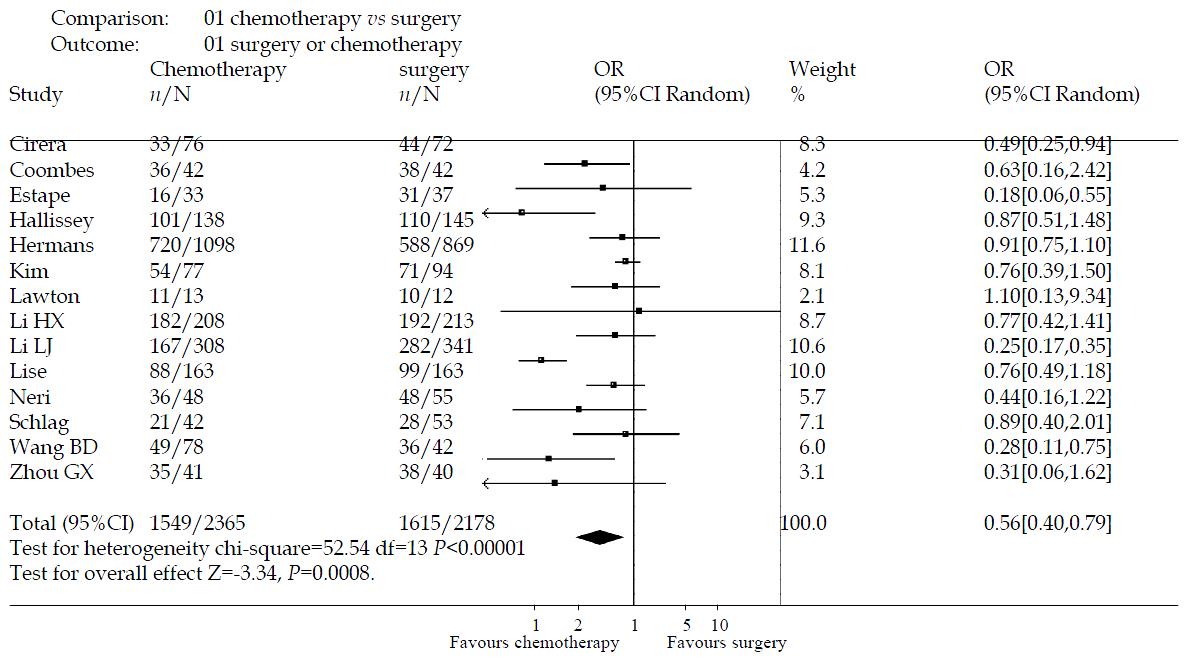
There were 1076 papers relevant to the searching words. Through the steps of screening the title, reading the abstract and the entire article, twenty-seven randomized trials were identified. Only fourteen randomized trials comparing the efficacy of intravenous chemotherapy after gastrectomy with that of surgery alone in patients with confirmed gastric carcinomas, including 4543 patients, met the inclusion criteria(1A-5A)[47,49-56]. There were six trials which were excluded for repetitive studies(6A-7A)[57-60], five for having been included in the result of the Hermans’ meta-analysis[61-65], two for no available data[66,67]. Of fourteen included trials, four trials were conducted in China (see appendix)(2A-5A), three in England(1A)[50,55], two in Italy[49,54], two in Spain[51,56], one in Korea[52], Germany[53] and Netherlands[47] respectively. The average sample size was 324 patients (from 25 to 1967 patients). The follow-up time was from forty-eight months to one hundred and twenty months. The chemotherapy regimens used were FAM, MMC, MFV, MFC, FEM and 5-FU + BCNU(Table 1). All the baselines of the trials were parallel. None of them performed the cost- effectiveness analysis.
| Author | Published time | Chemotherapy regimens | Chemotherapy group (number of death/total) | Surgery group (number of death/total) | Follow -uptime (months) |
| Lise | 1995 | FAM | 88/163 | 99/163 | 78 |
| Hallissey | 1994 | FAM | 101/138 | 110/145 | 60 |
| Estape | 1991 | MMC | 16/33 | 31/37 | 120 |
| Kim | 1992 | FM | 54/77 | 71/94 | 60 |
| Li LJ | 1994 | MFV/MFC/FAM | 167/308 | 282/341 | 60 |
| Wang BD | 1994 | FM+Ara-C | 49/78 | 36/42 | 36 |
| Li HX | 1994 | FM | 182/208 | 192/213 | 60 |
| Coombes | 1998 | FEM | 36/42 | 38/42 | 60 |
| Schlag | 1987 | 5Fu + BCNU | 21/42 | 28/53 | 72 |
| Neri | 1996 | Epidoxorubicin | 36/48 | 48/55 | 36 |
| Zhou GX | 1998 | FM | 35/41 | 38/40 | 60 |
| Lawton | 1981 | 5Fu + BCNU | 11/13 | 10/12 | 60 |
| Cirera | 1999 | MMC + Tegafur | 33/76 | 44/72 | 37 |
| Hermans | 1993 | Meta-analysis | 720/1098 | 588/869 | NA |
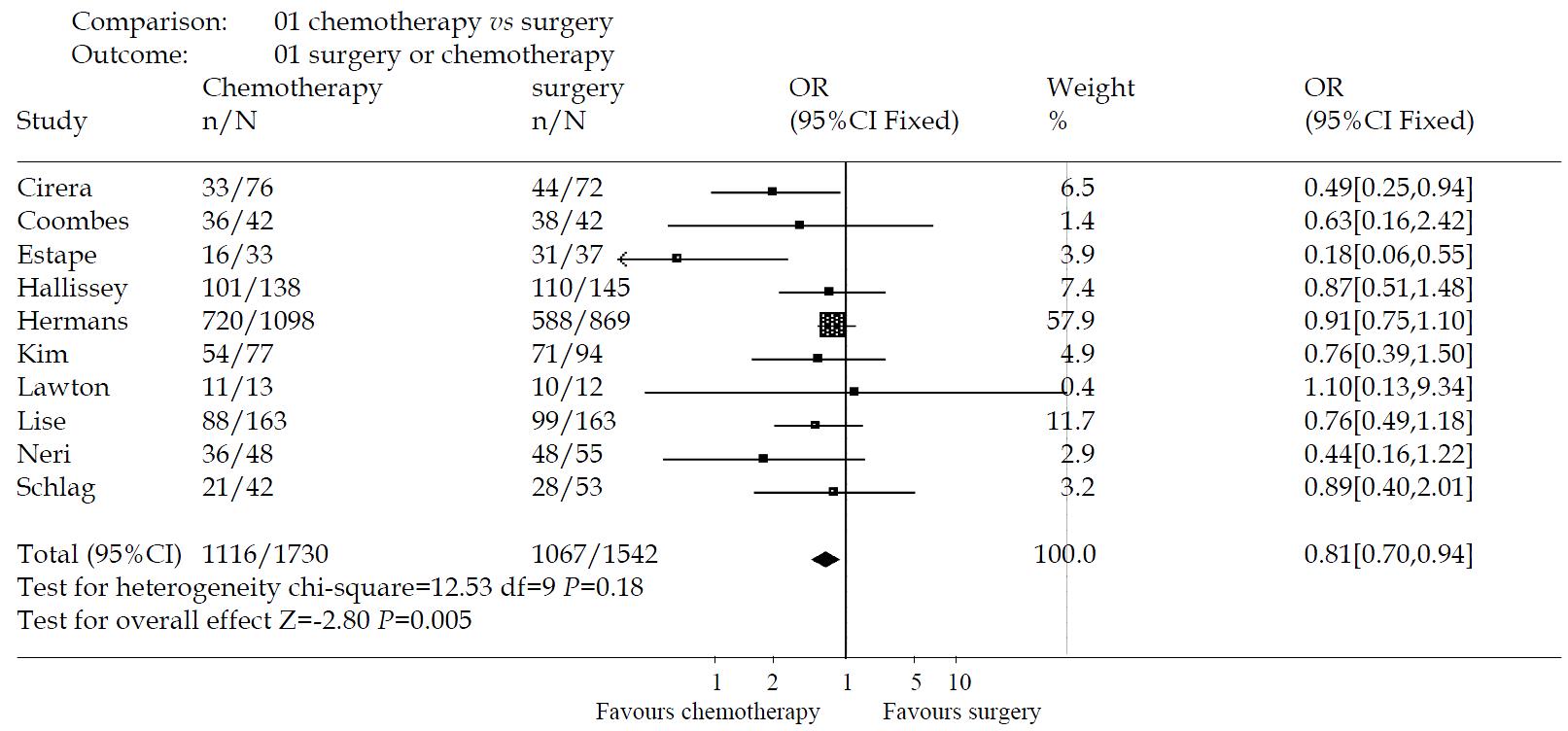
The effectiveness of intravenous chemotherapy after gastrectomy was better than surgery alone (odds ratio 0.56, 95%CI 0.40-0.79). The results of the trials showed inconsistency, as checked by the chi-square test (χ2 = 52.54, P < 0.00001). There was a significant difference between the two groups by u-test (P = 0.0008) (Figure 1). By excluding the low quality trials(2A-5A), the sensitivity analysis was performed and revealed the same difference between chemotherapy and surgery alone (odds ratio 0.81, 95%CI 0.70-0.94, P = 0.005) (Figure 2).
Of fourteen trials, only three studies[49,50,56] were of high quality according to the Jadad-scale (with three score). There was one meta-analysis trial[47] and the others, about ten trials were of low quality. There was no trial which mentioned sample-size calculation, allocation concealment, intention-to-treat analysis. Most of the trials didn’t describe the blind-procedure. Therefore, the methodologic quality of the RCTs is not strong enough to testify the conclusion.
There were five trials[49,53-56] which detailed the side-effects of medicine according to World Health Organization grade. The side-effects halting treatment were haematologic and biochemical toxicity, debilitating nausea and vomiting. There were two patients died of chemotherapeutic toxicity (one died of cardiac toxicity and the other of massive alimentary tract hemorrhage because of thrombopenia). Severe toxicity (grade 3 or 4 according to the WHO scale) occurred in 5.33%, with alopecia in 39 patients, leucopenia (WBC values less than 2000/μL) in 18, nausea in 21, thrombopenia (platelet count less than 50000/μL) in 13, anemia in 9, vomiting in 5, diarrhea in 5, gastritis in 5, stomatitis in 4, cardiac toxicity in 4, septicemia in 2 and neural toxicity in 1.
It is well recognized that most patients who undergo curative resection of gastric carcinoma remain at high risk of local and systematic relapse. Thus, a worldwide effort has been done to develop effective adjuvant therapy to reduce this risk[68].
The aim of meta-analysis is to summarize the results of randomized trials performed to evaluate the effect of intravenous chemotherapy for gastric cancer. Surgical resection without any adjuvant therapy is considered standard treatment. Only intravenous chemotherapy trials with gastrectomy control arm were taken into consideration in this meta-analysis. There are two meta-analyses to assess the effect of intravenous chemotherapy for gastric cancer with gastrectomy. Hermans et al[47] researched the randomized controlled trials by meta-analysis; the results indicated that postoperative chemotherapy in general offered no additional survival benefit for patients with curatively resected gastric cancer. Janunger et al[48] performed a systematic overview of chemotherapy effects in gastric cancer by the Swedish Council of Technology Assessment in Health Care (SBU). A meta-analysis of 21 randomised adjuvant studies revealed a statistically significant survival benefit (OR = 0.84, 95%CI 0.74-0.96). But We couldn’t get the original article of Janunger, therefore we didn’t include the trials in this meta-analysis.
Measuring an effect on survival by calculating the odds ratios was proved to be effective in an analysis[47]. Only four trials which were performed by Cirera[56], Estape[51], Li et al(2A) and Wang et al(4A) respectively, demonstrated a positive effect of intravenous chemotherapy versus the controlled group by calculating the odds ratios.
Of included fourteen trials, only three studies were of high quality according to the Jadad-scale. There was one meta-analysis trial and the others, about ten trials were of low quality. There was no trial which mentioned sample-size calculation, allocation concealment, intention-to- treat analysis. Therefore, the methodologic quality of the RCTs is not strong enough to testify the conclusion. Based on the review, intravenous chemotherapy after gastrectomy may have positive treatment effect on gastric cancer. However, the evidence is not strong because of the general low methodologic quality of the RCTs. Rigorously designed, randomised, double-blind, placebo-controlled trials are required.
The toxicity of medicine is an important factor to influence the outcome of the chemotherapy. But unfortunately, there were only five trials which detailed the side effects of medicine according to World Health Organization grade in this meta-analysis. Hence, in the future research, we should put in mind to observe the side effects carefully and describe them by the WHO grade standard.
Recently, such therapies as intraperitoneal hyperthermic perfusion[69-74], neo-adjuvant chemotherapy[75-84], radiotherapy[85-89] and chemoimmunotherapy[52] are demonstrated with a positive effect to reduce the relapse risk. Tao et al[90] revealed that preoperative regional artery chemotherapy had the effect to induce growth inhibition and apoptosis of gastric carcinoma cells. Cao et al[91] found that human primary gastric cancer cell in vitro were methionine-dependent; methionine-free environment might strengthen the killing effect of chemotherapy on human primary gastric cancer cells. But, the scientific conclusion should be supported by the high quality randomized, double-blind, controlled trials.
| 1. | Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54-59. [PubMed] |
| 2. | Zhang XY. Some recent works on diagnosis and treatment of gastric cancer. World J Gastroenterol. 1999;5:1-3. [PubMed] |
| 3. | Zou SC, Qiu HS, Zhang CW, Tao HQ. A clinical and long-term follow-up study of peri-operative sequential triple therapy for gastric cancer. World J Gastroenterol. 2000;6:284-286. [PubMed] |
| 4. | Li Y, Yang L, Cui JT, Li WM, Guo RF, Lu YY. Construction of cDNA representational difference analysis based on two cDNA libraries and identification of garlic inducible expression genes in human gastric cancer cells. World J Gastroenterol. 2002;8:208-212. [PubMed] |
| 5. | Li Y, Lu YY. Applying a highly specific and reproducible cDNA RDA method to clone garlic up-regulated genes in human gastric cancer cells. World J Gastroenterol. 2002;8:213-216. [PubMed] |
| 6. | Liu JR, Li BX, Chen BQ, Han XH, Xue YB, Yang YM, Zheng YM, Liu RH. Effect of cis-9, trans-11-conjugated linoleic acid on cell cycle of gastric adenocarcinoma cell line (SGC-7901). World J Gastroenterol. 2002;8:224-229. [PubMed] |
| 7. | Tovey FI, Hobsley M. Post-gastrectomy patients need to be followed up for 20-30 years. World J Gastroenterol. 2000;6:45-48. [PubMed] |
| 8. | Hou P, Tu ZX, Xu GM, Gong YF, Ji XH, Li ZS. Helicobacter pylori vacA genotypes and cagA status and their relationship to associated diseases. World J Gastroenterol. 2000;6:605-607. [PubMed] |
| 9. | Deng DJ. progress of gastric cancer etiology: N-nitrosamides 1999s. World J Gastroenterol. 2000;6:613-618. [PubMed] |
| 10. | Yin F, Shi YQ, Zhao WP, Xiao B, Miao JY, Fan DM. Suppression of P-gp induced multiple drug resistance in a drug resistant gastric cancer cell line by overexpression of Fas. World J Gastroenterol. 2000;6:664-670. [PubMed] |
| 11. | Cai L, Yu SZ, Ye WM, Yi YN. Fish sauce and gastric cancer: an ecological study in Fujian Province,China. World J Gastroenterol. 2000;6:671-675. [PubMed] |
| 12. | Li QF, Ou Yang GL, Li CY, Hong SG. Effects of tachyplesin on the morphology and ultrastructure of human gastric carcinoma cell line BGC-823. World J Gastroenterol. 2000;6:676-680. [PubMed] |
| 13. | Zhu JS, Su Q, Zhou JG, Hu PL, Xu JH. Study of primary leiomyosarcoma induced by MNNG in BALB/C nude mice. World J Gastroenterol. 2000;6:128-130. [PubMed] |
| 14. | Cao WX, Cheng QM, Fei XF, Li SF, Yin HR, Lin YZ. A study of preoperative methionine-depleting parenteral nutrition plus chemotherapy in gastric cancer patients. World J Gastroenterol. 2000;6:255-258. [PubMed] |
| 15. | Xue JT, Wu J, Meng L, Dong ZW, Shou CC. Expression of VEGF(121) in gastric carcinoma MGC803 cell line. World J Gastroenterol. 2000;6:281-283. [PubMed] |
| 16. | Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County,Fujian Province,China. World J Gastroenterol. 2000;6:374-376. [PubMed] |
| 17. | Zhang FX, Zhang XY, Fan DM, Deng ZY, Yan Y, Wu HP, Fan JJ. Antisense telomerase RNA induced human gastric cancer cell apoptosis. World J Gastroenterol. 2000;6:430-432. [PubMed] |
| 18. | Gu QL, Li NL, Zhu ZG, Yin HR, Lin YZ. A study on arsenic trioxide inducing in vitro apoptosis of gastric cancer cell lines. World J Gastroenterol. 2000;6:435-437. [PubMed] |
| 19. | Wang ZN, Xu HM. Relationship between collagen IV expression and biological behavior of gastric cancer. World J Gastroenterol. 2000;6:438-439. [PubMed] |
| 20. | Han FC, Yan XJ, Su CZ. Expression of the CagA gene ofH. pylori and application of its product. World J Gastroenterol. 2000;6:122-124. [PubMed] |
| 21. | Chen GY, Wang DR. The expression and clinical significance of CD44v in human gastric cancers. World J Gastroenterol. 2000;6:125-127. [PubMed] |
| 22. | Tuo BG, Yan YH, Ge ZL, Ou GW, Zhao K. Ascorbic acid secretion in the human stomach and the effect of gastrin. World J Gastroenterol. 2000;6:704-708. [PubMed] |
| 23. | Huang XQ. Helicobacter pylori infection and gastrointestinal hormones: a review. World J Gastroenterol. 2000;6:783-788. [PubMed] |
| 24. | Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848-854. [PubMed] |
| 25. | Chen JP, Lin C, Xu CP, Zhang XY, Wu M. The therapeutic effects of recombinant adenovirus RA538 on human gastric carcinoma cells in vitro and in vivo. World J Gastroenterol. 2000;6:855-860. [PubMed] |
| 26. | Zhou HP, Wang X, Zhang NZ. Early apoptosis in intestinal and diffuse gastric carcinomas. World J Gastroenterol. 2000;6:898-901. [PubMed] |
| 27. | Chen XQ, Zhang WD, Song YG, Zhou DY. Induction of apoptosis of lymphocytes in rat mucosal immune system. World J Gastroenterol. 1998;4:19-23. [PubMed] |
| 28. | Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of Fas gene or Bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. World J Gastroenterol. 1998;4:421-425. [PubMed] |
| 29. | Wang XW, Xie H. Presence of Fas and Bcl-2 proteins in BEL-7404 human hepatoma cells. World J Gastroenterol. 1998;4:540-543. [PubMed] |
| 30. | Lu YF, Zhao G, Guo CY, Jia SR, Hou YD. Vagus effect on pylorus-preserving gastrectomy. World J Gastroenterol. 1999;5:177-178. [PubMed] |
| 31. | Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene Bax in gastric carcinoma. World J Gastroenterol. 1999;5:15-17. [PubMed] |
| 32. | Li HL, Chen DD, Li XH, Zhang HW, Lu YQ, Ye CL, Ren XD. Changes of NF-kB, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. World J Gastroenterol. 2002;8:431-435. [PubMed] |
| 33. | He XS, Su Q, Chen ZC, He XT, Long ZF, Ling H, Zhang LR. Expression, deletion [was deleton] and mutation of p16 gene in human gastric cancer. World J Gastroenterol. 2001;7:515-521. [PubMed] |
| 34. | Han Y, Han ZY, Zhou XM, Shi R, Zheng Y, Shi YQ, Miao JY, Pan BR, Fan DM. Expression and function of classical protein kinase C isoenzymes in gastric cancer cell line and its drug-resistant sublines. World J Gastroenterol. 2002;8:441-445. [PubMed] |
| 35. | Ji F, Peng QB, Zhan JB, Li YM. Study of differential polymerase chain reaction of C-erbB-2 oncogene amplification in gastric cancer. World J Gastroenterol. 1999;5:152-155. [PubMed] |
| 36. | Xia L, Yuan YZ, Xu CD, Zhang YP, Qiao MM, Xu JX. Effects of epidermal growth factor on the growth of human gastric cancer cell and the implanted tumor of nude mice. World J Gastroenterol. 2002;8:455-458. [PubMed] |
| 37. | Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and p53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779-782. [PubMed] |
| 38. | Liu LX, Liu ZH, Jiang HC, Qu X, Zhang WH, Wu LF, Zhu AL, Wang XQ, Wu M. Profiling of differentially expressed genes in human gastric carcinoma by cDNA expression array. World J Gastroenterol. 2002;8:580-585. [PubMed] |
| 39. | Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801-804. [PubMed] |
| 40. | Miehlke S, Kirsch C, Dragosics B, Gschwantler M, Oberhuber G, Antos D, Dite P, Läuter J, Labenz J, Leodolter A. Helicobacter pylori and gastric cancer: current status of the Austrain Czech German gastric cancer prevention trial (PRISMA Study). World J Gastroenterol. 2001;7:243-247. [PubMed] |
| 41. | Yao XX, Yin L, Sun ZC. The expression of hTERT mRNA and cellular immunity in gastric cancer and precancerosis. World J Gastroenterol. 2002;8:586-590. [PubMed] |
| 42. | Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS,VEGF,tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591-595. [PubMed] |
| 43. | Ren J, Dong L, Xu CB, Pan BR. The role of KDR in the interactions between human gastric carcinoma cell and vascular endothelial cell. World J Gastroenterol. 2002;8:596-601. [PubMed] |
| 44. | Ren J, Dong L, Xu CB, Pan BR. Expression of sphingosine kinase gene in the interactions between human gastric carcinoma cell and vascular endothelial cell. World J Gastroenterol. 2002;8:602-607. [PubMed] |
| 45. | Cunningham D, Hole D, Taggart DJ, Soukop M, Carter DC, McArdle CS. Evaluation of the prognostic factors in gastric cancer: the effect of chemotherapy on survival. Br J Surg. 1987;74:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Averbach AM, Jacquet P. Strategies to decrease the incidence of intra-abdominal recurrence in resectable gastric cancer. Br J Surg. 1996;83:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, Van de Velde CJ. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441-1447. [PubMed] |
| 48. | Janunger KG, Hafström L, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol. 2001;40:309-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Lise M, Nitti D, Marchet A, Sahmoud T, Buyse M, Duez N, Fiorentino M, Dos Santos JG, Labianca R, Rougier P. Final results of a phase III clinical trial of adjuvant chemotherapy with the modified fluorouracil, doxorubicin, and mitomycin regimen in resectable gastric cancer. J Clin Oncol. 1995;13:2757-2763. [PubMed] |
| 50. | Hallissey MT, Dunn JA, Ward LC, Allum WH. The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: five-year follow-up. Lancet. 1994;343:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 187] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Estape J, Grau JJ, Lcobendas F, Curto J, Daniels M, Viñolas N, Pera C. Mitomycin C as an adjuvant treatment to resected gastric cancer. A 10-year follow-up. Ann Surg. 1991;213:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Kim JP, Kwon OJ, Oh ST, Yang HK. Results of surgery on 6589 gastric cancer patients and immunochemosurgery as the best treatment of advanced gastric cancer. Ann Surg. 1992;216:269-278; discussion 278-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Schlag P. Adjuvant chemotherapy in gastric cancer. World J Surg. 1987;11:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Neri B, de Leonardis V, Romano S, Andreoli F, Pernice LM, Bruno L, Borrelli D, Valeri A, Fabbroni S, Intini C. Adjuvant chemotherapy after gastric resection in node-positive cancer patients: a multicentre randomised study. Br J Cancer. 1996;73:549-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Lawton JO, Giles GR, Hall R, Bird GG, Matheson T. Chemotherapy following palliative resection of gastric cancer. Br J Surg. 1981;68:397-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 56. | Cirera L, Balil A, Batiste-Alentorn E, Tusquets I, Cardona T, Arcusa A, Jolis L, Saigí E, Guasch I, Badia A. Randomized clinical trial of adjuvant mitomycin plus tegafur in patients with resected stage III gastric cancer. J Clin Oncol. 1999;17:3810-3815. [PubMed] |
| 57. | Allum WH, Hallissey MT, Ward LC, Hockey MS. A controlled, prospective, randomised trial of adjuvant chemotherapy or radiotherapy in resectable gastric cancer: interim report. British Stomach Cancer Group. Br J Cancer. 1989;60:739-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Allum WH, Hallissey MT, Kelly KA. Adjuvant chemotherapy in operable gastric cancer. 5 year follow-up of first British Stomach Cancer Group trial. Lancet. 1989;1:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Yu CC, Levison DA, Dunn JA, Ward LC, Demonakou M, Allum WH, Hallisey MT. Pathological prognostic factors in the second British Stomach Cancer Group trial of adjuvant therapy in resectable gastric cancer. Br J Cancer. 1995;71:1106-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Pignon JP, Ducreux M, Rougier P. Meta-analysis of adjuvant chemotherapy in gastric cancer: a critical reappraisal. J Clin Oncol. 1994;12:877-878. [PubMed] |
| 61. | Engstrom PF, Lavin PT, Douglass HO, Brunner KW. Postoperative adjuvant 5-fluorouracil plus methyl-CCNU therapy for gastric cancer patients. Eastern Cooperative Oncology Group study (EST 3275). Cancer. 1985;55:1868-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Higgins GA, Amadeo JH, Smith DE, Humphrey EW, Keehn RJ. Efficacy of prolonged intermittent therapy with combined 5-FU and methyl-CCNU following resection for gastric carcinoma. A Veterans Administration Surgical Oncology, Group report. Cancer. 1983;52:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Bonfanti G, Tumori N. Adjuvant treatments following curative resection for gastric cancer. The Italian Gastrointestinal Tumor Study Group. Br J Surg. 1988;75:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Douglass HO, Stablein DM. Controlled trial of adjuvant chemotherapy following curative resection for gastric cancer. The Gastrointestinal Tumor Study Group. Cancer. 1982;49:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Coombes RC, Schein PS, Chilvers CE, Wils J, Beretta G, Bliss JM, Rutten A, Amadori D, Cortes-Funes H, Villar-Grimalt A. A randomized trial comparing adjuvant fluorouracil, doxorubicin, and mitomycin with no treatment in operable gastric cancer. International Collaborative Cancer Group. J Clin Oncol. 1990;8:1362-1369. [PubMed] |
| 66. | Fielding JW, Fagg SL, Jones BG, Ellis D, Hockey MS, Minawa A, Brookes VS, Craven JL, Mason MC, Timothy A. An interim report of a prospective, randomized, controlled study of adjuvant chemotherapy in operable gastric cancer: British Stomach Cancer Group. World J Surg. 1983;7:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Krook JE, O'Connell MJ, Wieand HS, Beart RW, Leigh JE, Kugler JW, Foley JF, Pfeifle DM, Twito DI. A prospective, randomized evaluation of intensive-course 5-fluorouracil plus doxorubicin as surgical adjuvant chemotherapy for resected gastric cancer. Cancer. 1991;67:2454-2458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Shimada K, Ajani JA. Adjuvant therapy for gastric carcinoma patients in the past 15 years: a review of western and oriental trials. Cancer. 1999;86:1657-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 69. | Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 70. | Yu W, Whang I, Suh I, Averbach A, Chang D, Sugarbaker PH. Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg. 1998;228:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 135] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Rosen HR, Jatzko G, Repse S, Potrc S, Neudorfer H, Sandbichler P, Zacherl J, Rabl H, Holzberger P, Lisborg P. Adjuvant intraperitoneal chemotherapy with carbon-adsorbed mitomycin in patients with gastric cancer: results of a randomized multicenter trial of the Austrian Working Group for Surgical Oncology. J Clin Oncol. 1998;16:2733-2738. [PubMed] |
| 72. | Sautner T, Hofbauer F, Depisch D, Schiessel R, Jakesz R. Adjuvant intraperitoneal cisplatin chemotherapy does not improve long-term survival after surgery for advanced gastric cancer. J Clin Oncol. 1994;12:970-974. [PubMed] |
| 73. | Yonemura Y, Fujimura T, Fushida S, Takegawa S, Kamata T, Katayama K, Kosaka T, Yamaguchi A, Miwa K, Miyazaki I. Hyperthermo-chemotherapy combined with cytoreductive surgery for the treatment of gastric cancer with peritoneal dissemination. World J Surg. 1991;15:530-55; discussion 530-55;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Hagiwara A, Takahashi T, Ueda T, Lee R, Takeda M, Itoh T. Intraoperative chemotherapy with carbon particles adsorbing mitomycin C for gastric cancer with peritoneal dissemination in rabbits. Surgery. 1988;104:874-881. [PubMed] |
| 75. | Leichman L, Silberman H, Leichman CG, Spears CP, Ray M, Muggia FM, Kiyabu M, Radin R, Laine L, Stain S. Preoperative systemic chemotherapy followed by adjuvant postoperative intraperitoneal therapy for gastric cancer: a University of Southern California pilot program. J Clin Oncol. 1992;10:1933-1942. [PubMed] |
| 76. | Kiyabu M, Leichman L, Chandrasoma P. Effects of preoperative chemotherapy on gastric adenocarcinomas. A morphologic study of 25 cases. Cancer. 1992;70:2239-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 77. | Ajani JA, Ota DM, Jessup JM, Ames FC, McBride C, Boddie A, Levin B, Jackson DE, Roh M, Hohn D. Resectable gastric carcinoma. An evaluation of preoperative and postoperative chemotherapy. Cancer. 1991;68:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 78. | Yonemura Y, Sawa T, Kinoshita K, Matsuki N, Fushida S, Tanaka S, Ohoyama S, Takashima T, Kimura H, Kamata T. Neoadjuvant chemotherapy for high-grade advanced gastric cancer. World J Surg. 1993;17:256-261; discussion 261-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Ajani JA, Mansfield PF, Lynch PM, Pisters PW, Feig B, Dumas P, Evans DB, Raijman I, Hargraves K, Curley S. Enhanced staging and all chemotherapy preoperatively in patients with potentially resectable gastric carcinoma. J Clin Oncol. 1999;17:2403-2411. [PubMed] |
| 80. | Fink U, Schuhmacher C, Stein HJ, Busch R, Feussner H, Dittler HJ, Helmberger A, Böttcher K, Siewert JR. Preoperative chemotherapy for stage III-IV gastric carcinoma: feasibility, response and outcome after complete resection. Br J Surg. 1995;82:1248-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Wilke H, Preusser P, Fink U, Gunzer U, Meyer HJ, Meyer J, Siewert JR, Achterrath W, Lenaz L, Knipp H. Preoperative chemotherapy in locally advanced and nonresectable gastric cancer: a phase II study with etoposide, doxorubicin, and cisplatin. J Clin Oncol. 1989;7:1318-1326. [PubMed] |
| 82. | Melcher AA, Mort D, Maughan TS. Epirubicin, cisplatin and continuous infusion 5-fluorouracil (ECF) as neoadjuvant chemotherapy in gastro-oesophageal cancer. Br J Cancer. 1996;74:1651-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 83. | Sugamura K, Makino M, Shirai H, Kimura O, Maeta M, Itoh H, Kaibara N. Enhanced induction of apoptosis of human gastric carcinoma cells after preoperative treatment with 5-fluorouracil. Cancer. 1997;79:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 84. | Becker K, Fumagalli U, Mueller JD, Fink U, Siewert JR, Höfler H. Neoadjuvant chemotherapy for patients with locally advanced gastric carcinoma: effect on tumor cell microinvolvement of regional lymph nodes. Cancer. 1999;85:1484-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 85. | Lokich JJ, Shea M, Chaffey J. Sequential infusional 5-fluorouracil followed by concomitant radiation for tumors of the esophagus and gastroesophageal junction. Cancer. 1987;60:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 86. | Gill PG, Jamieson GG, Denham J, Devitt PG, Ahmad A, Yeoh E, Jones AM. Treatment of adenocarcinoma of the cardia with synchronous chemotherapy and radiotherapy. Br J Surg. 1990;77:1020-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 87. | Coia LR, Paul AR, Engstrom PF. Combined radiation and chemotherapy as primary management of adenocarcinoma of the esophagus and gastroesophageal junction. Cancer. 1988;61:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 88. | Haas CD, Mansfield CM, Leichman LP, Considine B, Bukowski RM. Combined nonsimultaneous radiation therapy and chemotherapy with 5-FU, doxorubicin, and mitomycin for residual localized gastric adenocarcinoma: a Southwest Oncology Group pilot study. Cancer Treat Rep. 1983;67:421-424. [PubMed] |
| 89. | Weissberg JB. Role of radiation therapy in gastrointestinal cancer. Arch Surg. 1983;118:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 90. | Tao HQ, Zou SC. Effect of preoperative regional artery chemotherapy on proliferation and apoptosis of gastric carcinoma cells. World J Gastroenterol. 2002;8:451-454. [PubMed] |
| 91. | Cao WX, Ou JM, Fei XF, Zhu ZG, Yin HR, Yan M, Lin YZ. Methionine-dependence and combination chemotherapy on human gastric cancer cells in vitro. World J Gastroenterol. 2002;8:230-232. [PubMed] |
Appendix A. RCT reports retrieved in Chinese of chemotherapy for resected gastric cancer
1A Zhai Y, Ding DS. A controlled, randomised trial of adjuvant che-motherapy using FEM combination protocol in resectable gas-tric cancer.
2A Li LJ, Wang LY, Cai L, Chao GF, Shi YQ, Wang ZY, Lin YJ. Com-bined treatment of surgery with chemotherapy of stomach cancer: an analysis 5-year following up of 649 patients.
3A Li HX, Wang YB, Zhuang YZ. Clinical trial of perioperative che-motherapy using FM combination protocol in patient with gas-tric cancer.
4A Wang BD, Zhang GY, Leng GZ. The effect of chemotherapy in respectable gastric cancer.
5A Zhou GX, Peng YM. Clinical study on the effect of chemical therapy to stomach cancer after operation.
6A Wang ZY, Jia SW, Li LJ, Cai YH, Bai YX, Li L, Yan ZJ, Dai HX. Combined treatment of surgery with chemotherapy of stomach cancer: an analysis 5-year following up of 170 patients.
7A Chen W, Li RL, Sui GJ. Adjuvant chemotherapy can improve the late result of radical operation in patients with gastric cancer.
Edited by Lu HM