Published online Oct 15, 2002. doi: 10.3748/wjg.v8.i5.815
Revised: February 1, 2002
Accepted: February 9, 2002
Published online: October 15, 2002
AIM: To clarify the significance of cyclooxygenase-2 (COX-2) expression in human primary hepatocellular carcinoma (HCC) and adjacent nontumorous tissues.
METHODS: The COX-2 protein and mRNA were investigated in 27 HCC tissues with adjacent nontumorous tissues, and 5 histologically normal liver tissues, using immunohistochemistry and in situ hybridization.
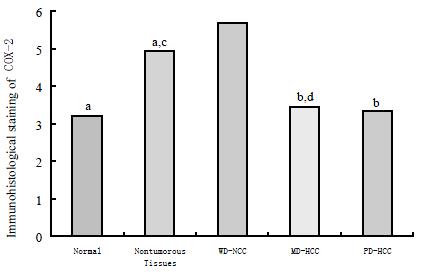
RESULTS: The well-differentiated HCC expressed COX-2 protein (5.68 ± 1.19) more strongly than moderated HCC (3.43 ± 1.98) and poor differentiated HCC (3.33 ± 1.50) (P < 0.05 respectively), adjacent nontumorous tissues (4.93 ± 1.05) and normal liver tissues (3.20 ± 1.92) (P < 0.01 respectively); More intensive staining of COX-2 in adjacent nontumorous tissues was observed than that in normal liver tissues (P < 0.05). There was no significant difference among adjacent nontumorous tissues, moderately differentiated HCC and poorly differentiated HCC (P > 0.05). The expression of COX-2 mRNA was observed in the cytoplasm of the cells of HCC and of the hepatocytes in adjacent nontumorous tissues in which COX-2 protein was positive.
CONCLUSION: The overexpression of COX-2 in well-differentiated HCC suggests that COX-2 may play a role in the early stages of hepatocarcinogensis.
- Citation: Qiu DK, Ma X, Peng YS, Chen XY. Significance of cyclooxygenase-2 expression in human primary hepatocellular carcinoma. World J Gastroenterol 2002; 8(5): 815-817
- URL: https://www.wjgnet.com/1007-9327/full/v8/i5/815.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i5.815
Cyclooxygenase (COX) is the rate-limiting enzyme involved in the conversion of arachidonic acid to prostaglandin H2, the precursor of various compounds including prostaglandins, prostacyclin and thromboxanes. Two COX genes, COX-1 and COX-2, have been identified, which share greater than 60% identity at the amino acid level. COX-1 is constitutively expressed in a number of cell types, whereas COX-2 is inducible by a variety of factors such as mitogen, cytokines, growth factor, and tumor promoters[1]. The expression of COX-2 is significantly increased in various types of carcinoma. There is also sufficient evidence indicating that selective COX-2 inhibitors produce effective prevention of carcinogenesis and that these compounds act by inducing of apoptosis of various cancer cells. These findings suggest that COX-2 may be involved in carcinogenesis and/or progression of certain types of human malignancies[2]. In this study, we examined the expression of COX-2 in human primary hepatocellular carcinoma (HCC) and adjacent nontumorous tissues.
HCC tissue and adjacent nontumorous liver tissues were obtained from 27 patients with hepatic tumor, who received hepatectomy at Renji hospital. Five specimens of grossly normal liver tissues from the area surrounding benign angiomas were used as controls. Specimens were fixed in 10% neutral formalin and embedded in paraffin.
Serial 5-um sections were stained with hematoxylin and eosin. Each HCC was histologically graded into one of three categories: well-differentiated, moderately differentiated, or poorly differentiated, according to the criteria proposed by the Liver Cancer Study Group of Japan[3]. The tumor tissues consisted of 11 well-differentiated, 7 moderately differentiated, and 9 poorly differentiated HCCs, and the nontumorous sites consisted of 3 chronic hepatitis and 24 cirrhosis.
Immunohistochemical staining was performed on serial sections at room temperature, using the peroxidase method with the polyclonal antibody against human COX-2 (Santa Cruz Co.). The sections were deparaffinized in xylene and rehydrated through graded alcohol, immersed the sections in 3% hydrogen peroxide for 10 min to inactivate endogenous peroxidases, then were incubated for 10 min with 10% normal swine serum in Tris-buffered saline to block non-specific binding, subsequently incubated overnight at 4 °C with relevant antibody. The following day, the sections were incubated with biotinylated anti-mouse IgG (Maxim Biotech Inc. USA) for 45 min, followed by peroxidase-conjugated streptavidin (Maxim Biotech Inc.). The chromogenic reaction was developed with diaminobenzidine for 10 min, and all sections were counterstained with hematoxylin. In controls, the primary antibody was omitted.
The intensity of staining for COX-2 in HCC tissues and nontumorous adjacent tissues was scored in each specimen on a scale of 0 to 3, in which 0 = negative staining, 1 = weakly positive staining, 2 = moderately positive staining, and 3 = strongly positive staining. The percentage of positive cells in each specimen was estimated and scored on a scale of 0 to 4, in which 0 = negative, 1 = positive staining in 1% to 25% of cells counted, 2, in 26% to 50%; 3, in 51% to 75%; and 76% to 100%. Each section was evaluated for the sum of these two parameters.
Deparaffinized sections of liver tissue were treated with proteinase K (70 mg/mL) for 30 min at 37 °C, followed by fixation with paraformaldehyde (0.4%, 20 min). Sections were prehybridized for 1-hour, then hybridized at 55 °C overnight with digoxigenin-labeled (labeling-kit: Boehringer Mannheim) fragments of COX-2 (Cayman Chemical). After extensive washing of the tissue section, hybridization was visualized using anti-digoxiganin, alkaline phosphatase-conjugated antibody and NBT/BCIP.
Data are presented as the mean ± SD. Statistical significances were assessed using Mann-Whitney's U test. Significance was accepted when P < 0.05.
In immunohistochemical analysis, cytoplasmic staining for COX-2 was observed in HCC cells and nontumorous hepatocytes. The distribution of positive cells was mostly extensive, and occasionally focal or scattered. Different histological grades of HCC demonstrated different immunoreactivity for COX-2. A significantly high expression level of COX-2 (5.68 ± 1.19) was found in well-differentiated HCC, compared with that of normal tissue (3.20 ± 1.92), adjacent nontumorous tissues (4.93 ± 1.05) (P < 0.01, respectively), moderately differentiated HCC (3.43 ± 1.98) and poorly differentiated HCC (3.33 ± 1.50) (P < 0.05, respectively). More intensive staining of COX-2 in adjacent nontumorous tissues was observed than that in normal liver tissues (P < 0.05). These is no significant difference among adjacent nontumorous tissue, moderately differentiated HCC and poorly differentiated HCC (P > 0.05, respectively) (Figure 1). The expression of COX-2 mRNA was observed in the cytoplasm of the cells of HCC and of the hepatocytes in adjacent nontumorous tissues in which COX-2 protein was positive.
Primary HCC is one of the most common tumors in China. The prognosis of HCC is generally poor, and the 5-year survival rate is limited to 25%-39% after surgery[4]. The present study showed that a significantly increased expression of COX-2 was observed in HCC, suggesting that COX-2 was being involved in hepatocarcinogenesis. Of interest, a profound expression of COX-2 was demonstrated in well-differentiated HCC. It is known that early HCC is usually a well-differentiated carcinoma and then gradually changes into a poorly differentiated phenotype during tumor progression[5]. In this context, it is suggested that COX-2 may be associated with the early process of the progression of HCCs. This result is consistent with those reported by Koga[6].
Recent studies have highlighted the relevance of COX-2 in human carcinogenesis. Epidemiological studies indicate that NSAIDs lead to a regression of colonic polyps in patients with familial adenomatous polyposis. NSAIDs are also known to reduce the risk of colorectal cancers, breast and lung cancers[7]. There is also a sufficient evidence from animal studies indicating that selective COX-2 inhibitors produce effective prevention of carcinogenesis. Introduction of COX-2 cDNA into colon carcinoma cells facilitated growth. COX-2 appears to prevent apoptosis because selective COX-2 inhibitors induced apoptosis in various cell culture systems[8]. Furthermore, it was shown that COX-2 promoted angiogenesis in malignant cells[9]. COX-2 mRNA and protein were recently found to be expressed in human colon carcinoma[10] and gastric carcinoma[11]. However, COX-2 protein was not expressed in human breast carcinoma or in human basal cell carcinoma. These observations have suggested that overexpression of COX-2 in carcinomas is not the universal event in carcinogenesis but may be specific. Recently, Kondo et al[12] showed that increased expression of COX-2 in nontumor liver tissue was associated with shorter disease-free survival in patients with hepatocellular carcinoma. Clinicopathological survey indicated a significant correlation between COX-2 expression and differentiated carcinoma. Moreover, high COX-2 expression in nontumorous tissue was significantly correlated with the presence of active inflammation. These findings suggest that COX-2 expression in nontumorous tissue may play a positive role in relapse of HCC after surgery.
Recent chemopreventive strategies for colon carcinogenesis have focused on using COX inhibitors, resulting in interesting data from animal models[13]. Denda et al[14] demonstrated that administration of NSAIDs suppressed cirrhosis and subsequent formation of HCC in the choline-deficient L-amino acid-defined rat model. The present finding of increased COX-2 expression in well-differentiated HCC tissues encourages chemopreventive studies for primary HCC. To clarity whether COX-2 is a principle enzyme involved in liver carcinogenesis, in vivo animal studies should be performed using a specific COX-2 inhibitor.
| 1. | Eberhart CE, Dubois RN. Eicosanoids and the gastrointestinal tract. Gastroenterology. 1995;109:285-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 272] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [PubMed] |
| 3. | Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277-287. [PubMed] |
| 4. | Colombo M. Hepatocellular carcinoma. J Hepatol. 1992;15:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Sugihara S, Nakashima O, Kojiro M, Majima Y, Tanaka M, Tanikawa K. The morphologic transition in hepatocellular carcinoma. A comparison of the individual histologic features disclosed by ultrasound-guided fine-needle biopsy with those of autopsy. Cancer. 1992;70:1488-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 304] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 506] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245-4249. [PubMed] |
| 9. | Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1650] [Cited by in RCA: 1647] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 10. | Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785-3789. [PubMed] |
| 11. | Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276-1280. [PubMed] |
| 12. | Kondo M, Yamamoto H, Nagano H, Okami J, Ito Y, Shimizu J, Eguchi H, Miyamoto A, Dono K, Umeshita K. Increased expression of COX-2 in nontumor liver tissue is associated with shorter disease-free survival in patients with hepatocellular carcinoma. Clin Cancer Res. 1999;5:4005-4012. [PubMed] |
| 13. | Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, Abreu-Goris M, Newmark HL, Lipkin ML, DeCosse JJ. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996;56:2556-2560. [PubMed] |
| 14. | Denda A, Endoh T, Kitayama W, Tang Q, Noguchi O, Kobayashi Y, Akai H, Okajima E, Tsujiuchi T, Tsutsumi M. Inhibition by piroxicam of oxidative DNA damage, liver cirrhosis and development of enzyme-altered nodules caused by a choline-deficient, L-amino acid-defined diet in rats. Carcinogenesis. 1997;18:1921-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Edited by Zhao P