Published online Jun 15, 2002. doi: 10.3748/wjg.v8.i3.551
Revised: November 23, 2001
Accepted: December 10, 2001
Published online: June 15, 2002
AIM: To observe expression of CD14 protein and CD14 gene in rat liver sinusoidal endothelial cells (LSECs) during endotoxemia, and the role of CD14 protein in the activation of lipopolysaccharide (LPS)-induced LSECs.
METHODS: Wistar rat endotoxemia model was established first by injection of a dose of LPS (5 mg/kg, Escherichia coli O111:B4) via the tail vein, then sacrificed after 0 h, 3 h, 6 h, 12 h, and 24 h, respectively. LSECs were isolated from normal and LPS-injected rats by an in situ collagenase perfusion technique. The isolated LSECs were incubated with rabbit anti-rat CD14 polyclonal antibody, then stained with goat anti rabbit IgG conjugated fluorescein isothiocyanate (FITC) and flow cytometric analysis (FCM) was performed. The percentage and mean fluorescence intensity (MFI) of CD14-positive cells were taken as the indexes. LSECs were collected to measure the expression of CD14 mRNA by in situ hybridization analysis. The isolated LSECs from normal rats were incubated firstly with anti-CD14 antibody, then stimulated with different concentrations of LPS, and the supernatants of these cells were then collected for measuring the levels of tumor necrosis factor (TNF)-α and Interleukin (IL)-6 with ELISA.
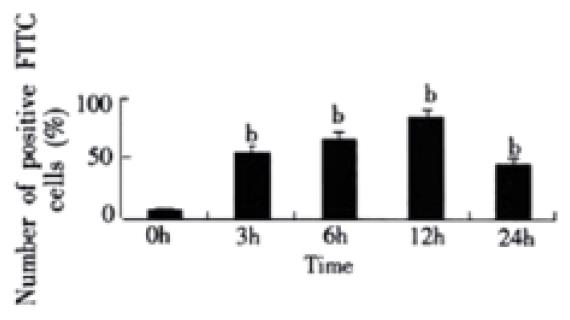
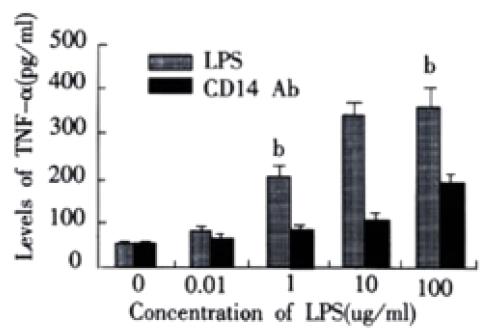
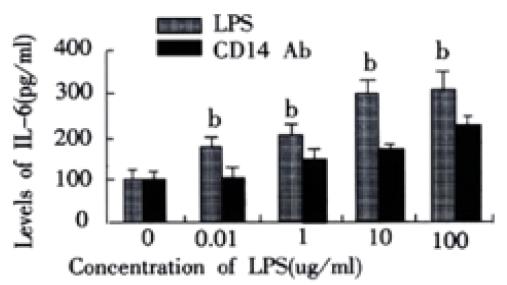
RESULTS: In rats with endotoxemia, LSECs displayed a strong MFI distinct from that of control rats. CD14 positive cells in rats with endotoxemia were 54.32%, 65.83%, 85.64%, and 45.65% at 3 h, 6 h, 12 h, and 24 h respectively, there was significant difference when compared to normal group of animals (4.45%) (P < 0.01). The expression of CD14 mRNA in isolated LSECs was stronger than that in control rats. In LPS group, the levels of TNF-α and IL-6 were 54 ± 6 ng·L-1, 85 ± 9 ng·L-1, 206 ± 22 ng·L-1, 350 ± 41 ng·L-1, 366 ± 42 ng. L-1 and 103 ± 11 ng·L-1, 187 ± 20 ng·L-1, 244 ± 26 ng·L-1, 290 ± 31 ng·L-1, and 299 ± 34 ng·L-1, respectively at different concentration points. In anti-CD14 group, the levels of TNF-α and IL-6 were 56 ± 5 ng·L-1, 67 ± 8 ng·L-1, 85 ± 10 ng·L-1, 113 ± 12 ng·L-1, 199 ± 22 ng·L-1 and 104 ± 12 ng·L-1, 125 ± 12 ng·L-1, 165 ± 19 ng·L-1, 185 ± 21 ng·L-1, and 222 ± 23 ng·L-1, respectively at different concentration points. There was significant difference between the two groups (P < 0.01).
CONCLUSION: LSECs can synthesize CD14 protein and express CD14 gene during endotoxemia. CD14 protein plays an important role in the activation of LPS-induced LSECs. This finding has important implications for the understanding of the mechanisms by which LPS may injure liver sinusoidal endothelial cells during sepsis.
- Citation: Gong JP, Dai LL, Liu CA, Wu CX, Shi YJ, Li SW, Li XH. Expression of CD14 protein and its gene in liver sinusoidal endothelial cells during endotoxemia. World J Gastroenterol 2002; 8(3): 551-554
- URL: https://www.wjgnet.com/1007-9327/full/v8/i3/551.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i3.551
Lipopolysaccharide (LPS) has been shown to play a key role in the pathogenesis of severe sepsis and septic shock caused by gram-negative bacteria. LPS stimulates monocytes and macrophages to release proinflammatory mediators, such as tumor necrosis factor (TNF)-α and interleukins[1-10]. Recent studies have reported that LPS-binding protein (LBP) and LPS receptor CD14 mediate responses of activated monocytes, macrophages and other cells to LPS[11-13]. CD14 is a 55-kDa glycoprotein with multiple leucine-rich repeats and was first described as a myeloid differentiation antigen[14]. CD14 has been identified as receptor for complexes of LPS and LBP. It is known that CD14 is linked to the cell membrane by a glycosylphosphatidylinositol anchor in myeloid lineage cells, and it plays a pivotal role in the activation of LPS-induced monocytes and macrophages[15,16]. But it is not yet clear whether CD14 is expressed by vascular endothelial cells. Indeed, it has been generally accepted that endothelial cells do not express CD14[17]. Soluble CD14 (sCD14) is thought to facilitate LPS-induced activation of endothelial cells[18]. However, recent studies have shown that endothelial cells are sensitive to low concentration of LPS and anti-CD14 antibodies can block endothelial cell activation even in the absence of serum, which is an observation inconsistent with the concept that endothelial cells do not express CD14[19]. Our aim was to demonstrate that liver sinusoidal endothelial cells (LSECs) synthesize CD14 protein and express CD14 gene in rats with endotoxemia, and the role of CD14 protein in the activation of LPS-induced LSECs.
LPS (Escherichia coli O111: B4) and collagenase (type IV) were purchased from Sigma Chemical Company (St. Louis, Mo.). A rabbit anti-rat CD14 polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif). Fluorescein isothiocyanate (FITC)-IgG were purchased from Zhongshan Biotechnology Company (Beijing, China). In situ hybridization analysis kit of CD14 mRNA was purchased from Boshide Biotechnology Company (Wuhan, China).
Male Wistar rats, which were pathogen-free and weighed approximately 225 g each, were purchased from the Animal Center of Chongqing University of Medical Science. The rats were exposed each day to 12 h of light and darkness respectively. Rodent chow and water were provided ad libitum. Experimental protocols were approved by the Institutional Care and Use Committee of Chongqing University of Medical Science.
The Wistar rat endotoxemia model was established as described previously[20].In brief, animals were injected with a dose of LPS (5 mg/kg, Escherichia coli O111:B4) via the tail vein, then the sacrificed after 3 h, 6 h, 12 h, and 24 h, respectively. There were six rats at each time point. Other six rats were used as control group (0 h).
LSECs were isolated from normal and LPS-injected rats by an in situ collagenase perfusion technique, modified as described previously[21]. In brief, livers were removed after a portal vein perfusion with Hanks’ balanced salt solution (HBSS) and the homogenate was digested in a solution of 0.5% collagenase. LSECs were separated from other nonparenchymal cells by two cycles of differential centrifugation (50 × g for 2 min) and further purified over a 30% Percoll gradient. LSECs purity exceeded 90% as assessed by light microscopy, and viability was typically greater than 95% as determined by trypan blue exclusion assay.
In situ hybridization was performed as described previously[22]. Positive result: positive location was blue.
Expression of CD14 protein in LSECs was examined by flow cytometric analysis as described previously[23]. In brief, LSECs were incubated with rabbit anti-rat CD14 polyclonal antibody (1 ug/mL) after washing, and then cells were incubated with goat anti-rabbit immunoglobulin G labeled with FITC. After being washed three times, 10000 cells were analyzed by flow cytometry (Coulter, USA), and the percentage and mean fluorescence intensity (MFI) of CD14-positive cells were taken as the indexes.
To determinate the role of CD14 in the activation of LPS-induced LSECs, LSECs were isolated from normal rats. These cells were harvested and adjusted to a concentration of 1 × 106/mL/well and were divided into two groups. Group of LPS: LSECs were incubated at different concentrations of LPS (0, 0.01 ug/mL, 1 ug/mL, 10 ug/mL, and 100 ug/mL). Group of anti-CD14 antibody blockade: LSECs were pre-incubated for 30 min with 0.2 mL CD14 antibody (1:100 dilution) before different concentrations of LPS were added. Supernatants were then collected for measuring the levels of TNF-α and IL-6 with ELISA.
All results were expressed as mean ± SEM. Statistical differences between means were determined by using Student’s t test. The value of P < 0.01 was considered significant.
To confirm expression of CD14 on LSECs, we examined the binding of FITC to the cells. CD14 positive cells were 4.45% in rats of normal group. But in rats with endotoxemia, CD14 positive cells were 54.32%, 65.83%, 85.64%, and 45.65% at 3 h, 6 h, 12 h, and 24 h respectively after stimulation of LPS, which were significant different when compared with normal group of animals (P < 0.01) (Figure 1).
We postulated that LSECs could express CD14 mRNA during endotoxemia. In order to examine the cell-specific expression of CD14 mRNA, freshly isolated and purified LSECs were analyzed by in situ hybridization with a riboprobe specific for rat CD14. Our analysis showed that LSECs from controls had no detectable level of CD14 mRNA. LPS treatment increased the level of CD14 mRNA in LSECs, inducing expression as early as 3 h after LPS treatment. The expression of CD14 gene increased with time, reaching a maximum induction by 12 h after treatment of LPS, and subsequently declined to low level by 24 h.
In LPS group, with increasing of LPS concentrations, the levels of TNF-a and IL-6 in supernatant of LSECs also increased. In group of anti-CD14 antibody blockade, productions of TNF-α and IL-6 in supernatants of LSECs were obviously inhibited by Ab against CD14 when compared with LPS group (P < 0.01). (Figure 2 and Figure 3)
CD14 as a key LPS signaling molecule was first reported to be expressed in monocyte-macrophage system[4,12,23]. Recent works have showed that the CD14 antigen is expressed in many types of cells and tissues[20,24-32]. But it is not yet clear whether vascular endothelial cells could synthesize CD14 protein and express CD14 gene. Beekhuizen et al reported endothelial cells did not express CD14. With method of in situ hybridization, Fearns et al[24] found that endothelial cells did not express CD14 protein. Wang et al[18] considered that sCD14 was thought to facilitate LPS-induced activation of endothelial cells. But, Lee et al[33] found CD14-negative murine pre-B cells (70Z/3), which were unresponsive to low concentrations of LPS (0.1 ng/mL) even in the presence of serum, showed responses to LPS when transfected with CD14. Surprisingly, anti-CD14 antibody blocked endothelial cell activation by LPS even in the absence of serum, which is an observation inconsistent with the concept that endothelial cells do not express CD14 protein.
In this experiment, we selected LSECs to represent vascular endothelial cells as targets of our experiment, and determined whether LSECs could synthesize CD14 protein and express CD14 gene. We found: (1) LSECs from normal rats did not synthesize CD14 protein and express CD14 gene, but the synthesis and expression of CD14 were markedly upregulated by LPS during endotoxemia, accompanied with the expression of CD14 mRNA, which showed that CD14 protein in LSECs was not passively acquired from serum. (2) Anti-CD14 antibody could block LSECs activation by LPS in the absence of serum, which further indicated that LSECs could synthesize and express CD14 molecules.
Why were our findings different from previously published data that endothelial cells were CD14 negative? We think there were a few possibilities: (1) many authors used routine passaging of multiple culture of human vascular endothelial cells (HUVEC) or HUVEC purchased from tissue culture laboratories to observe whether these cells expressed CD14, but these cells might lose CD14 gene when they were cultured at multiple passages. Jersmann et al[34] reported when HUVES were cultured at passages 3 to 5, these cells were indistinguishable from passage 1 HUVEC in a number of properties and displayed normal morphology and viability and response to TNF to the same extent as passage 1 cells. However, unlike passage 1 cells, HUVEC that had undergone multiple passing expressed extremely low amounts of CD14 protein. We used freshly isolated primary rat LSECs to study the expression of CD14 and found they could obviously synthesize and express CD14 during endotoxemia. (2) LSECs were different from other endothelial cells in location, construction, and function. LSECs are located in hepatic sinus and stimulated by LPS from gut via portal vein blood, so these cells have their property which are different from other endothelial cells[35]. (3) The choice of Ab against CD14 for the flow cytometric analysis may have been an additional explanation for the previously reported lack of CD14 on the endothelial cells’ surface. Jersmann et al[34] stained HUVEC with five different primary mAbs (MY4, 2D-15C, TUK4, LeuM3, and Rmo52) against CD14, and found only MY4 and TUK4 produced a positive stain and MY4 was the most effective mAb for detection of CD14 expression in endothelial cells. We stained LSECs with rabbit anti-rats primary antibody against CD14 from Santa Cruz Biotechnology and found this Ab against CD14 was effective for detecting the expression of CD14 protein. As expression of CD14 in animals is probably different from that in humans, further investigation of the expression of CD14 among animals is going on actively in our laboratory.
| 1. | Gong JP, Liu CA, Wu CX, Li SW, Shi YJ, Li XH. Nuclear factor kB activity in patients with acute severe cholangitis. World J Gastroenterol. 2002;8:346-349. [PubMed] |
| 2. | Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Yang K, Li Y, Li XH. Intestinal damage mediated by Kupffer cells in rats with endotoxemia. World J Gastroenterol. 2002;8:923-927. [PubMed] |
| 3. | Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Li XH, Peng Y. Liver sinusoidal endothelial cell injury by neutrophils in rats with acute obstructive cholangitis. World J Gastroenterol. 2002;8:342-345. [PubMed] |
| 4. | Heumann D, Adachi Y, Le Roy D, Ohno N, Yadomae T, Glauser MP, Calandra T. Role of plasma, lipopolysaccharide-binding protein, and CD14 in response of mouse peritoneal exudate macrophages to endotoxin. Infect Immun. 2001;69:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Merkel SM, Alexander S, Zufall E, Oliver JD, Huet-Hudson YM. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun. 2001;69:6119-6122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Rabehi L, Irinopoulou T, Cholley B, Haeffner-Cavaillon N, Carreno MP. Gram-positive and gram-negative bacteria do not trigger monocytic cytokine production through similar intracellular pathways. Infect Immun. 2001;69:4590-4599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Hotchkiss RS, Karl IE. Cytokine blockade in sepsis--Are two better than one? Crit Care Med. 2001;29:671-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Parker SJ, Watkins PE. Experimental models of gram-negative sepsis. Br J Surg. 2001;88:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Nanbo A, Nishimura H, Muta T, Nagasawa S. Lipopolysaccharide stimulates HepG2 human hepatoma cells in the presence of lipopolysaccharide-binding protein via CD14. Eur J Biochem. 1999;260:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Gutsmann T, Müller M, Carroll SF, MacKenzie RC, Wiese A, Seydel U. Dual role of lipopolysaccharide (LPS)-binding protein in neutralization of LPS and enhancement of LPS-induced activation of mononuclear cells. Infect Immun. 2001;69:6942-6950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Hiki N, Berger D, Mimura Y, Frick J, Dentener MA, Buurman WA, Seidelmann M, Kaminishi M, Beger HG. Release of endotoxin-binding proteins during major elective surgery: role of soluble CD14 in phagocytic activation. World J Surg. 2000;24:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1089] [Article Influence: 35.1] [Reference Citation Analysis (12)] |
| 15. | Enomoto N, Yamashina S, Kono H, Schemmer P, Rivera CA, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Thurman RG. Development of a new, simple rat model of early alcohol-induced liver injury based on sensitization of Kupffer cells. Hepatology. 1999;29:1680-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Li SW, Gong JP, Wu CX, Shi YJ, Liu CA. Lipopolysaccharide induced synthesis of CD14 proteins and its gene expression in hepatocytes during endotoxemia. World J Gastroenterol. 2002;8:124-127. [PubMed] |
| 17. | Kono H, Wheeler MD, Rusyn I, Lin M, Seabra V, Rivera CA, Bradford BU, Forman DT, Thurman RG. Gender differences in early alcohol-induced liver injury: role of CD14, NF-kappaB, and TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2000;278:G652-G661. [PubMed] |
| 18. | Wong PM, Chugn SW, Sultzer BM. Genes, receptors, signals and responses to lipopolysaccharide endotoxin. Scand J Immunol. 2000;51:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | von Asmuth EJ, Dentener MA, Bazil V, Bouma MG, Leeuwenberg JF, Buurman WA. Anti-CD14 antibodies reduce responses of cultured human endothelial cells to endotoxin. Immunology. 1993;80:78-83. [PubMed] |
| 20. | Gong JP, Xu MQ, Li K, Zhu J, Han BL. Expression of CD14 in Kupffer cells induced by lipopolysaccharide. Di-San Junyi Daxue Xuebao. 2001;23:425-428. |
| 21. | Gong JP, Han BL. Technique of isolation, culture and identification of liver cells. Shijie Huaren Xiaohua Zazhi. 1999;7:417-419. |
| 22. | Fearns C, Loskutoff DJ. Role of tumor necrosis factor alpha in induction of murine CD14 gene expression by lipopolysaccharide. Infect Immun. 1997;65:4822-4831. [PubMed] |
| 23. | Gong JP, Han BL. Effects of CD14 in LPS mediating activation of Kupffer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:875-877. |
| 24. | Fearns C, Kravchenko VV, Ulevitch RJ, Loskutoff DJ. Murine CD14 gene expression in vivo: extramyeloid synthesis and regulation by lipopolysaccharide. J Exp Med. 1995;181:857-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 117] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Li S, Wu C, Shi Y, Liu C. [Lipopolysaccharide upregulates expression of CD14 gene and CD14 proteins of hepatocytes in rats]. Zhonghua Ganzangbing Zazhi. 2001;9:103-104. [PubMed] |
| 26. | Zuo GQ, Gong JP, Liu CA, Li SW, Wu XC, Yang K, Li Y. Expression of lipopolysaccharide binding protein and its receptor CD14 in experimental alcoholic liver disease. World J Gastroenterol. 2001;7:836-840. [PubMed] |
| 27. | Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol. 2000;165:3541-3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 251] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 28. | Ikejima K, Enomoto N, Seabra V, Ikejima A, Brenner DA, Thurman RG. Pronase destroys the lipopolysaccharide receptor CD14 on Kupffer cells. Am J Physiol. 1999;276:G591-G598. [PubMed] |
| 29. | Scott MG, Vreugdenhil AC, Buurman WA, Hancock RE, Gold MR. Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol. 2000;164:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1205] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 31. | Haziot A, Hijiya N, Gangloff SC, Silver J, Goyert SM. Induction of a novel mechanism of accelerated bacterial clearance by lipopolysaccharide in CD14-deficient and Toll-like receptor 4-deficient mice. J Immunol. 2001;166:1075-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol. 2001;166:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 295] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 33. | Lee JD, Kato K, Tobias PS, Kirkland TN, Ulevitch RJ. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992;175:1697-1705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 183] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Jersmann HP, Hii CS, Hodge GL, Ferrante A. Synthesis and surface expression of CD14 by human endothelial cells. Infect Immun. 2001;69:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Bone-Larson CL, Simpson KJ, Colletti LM, Lukacs NW, Chen SC, Lira S, Kunkel SL, Hogaboam CM. The role of chemokines in the immunopathology of the liver. Immunol Rev. 2000;177:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Edited by Hu DK