Published online Jun 15, 2002. doi: 10.3748/wjg.v8.i3.531
Revised: December 23, 2001
Accepted: January 28, 2002
Published online: June 15, 2002
AIM: Critical illnesses such as sepsis, trauma, and burns cause a growth hormone insensitivity, which leads to an increased negative nitrogen balance. Endotoxin is generously released into blood under these conditions and stimulates the production of proinflammatory cytokines such as TNF-α, IL-6, and IL-1, which may play a very important role in inducing the growth hormone insensitivity. The objective of this current study was to investigate the role of endotoxin, TNF-α and IL-6 in inducing the growth hormone insensitivity at the receptor and post-receptor levels.
METHODS: Spague-Dawley rats were injected with endotoxin, TNF-α, and IL-6, respectively and part of rats injected with endotoxin was treated with exogenous somatotropin simultaneously. All rats were killed at different time points. The expression of IGF-I, GHR, SOCS-3 and β-actin mRNA in the liver was detected by RT-PCR and the GH levels were measured by radioimmunoassay, the levels of TNF-α and IL-6 were detected by ELISA.
RESULTS: There was no significant difference in serous GH levels between experimental group and control rats after endotoxin injection, however, liver IGF-I mRNA expression had been obviously down-regulated in endotoxemic rats. Liver GHR mRNA expression also had a predominant down-regulation after endotoxin injection. The lowest regulation of liver IGF-I mRNA expression occurred at 12 h after LPS injection, being decreased by 53% compared with control rats. For GHR mRNA expression, the lowest expression occurred at 8 h and had a 81% decrease. Although SOCS-3 mRNA was weakly expressed in control rats, it was strongly up-regulated after LPS injection and had a 7.84 times increase compared with control rats. Exogenous GH could enhance IGF-I mRNA expression in control rats, but it did fail to prevent the decline in IGF-I mRNA expression in endotoxemic rats. Endotoxin stimulated the production of TNF-α and IL-6, and the elevated IL-6 levels was shown a positive correlation with increased SOCS-3 mRNA expression. The liver GHR mRNA expression was obviously down-regulated after TNF-α iv injection and had a 40% decrease at 8 h, but the liver SOCS-3 mRNA expression was the 4.94 times up-regulation occurred at 40 min after IL-6 injection.
CONCLUSION: The growth hormone insensitivity could be induced by LPS injection, which was associated with down-regulated GHR mRNA expression at receptor level and with up-regulated SOCS-3 mRNA expression at post-receptor level. The in vivo biological activities of LPS were mediated by TNF-α and IL-6 indirectly, and TNF-α and IL-6 may exert their effects on the receptor and post-receptor levels respectively.
- Citation: Wang P, Li N, Li JS, Li WQ. The role of endotoxin, TNF-α, and IL-6 in inducing the state of growth hormone insensitivity. World J Gastroenterol 2002; 8(3): 531-536
- URL: https://www.wjgnet.com/1007-9327/full/v8/i3/531.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i3.531
Infection especially severe intra-abdominal infection is characterized by catabolic status associated with severe protein loss and negative nitrogen balance[1-7]. Meantime, the levels of many important hormones such as glucocorticoid, insulin and growth hormone (GH) do not decline, but their biological activities have reduced obviously. Critical illnesses such as sepsis, trauma and burns can usually cause a elevated level of growth hormone at early stage, however the insulin-like growth factor I (IGF-I), which is a growth hormone-dependent growth factor that inhibits protein breakdown, has been showing decreased predominantly, this phenomenon indicating a status of growth hormone insensitivity[8-12]. In this condition, the administration of high doses of recombinant human growth hormone could not improve negative nitrogen balance, in contrary, it may lead to other metabolic disorders and result in increased morbidity and mortality[13].
Endotoxin is generously released into blood under the infected condition and stimulates the production of proinflammatory cyotkines such as TNF-α, IL-6, and IL-1[14-18], which play very important roles in inducing the GH insensitivity. The GH insensitivity can occur at receptor and post-receptor levels, the receptor level associates with down-regulated GHR mRNA expression[19,20]; the post-receptor insensitivity mainly occurs on the intracellular signal transduction pathway of growth hormone. Recent studies have suggested that the SOCS protein family, especially SOCS-3 play a very important role on this level[21,22]. In this study, we investigated whether the GH insensitivity could be induced by LPS, TNF-α and IL-6 iv injection, and what kind of roles they played.
All experimental procedures were carried out in compliance with the appropriate institutional and national ethical guidelines for work with laboratory animals. 156 adolescent male Spague-Dawley rats (240-260 g) were obtained from animal center of Jinling Hospital (Nanjing, China). They were given free access to food and water for three days before experiments.
Escherichia coli lipopolysaccharide (LPS; serotype O111:B4 phenol extract), obtained from Sigma Chemical (St. Louis, MO), was resuspended in sterile endotoxin-free saline to obtain 4 mg/mL solutions. The recombinant rat TNF-α and IL-6 provided by Pepro Tech EC Ltd (London, England), were resuspended in sterile endotoxin-free saline to obtain 100000 U/mL solutions. Human growth hormone, kindly provided by Serono, was resuspended in sterile endotoxin-free saline to obtain a 1 mg/mL solution.
Male Spague-Dawley rats (provided by Animal Center of Jingling Hospital), weighing 250 ± 10 g, were given free access to food and water for three days before experiments. Rats were anesthetized with ether and received LPS, GH, TNF-α, IL-6, and saline injection, LPS, TNF-α, and IL-6 were administered through superficial dorsal veins of penis and GH was injected subcutaneously. All rats were killed at different time points; blood of rats with LPS injected was collected and centrifuged at 500 g for 10 min at 4 °C to collect serum. Livers were removed, flash-frozen in liquid nitrogen, and stored at -80 °C until homogenate preparation and RNA extraction.
After the 3-day adaptation period, 42 rats were randomly divided into laboratory group (n = 36) and control group (n = 6), LPS (7.5 mg.kg-1 iv) was administered to the laboratory group, every six rats were killed at 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h after injection. The control rats were given intravenous saline.
After 3-day adaptation period, 24 rats were divided into 4 groups (6 rats/group). The first group received one injection of LPS (7.5 mg.kg-1 iv) and one injection of saline (sc), the second group received one injection of GH (1.5 mg.kg-1 sc) and one injection of saline (iv), the third group received one injection of LPS (7.5 mg.kg-1 iv) and one injection of GH (1.5 mg.kg-1 sc), the fourth group received two injections of saline. All rats were killed at 10 h after injection.
After the 3-day adaptation period, 102 rats were randomly divided into laboratory group (n = 96) and control group (n = 6). Rat recombinant TNF-α and IL-6 (100000 U/kg.wt) were injected through the same pathway and every six rats were killed after 20 min, 40 min, 1 h, 2 h, 4 h, 8 h, 12 h and 24 h. The control rats were given intravenous saline.
Freshed-frozen liver samples were homogenized and total RNA was performed using TRIZOL Reagent (Biobasic Inc, Scarborough, Ontario, Canada). With Access RT-PCR system kit (Promega Coporation, Madison, WI), the cDNA synthesis and amplification was done in one tube following the manufacture's instructions. In brief, 1 μg RNA, 1 μM primers for SOCS-3, GHR, IGF-I and β-actin were added to each reaction mixture respectively, which included 0.2 mM dNTP, 1 mM MgSO4, AMV reverse transcriptase 5 U, Tfl DNA polymerase 5 U, and AMV/Tfl 5 × buffer 10 μL. The reaction final volume was 50 μL and was covered with 30 μL mineral oil. RT-PCR reaction was run in the following procedures: (1) Reverse transcription: 48 °C for 45 min, 1 cycle. (2) AMV RT inactivation and RNA/cDNA/primers denaturation: 94 °C for 2 min, 1 cycle. (3) Second strand cDNA synthesis and PCR amplification: denaturation 94 °C for 30 s, annealing 60 °C for 1 min, extension 68 °C for 2 min, 28 cycles for SOCS-3 and 21 cycles for GHR and IGF-I, β-actin as intra-control to be amplified along with SOCS-3, GHR and IGF-I. (4) Final extension: 68 °C for 7 min, 1 cycle. 5 μL each RT-PCR reaction was electrophoresed in a 1.7% Metaphor agarose (FMC Bioproducts, Rockland, ME) gel and stained with ethidium bromide. Products of RT-PCR reactions were photographed and analyzed by densitometry. The expression of IGF-I, GHR, and SOCS-3 mRNA in laboratory group is represented as a percentage or times compared with their expression in control group.
Polymerase chain reaction primers were as follows: IGF-I sense, 5'-CACATCTCTTCTACCTGGCACTC-3'; IGF-I antisense, 5'-GGATGGAACGAGCTGACTTTGTA-3', to give a 270 base pair product; GHR sense, 5'-CTGGGTTGAGTTCATTGAGCTGGAT-3'; GHR antisense, 5'-TGTAGAGGGGAGTTGGTGGGTTGAC-3', to give a 394 base pair product; SOCS-3 sense, 5'-ACCAGCGCCACTTCTTCACG-3'; and SOCS-3 antisense, 5'-GTGGAGCATCATACTGATCC-3', to give a 450 base pair product; β-actin sense, 5'-CATTTCCGGTGCACGATGGAG-3'; β-actin antisense, 5'-GCCATCCTGCGTCTGGACCTG-3', to give a 599 base pair production. All primers spanned at least one intron of genomic DNA.
Blood was obtained from the inferior vena cava at the time of sacrifice. Serum growth hormone levels was measured by radioimmunoassay according to manufacture's instructions (Northern Isotope Co, Beijing, China). Serum samples were analyzed for TNF-α and IL-6 content by enzyme-linked immunosorbent assay according to manufacture's instructions (BioSource International, Camarillo, CA).
All data are expressed as means ± SEM. Correlation between data was analyzed with linear regression. Comparisons between two groups were performed using an unpaired Student's t test. Differences were considered statistically significant when P < 0.05.
The levels of serum growth hormome at each time points after LPS injection had no significant difference compared with control rats, it maintained a relatively stable status (Table 1).
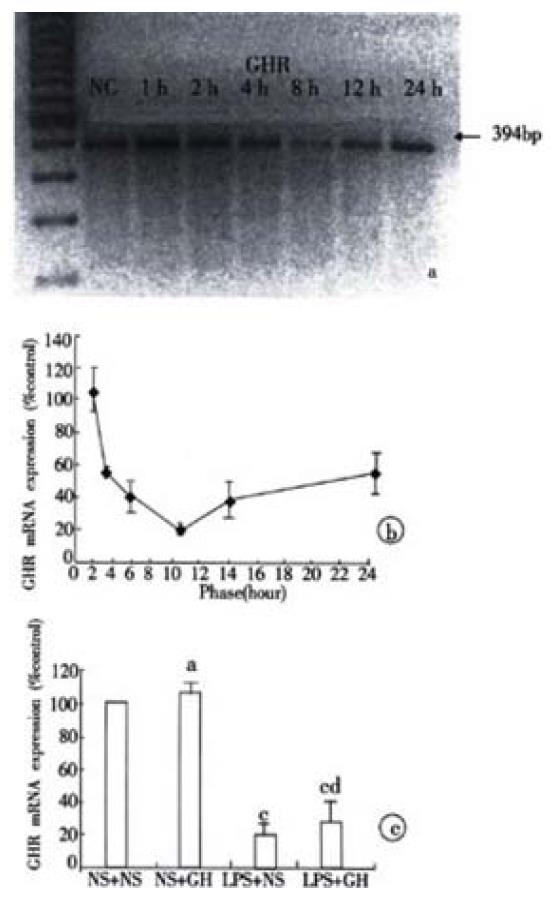
Liver IGF-I mRNA expression had already declined by 25% vs. control rats at 8 hours. On the time of 12 hours, we observed the lowest level of expression, which was a 53% decrease compared with control rats. It did not recover to the normal level and had a 15% reduction at 24 hours (Figure 1A, Figure 1B). Although exogenous GH administration in control rats significantly enhanced the liver IGF-I mRNA expression, it did fail to prevent its decline in endotoxemic rats (Figure 1C).
Liver GHR mRNA expression had already down-regulated by 45% at 2 hours after LPS injection, the lowest regulation occurred at 8 hours, which was a 89% decrease compared with control rats. After 24 hours, it did not recover to the normal level and had a 44% decrease (Figure 2A, Figure 2B). the exogenous GH administration had no effect on the liver GHR mRNA expression in control and endotoxemic rats (Figure 2C).
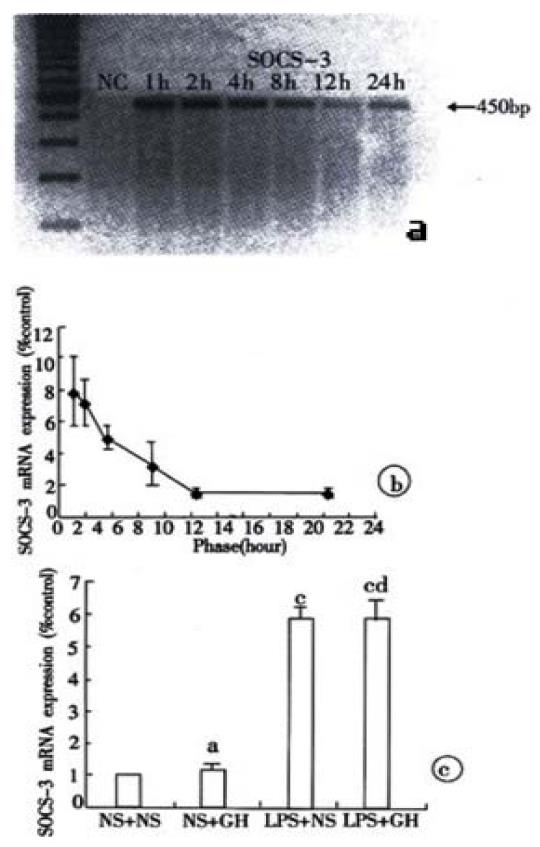
The liver SOCS-3 mRNA was weakly expressed in control rats, however, it was strongly up-regulated by 7.84 times vs. control rats at 1 hour after LPS injection. This level was maintained at 2 hours and it still had a 1.8 times increase at 24 hours (Figure 3A, Figure 3B). the exogenous GH infusion had no effect on the liver SOCS-3 mRNA expression in control and endotoxemic rats (Figure 3C).
The TNF-α level was increased rapidly after LPS injection, but it decreased obviously from the second hour and returned to the normal level at 4 h. The IL-6 level was also elevated rapidly after LPS injection; it got to the highest level at 2 h and then decreased gradually (Table 2). Linear regression analysis was shown a positive correlation of IL-6 with liver SOCS-3 mRNA expression (r = 0.935, P < 0.01).
| n | TNF-α levels (pg/mL) | IL-6 levels (pg/mL) | |
| Control | 6 | < 20 | < 8 |
| 1 h | 6 | 342.80 ± 50.01 | 1438.74 ± 323.07 |
| 2 h | 6 | 75.81 ± 11.50 | 1678.03 ± 126.57 |
| 4 h | 6 | < 20 | 1332.67 ± 120.95 |
| 8 h | 6 | < 20 | 142.59 ± 48.07 |
| 12 h | 6 | < 20 | 48.75 ± 10.57 |
| 24 h | 6 | < 20 | 46.82 ± 11.64 |
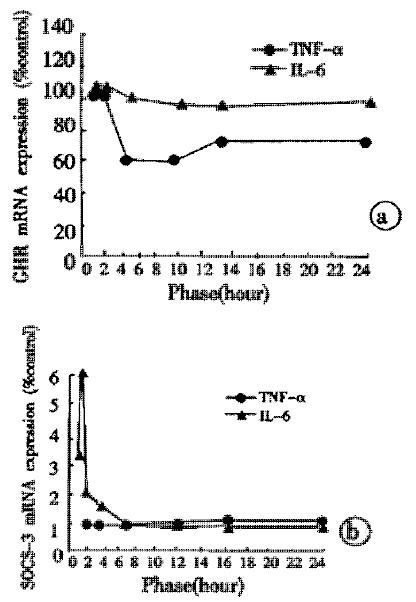
The liver GHR mRNA expression after TNF-α injection had already down-regulated at 4 hours and it reached the lowest level at 8 hours, which was a 40% decrease compared with control rats. At 24 hours, a 27% reduction still existed. The IL-6 injection had no effect on the liver GHR mRNA expression at different time points (Figure 4A). The liver SOCS-3 mRNA had weak expressions at all time points after TNF-α injection, no difference could be found compared with control rats. The IL-6 injection was able to up-ragulate rapidly the liver SOCS-3 mRNA expression, the latter showing a 2.73 times increase at 20 minutes and the highest level occurred at 40 minutes with a 4.94 times increase compared with control rats (Figure 4B).
In this report, using an experimental method of E. coli endotoxin infusion in laboratory rats, we have found endotoxin-induced growth hormone insensitivity. At 12 hours after LPS injection, there was no difference in serous growth hormone concentration between the experimental and control rats, however, the liver IGF-I mRNA expression had already declined obviously. In control rats, the liver IGF-I mRNA expression was up-regulated by 25% after exegeous GH administration, but in endotoxemic rats, GH did fail to prevent the decline in liver IGF-I mRNA expression. Several groups have observed that decreased IGF-I may result from a state of GH insensitivity. Ross et al[8] reported low circulating IGF-I levels in critically ill patients despite elevated GH secretion. More recently, the sdudy[19] showed that after a single injection of LPS in rats, plasma IGF-I level remained low despite the fact that GH level had returned to normal value. In agreement with these authors, our study support the possibility that the GH insensitivity maybe one of the important factors for the reduced liver IGF-I mRNA expression after LPS injection.
Growth hormone insensitivity can occur at receptor and post receptor levels, on the receptor level GH insensitivity is associated with the reduced GHR numbers on target cell sueface[19,20]. Because of the shorter half-life of liver GHR (30-40 min)[23] and the decreased liver GHR mRNA expression by endotoxin, these led to the reduced GHR synthesis. Our results shown that liver GHR mRNA expression was obviously down-regulated after LPS injection, manifested that LPS had effect on the receptor level GH insensitivity indeed.
The factor of post-receptor level GH insensitivity has caused more and more attention recently, and it is associated with a novel family of suppressor of cytokine signalling (SOCS) which includes eight members (SOCS-1 to SOCS-7 and CIS) that act in a classical negative feedback loop to regulate cytokine signal transduction[24-29]. SOCS-3 is a strong inhibitor on growth hormone intracellular signal transduction[30-32].
Once growth hormone binds to its receptor, the intracellular signal transduction is activated through JAK-STAT pathway[33,34]. The first activated tyrosine kinase is JAK2, which promotes the tyrosyl phosphorylation of both JAK2 itself and signal transducer and activator of transcription 5b (STAT 5b). Phosphorylated STAT 5b causes its dimerization and then the dimerized STAT 5b translocates into the nucleus, where it binds with high affinity to the promoters of various target genes and then activates the gene transcription such as IGF-I. SOCS-3 can block the GH intracellular JAK/STAT-dependent signaling pathway at different levels[35-44], including Competitively inhibits the phosphorylation of STAT 5b. Binds to GHR and leads to the degration of GHR-JAK2 compound directly or indirectly through Elongin B and Elongin C, in the end the JAK2 kinase loses its activity. Through binding to GHR, SOCS-3 can inhibit the JAK2 kinase activity directly. Our experiment observed that after LPS injection, liver SOCS-3 mRNA expression was rapidly up-regulated with a 7.84 times increase at 1 hour compared with the weak expression in control rats, this indicating that LPS induced the production of post-receptor GH insensitivity.
The in vivo biological activities of LPS are largely mediated by the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6[45-50], which is illustrated in our experiment by the marked stimulation of the secretion of TNF-α and IL-6 in blood. TNF-α and IL-6 had different roles in inducing the GH insensitivity, TNF-α injection leading to a reduced expression of liver GHR mRNA, and IL-6 being associated with the up-regulated SOCS-3 mRNA expression after injection. The elevated IL-6 levels stimulated by LPS had significant positive correlation with the increased liver SOCS-3 mRNA expression induced by LPS.
The mechanisms of TNF-α-induced GHR mRNA suppression was mostly mediated by inhibition of Sp transactivator binding to the L2 promoter of GHR gene[51], the other way might be associated with some other cytokines stimulated by TNF-α, such as IL-1[52].Both IL-6 and GH, belonging to the cytokine receptor superfamily, can transduce their signal from cell surface to nucleus through the same JAK-STAT pathway[53-55]. Hence, the elevated IL-6 levels stimulated by LPS promoted the increased expression of SOCS-3 mRNA, which not only had a negative feedback to IL-6 biological activities, but also inhibited the GH intracellular signal transduction[56-60].
In summary, our study observed that the growth hormone insensitivity could be induced by endotoxin, which suggested that the endotoxin played a very important role in inducing the GH insensitivity. The endotoxin not only had predominant effect on the GHR gene expression, but also induced the phenomenon of negative feedback loop at post-receptor level. The toxic effect of endotoxin was mostly mediated by TNF-α and IL-6 indirectly, and TNF-α maily had effect on the receptor gene expression, but for IL-6, it mainly caused the negative feedback loop at post-receptor level.
| 1. | Wu XN. Current concept of pathogenesis of severe acute pancreatitis. World J Gastroenterol. 2000;6:32-36. [PubMed] |
| 2. | Wu XN. Treatment revisited and factors affecting prognosis of severe acute pancreatitis. World J Gastroenterol. 2000;6:633-635. [PubMed] |
| 3. | Zhang JJ, Dong WF, Zhu ZY. The clinical significance and rational evaluation of early nutritional support in severe head-injured patients. World J Gastroenterol. 2000;6:20. |
| 4. | Chen QP. Enteral nutrition and acute pancreatitis. World J Gastroenterol. 2001;7:185-192. [PubMed] |
| 5. | Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am J Physiol. 1999;276:C1132-C1138. [PubMed] |
| 6. | Breuille D, Voisin L, Contrepois M, Arnal M, Rose F, Obled C. A sustained rat model for studying the long-lasting catabolic state of sepsis. Infect Immun. 1999;67:1079-1085. [PubMed] |
| 7. | Voisin L, Breuillé D, Combaret L, Pouyet C, Taillandier D, Aurousseau E, Obled C, Attaix D. Muscle wasting in a rat model of long-lasting sepsis results from the activation of lysosomal, Ca2+ -activated, and ubiquitin-proteasome proteolytic pathways. J Clin Invest. 1996;97:1610-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 181] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Ross RJ, Chew SL. Acquired growth hormone resistance. Eur J Endocrinol. 1995;132:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Bhutta ZA, Bang P, Karlsson E, Hagenäs L, Nizami SQ, Söder O. Insulin-like growth factor I response during nutritional rehabilitation of persistent diarrhoea. Arch Dis Child. 1999;80:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Vary TC, Dardevet D, Grizard J, Voisin L, Buffiere C, Denis P, Breuille D, Obled C. Differential regulation of skeletal muscle protein turnover by insulin and IGF-I after bacteremia. Am J Physiol. 1998;275:E584-E593. [PubMed] |
| 11. | Bjarnason R, Wickelgren R, Hermansson M, Hammarqvist F, Carlsson B, Carlsson LM. Growth hormone treatment prevents the decrease in insulin-like growth factor I gene expression in patients undergoing abdominal surgery. J Clin Endocrinol Metab. 1998;83:1566-1572. [PubMed] [DOI] [Full Text] |
| 12. | Hobler SC, Williams AB, Fischer JE, Hasselgren PO. IGF-I stimulates protein synthesis but does not inhibit protein breakdown in muscle from septic rats. Am J Physiol. 1998;274:R571-R576. [PubMed] |
| 13. | Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 658] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 14. | Wang JY, Wang XL, Liu P. Detection of serum TNF-alpha,IFN-beta,IL-6 and IL-8 in patients with hepatitis B. World J Gastroenterol. 1999;5:38-40. [PubMed] |
| 15. | Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Ritchie TC, Hunninghake GW, Zandi E, Engelhardt JF. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J Biol Chem. 2001;276:30188-30198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 342] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Ebong SJ, Goyert SM, Nemzek JA, Kim J, Bolgos GL, Remick DG. Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect Immun. 2001;69:2099-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Krakauer T. Suppression of endotoxin- and staphylococcal exotoxin-induced cytokines and chemokines by a phospholipase C inhibitor in human peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2001;8:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Massoudy P, Zahler S, Becker BF, Braun SL, Barankay A, Meisner H. Evidence for inflammatory responses of the lungs during coronary artery bypass grafting with cardiopulmonary bypass. Chest. 2001;119:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Defalque D, Brandt N, Ketelslegers JM, Thissen JP. GH insensitivity induced by endotoxin injection is associated with decreased liver GH receptors. Am J Physiol. 1999;276:E565-E572. [PubMed] |
| 20. | Hermansson M, Wickelgren RB, Hammarqvist F, Bjarnason R, Wennström I, Wernerman J, Carlsson B, Carlsson LM. Measurement of human growth hormone receptor messenger ribonucleic acid by a quantitative polymerase chain reaction-based assay: demonstration of reduced expression after elective surgery. J Clin Endocrinol Metab. 1997;82:421-428. [PubMed] [DOI] [Full Text] |
| 21. | Nicholson SE, Hilton DJ. The SOCS proteins: a new family of negative regulators of signal transduction. J Leukoc Biol. 1998;63:665-668. [PubMed] |
| 22. | Alexander WS, Starr R, Metcalf D, Nicholson SE, Farley A, Elefanty AG, Brysha M, Kile BT, Richardson R, Baca M. Suppressors of cytokine signaling (SOCS): negative regulators of signal transduction. J Leukoc Biol. 1999;66:588-592. [PubMed] |
| 23. | Frick GP, Tai LR, Baumbach WR, Goodman HM. Tissue distribution, turnover, and glycosylation of the long and short growth hormone receptor isoforms in rat tissues. Endocrinology. 1998;139:2824-2830. [PubMed] [DOI] [Full Text] |
| 24. | Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 633] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 25. | Stoiber D, Kovarik P, Cohney S, Johnston JA, Steinlein P, Decker T. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-gamma. J Immunol. 1999;163:2640-2647. [PubMed] |
| 26. | Pezet A, Favre H, Kelly PA, Edery M. Inhibition and restoration of prolactin signal transduction by suppressors of cytokine signaling. J Biol Chem. 1999;274:24497-24502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 158] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Bjørbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059-30065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 470] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 28. | Hilton DJ, Richardson RT, Alexander WS, Viney EM, Willson TA, Sprigg NS, Starr R, Nicholson SE, Metcalf D, Nicola NA. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 608] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 29. | Gisselbrecht S. The CIS/SOCS proteins: a family of cytokine-inducible regulators of signaling. Eur Cytokine Netw. 1999;10:463-470. [PubMed] |
| 30. | Mao Y, Ling PR, Fitzgibbons TP, McCowen KC, Frick GP, Bistrian BR, Smith RJ. Endotoxin-induced inhibition of growth hormone receptor signaling in rat liver in vivo. Endocrinology. 1999;140:5505-5515. [PubMed] [DOI] [Full Text] |
| 31. | Tollet-Egnell P, Flores-Morales A, Stavréus-Evers A, Sahlin L, Norstedt G. Growth hormone regulation of SOCS-2, SOCS-3, and CIS messenger ribonucleic acid expression in the rat. Endocrinology. 1999;140:3693-3704. [PubMed] |
| 32. | Adams TE, Hansen JA, Starr R, Nicola NA, Hilton DJ, Billestrup N. Growth hormone preferentially induces the rapid, transient expression of SOCS-3, a novel inhibitor of cytokine receptor signaling. J Biol Chem. 1998;273:1285-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 234] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Argetsinger LS, Carter-Su C. Mechanism of signaling by growth hormone receptor. Physiol Rev. 1996;76:1089-1107. [PubMed] |
| 34. | Han Y, Leaman DW, Watling D, Rogers NC, Groner B, Kerr IM, Wood WI, Stark GR. Participation of JAK and STAT proteins in growth hormone-induced signaling. J Biol Chem. 1996;271:5947-5952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Hansen JA, Lindberg K, Hilton DJ, Nielsen JH, Billestrup N. Mechanism of inhibition of growth hormone receptor signaling by suppressor of cytokine signaling proteins. Mol Endocrinol. 1999;13:1832-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Ram PA, Waxman DJ. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem. 1999;274:35553-35561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 280] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 37. | Zhang JG, Farley A, Nicholson SE, Willson TA, Zugaro LM, Simpson RJ, Moritz RL, Cary D, Richardson R, Hausmann G. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci USA. 1999;96:2071-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 503] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 38. | Boisclair YR, Wang J, Shi J, Hurst KR, Ooi GT. Role of the suppressor of cytokine signaling-3 in mediating the inhibitory effects of interleukin-1beta on the growth hormone-dependent transcription of the acid-labile subunit gene in liver cells. J Biol Chem. 2000;275:3841-3847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Schaefer F, Chen Y, Tsao T, Nouri P, Rabkin R. Impaired JAK-STAT signal transduction contributes to growth hormone resistance in chronic uremia. J Clin Invest. 2001;108:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Kamizono S, Hanada T, Yasukawa H, Minoguchi S, Kato R, Minoguchi M, Hattori K, Hatakeyama S, Yada M, Morita S. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem. 2001;276:12530-12538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 41. | Ram PA, Waxman DJ. Role of the cytokine-inducible SH2 protein CIS in desensitization of STAT5b signaling by continuous growth hormone. J Biol Chem. 2000;275:39487-39496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Sasaki A, Yasukawa H, Shouda T, Kitamura T, Dikic I, Yoshimura A. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J Biol Chem. 2000;275:29338-29347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 267] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 43. | Cohney SJ, Sanden D, Cacalano NA, Yoshimura A, Mui A, Migone TS, Johnston JA. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Mol Cell Biol. 1999;19:4980-4988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 216] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 44. | Song MM, Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem. 1998;273:35056-35062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 320] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 45. | Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1535] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 46. | Iwagaki A, Porro M, Pollack M. Influence of synthetic antiendotoxin peptides on lipopolysaccharide (LPS) recognition and LPS-induced proinflammatory cytokine responses by cells expressing membrane-bound CD14. Infect Immun. 2000;68:1655-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Bian Z, Brauner A, Li Y, Normark S. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J Infect Dis. 2000;181:602-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | van der Bruggen T, Nijenhuis S, van Raaij E, Verhoef J, van Asbeck BS. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect Immun. 1999;67:3824-3829. [PubMed] |
| 49. | Soltys J, Quinn MT. Modulation of endotoxin- and enterotoxin-induced cytokine release by in vivo treatment with beta-(1,6)-branched beta-(1,3)-glucan. Infect Immun. 1999;67:244-252. [PubMed] |
| 50. | Thissen JP, Verniers J. Inhibition by interleukin-1 beta and tumor necrosis factor-alpha of the insulin-like growth factor I messenger ribonucleic acid response to growth hormone in rat hepatocyte primary culture. Endocrinology. 1997;138:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Denson LA, Menon RK, Shaufl A, Bajwa HS, Williams CR, Karpen SJ. TNF-alpha downregulates murine hepatic growth hormone receptor expression by inhibiting Sp1 and Sp3 binding. J Clin Invest. 2001;107:1451-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Wolf M, Böhm S, Brand M, Kreymann G. Proinflammatory cytokines interleukin 1 beta and tumor necrosis factor alpha inhibit growth hormone stimulation of insulin-like growth factor I synthesis and growth hormone receptor mRNA levels in cultured rat liver cells. Eur J Endocrinol. 1996;135:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1651] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 54. | Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243-1254. [PubMed] |
| 55. | Chen T, Wang LH, Farrar WL. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 2000;60:2132-2135. [PubMed] |
| 56. | Paul C, Seiliez I, Thissen JP, Le Cam A. Regulation of expression of the rat SOCS-3 gene in hepatocytes by growth hormone, interleukin-6 and glucocorticoids mRNA analysis and promoter characterization. Eur J Biochem. 2000;267:5849-5857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Narazaki M, Fujimoto M, Matsumoto T, Morita Y, Saito H, Kajita T, Yoshizaki K, Naka T, Kishimoto T. Three distinct domains of SSI-1/SOCS-1/JAB protein are required for its suppression of interleukin 6 signaling. Proc Natl Acad Sci USA. 1998;95:13130-13134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 205] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Nicholson SE, Willson TA, Farley A, Starr R, Zhang JG, Baca M, Alexander WS, Metcalf D, Hilton DJ, Nicola NA. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 1999;18:375-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 322] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 59. | Terstegen L, Gatsios P, Bode JG, Schaper F, Heinrich PC, Graeve L. The inhibition of interleukin-6-dependent STAT activation by mitogen-activated protein kinases depends on tyrosine 759 in the cytoplasmic tail of glycoprotein 130. J Biol Chem. 2000;275:18810-18817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Schmitz J, Weissenbach M, Haan S, Heinrich PC, Schaper F. SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J Biol Chem. 2000;275:12848-12856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 310] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
Edited by Zhao P