Published online Jun 15, 2002. doi: 10.3748/wjg.v8.i3.469
Revised: February 13, 2002
Accepted: March 5, 2002
Published online: June 15, 2002
AIM: The goal of this study was to characterize the AFP receptor, its possible signal transduction pathway and its proliferative functions in human hepatoma cell line Bel 7402.
METHODS: Cell proliferation enhanced by AFP was detected by MTT assay, 3H-thymidine incorporation and S-stage percentage of cell cycle analysis. With radioactive labeled 125I-AFP for receptor binding assay; cAMP accumulation, protein kinase A activity were detected by radioactive immunosorbent assay and the change of intracellular free calcium ([Ca2+]i) was monitored by scanning fluorescence intensity under TCS-NT confocal microscope. The expression of oncogenes N-ras, p53, and p21ras in the cultured cells in vitro were detected by Northern blotting and Western blotting respectively.
RESULTS: It was demonstrated that AFP enhanced the proliferation of human hepatoma Bel 7402 cell in a dose dependent fashion as shown in MTT assay, 3H-thymidine incorporation and S-phase percentage up to 2-fold. Two subtypes of AFP receptors were identified in the cells with Kds of 1.3 × 10-9 mol·L-1 and 9.9 × 10-8 mol·L-1 respectively. Pretreatment of cells with AFP resulted in a significant increase (625%) in cAMP accumulation. The activity of protein kinase A activity were increased up to 37.5, 122.6, 73.7 and 61.2% at treatment time point 2, 6, 12 and 24 hours. The level of intracellular calcium were elevated after the treatment of alpha-fetoprotein and achieved to 204% at 4 min. The results also showed that AFP (20 mg·L-1) could upregulate the expression of N-ras oncogenes and p53 and p21ras in Bel 7402 cells. In the later case, the alteration were 81.1% (12 h) and 97.3% (12 h) respectively compared with control.
CONCLUSION: These results demonstrate that AFP is a potential growth factor to promote the proliferation of human hepatoma Bel 7402 cells. Its growth-regulatory effects are mediated by its specific plasma membrane receptors coupled with its transmembrane signaling transduction through the pathway of cAMP-PKA and intracellular calcium to regulate the expression of oncogenes.
- Citation: Li MS, Li PF, He SP, Du GG, Li G. The promoting molecular mechanism of alpha-fetoprotein on the growth of human hepatoma Bel7402 cell line. World J Gastroenterol 2002; 8(3): 469-475
- URL: https://www.wjgnet.com/1007-9327/full/v8/i3/469.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i3.469
Alpha-fetoprotein (AFP) is an oncofetal protein normally produced in the fetal liver and yolk sac, whose higher serum level is a useful marker for hepatocellular carcinoma and yolk sac tumors. Although the physicochemical and structural properties of this 70 kDa glycoprotein have been largely documented, its pathophysiological functions were limited in in vitro studies. In the last decade, the growth regulatory properties of AFP have aroused interest as a result of studies involving ontogenetic and oncogenic growth in both cell culture and animal models[1-3]. A myriad of studies has now described that AFP is capable of regulating growth in ovarian, placental, uterine, hepatic phagocyte, bone marrow, and lymphatic cells[4] in addition to various neoplastic cells[5]. This suggests that AFP is not merely a fetal form of albumin-like carrier protein and a marker for cancer and fetal disorders, but should rather now be considered as a potential factor associated with the regulation of growth, differentiation, regeneration, apoptosis and transformation in both ontogenetic and oncogenic growth processes. Although it is currently thought that a 62 to 67 kDa membrane protein on the surface of monocytes and phagocytes is specific for AFP binding[6,7], the properties of the binding sites were still unknown in most tumor cell lines. Furthermore, few studies have focused on its intracellular signaling events and gene expression. The goal of this study is to characterize the AFP receptor, its possible signal transduction pathway and its proliferative functions in human hepatoma Bel 7402 cells.
Purified AFP was from Sigma (USA). Monoclonal antibod against AFP (anti-AFP) was prepared in this laboratory and used to block AFP. The cAMP kit and Na [125I] were purchased from Amersham, UK. Fluo-3 AM was from BIORAD (USA). Monoclonal antibodies for p53 and p21 were purchased from MBI (USA).
Purification of human AFP Human AFP was prepared as previously described[8]. Briefly, human cord blood AFP was precipitated by ammonium sulphate and passed through an anti-AFP affinity chromatography column. AFP-positive fractions were collected and concentrated. The purity of prepared AFP was 92.7% as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein was stored at -80 °C until use.
Effect of AFP on the cell proliferation Total 1.5 × 104 cells per well of Bel 7402 cells were plated into 96-well plates and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) at 37 °C in a humidified atmosphere of 5% CO2 for 48 h. The cultures were replaced with medium without FCS for another 24 h, and treated with different concentration of AFP (1-80 mg·L-1) for 48 h. The effects of AFP on the proliferation of cells were measured by MTT assay and 3H-Thymidine incorporation, which were performed following a regular procedure.
Effect of AFP on the cell cycle First, 3 × 104 cells per well of Bel 7402 cells were plated into 6-well plates. Culture and AFP treatment were then performed as described above. After being treated for 24 h, the cells were digested with 0.25% trypsin/0.02% EDTA and washed three times with PBS. A final density of 1 × 106 cells in 1 ml was added 20 µl (10 mg·mL-1) RNase (Promega USA) solution and incubated at 37 °C for 30 min. The effects of AFP on the cell cycle were detected with flow cytometry.
AFP receptor binding assay Bel-7402 cells were maintained in a humidified atmosphere of 5% CO2 at 37 °C in RPMI-1640 medium supplemented with 10% FCS. The cells were initially depleted of serum for 12 h and then washed with cold medium. Resuspended cells were passed through a 300-mesh screen and adjusted to 1 × 106 cells per ml. 125I-AFP was radioiodinated by the iodogen method and run through a column of Sephadex-G25 to remove free 125I. The specific activity of 125I-AFP was 2715 Ci per mmol and the purity of radioactivity ratio was 99.4%. Each reaction contained 7 × 105 cells, 125I-AFP of 5 × 104 cpm and different concentrations of non-labeled AFP (0.25-64.5 ng). The reaction was triplicated and performed at 4 °C for 2 h. All samples were collected onto glassfiber membrane (presaturated with 0.5% albumin) and washed three times with 15 ml of PBS. The radioactivity of 125I was detected by a γ-counter. Human serum albumin (HSA) as a non-labeled ligand was utimized for measuring IC50. The parameters of binding were ed using a program of Radioligand Binding Assay of Receptors (RBA).
Extraction and measurement of cAMP The cells were adjusted to 4 × 104 cells per ml and cultured in 24 well plates. After 24 h incubation, the cells were collected and resuspended in the medium supplemented with 0.1% egg albumin and 25 mmol·L-1 of HEPES (pH7.4) and 2 mmol·L-1 IBMX (3-methyl-1-isobutyl-xanthine) at 37 °C for 15 min. AFP (20 mg·L-1) and/or anti-AFP (40 mg·L-1) was added into each well respectively for 4 h. Extraction of cAMP was performed according to the method described by Iwashia[9]. In short, the supernatant was removed and replaced with 1 ml of cold PBS per well. After wash, the pellet was frozen in -80 °C for 30 min and then 0.5 ml of HCl (0.05N) was added into each well for another 30 min. The samples were thawed and spun at 10000 g for 5 min. The supernatants were lyophilized, and the content of cAMP was measured by the radio immunoassay following the instruction of cAMP assay kit.
Determination of protein kinase A activity Total 4 × 105 cells per well were cultured in 24-well plates for 48 h, changed to fresh medium without FCS for another 24 h, and then treated with either AFP (20 mg·L-1), anti-AFP (40 mg·L-1) or AFP (20 mg·L-1) plus anti-AFP (40 mg·L-1) respectively. After 2, 6, 12 and 24 h treatment, the cells were washed and resuspended in 1 ml PBS. The measurement of PKA activity has been described by Plet[10]. Briefly, 40 µl of cell extract was mixed with 160 µl of the reaction mixture at the final concentration of 20 mmol·L-1 Tris-HCl (pH7.5), 5 mmol·L-1 MgCl2, 0.25 g·L-1 BSA, 0.5 g·L-1 histone, 2 × 10-7 mol·L-1 ATP (γ-32P ATP, 3.7 × 104 Bq) and 8.0 µmol·L-1 cAMP at 37 °C for 10 min. Followed by incubation on ice for 5 min, the reaction mixture was filtered through Whatmen GF/C filter, washed with 10% TCA-2% phosphoric acid and 5% TCA for 30 min. The radioactivities were measured by a liquid scintillation counter, and PKA activity was expressed as pmol value of 32P in histone catalyzed by per mg protein per min.
Determination of intracellular calcium concentration The cell suspension was dispensed into specific culture plates at a density of 2 × 104 cells per ml and incubated at 37 °C in a humidified atmosphere of 5% CO2 for 48 h. The supernatant was removed and replaced with medium without FCS for 6 h, followed by washing three times with Hank’s solution. The measurement of intracellular calcium concentration has been described by Tsugorka and Petti et al[11,12]. Briefly, the cells were loaded with 10 ml of Fluo-3AM in Hank's solution at a final concentration of 5 µmol·L-1 and incubated at 37 °C for 30 min. After washing 3 times with Hank’s solution, either AFP (20 mg·L-1) or anti-AFP (40 mg·L-1) was loaded into each well. The change of intracellular free calcium ([Ca2+]i) was monitored by scanning fluorescence intensity under TCS-NT confocal microscopy every 10 s.
RNA isolation and Northern blotting Cells were treated with either AFP (20 mg·L-1), anti-AFP (40 mg·L-1) or AFP (20 mg·L-1) plus anti-AFP (40 mg·L-1) for 24 h. Total cellular RNA was isolated from cell lines with TRIzol reagent (Promege, Madison, WI, U.S.A) according to the manufacturer's protocol. RNA (10-20 µg/lane), quantitated by absorbance at 260 nm, and fractionated by eletrophoresis through a 1% formaldehyde agarose gel, and the fractionated RNA was transferred (in 20 × SSC) to nitrocellulose membranes (Millipore corporation Bedford, MA; U.S.A), by standard procedure[13] These membranes were hybridized with a 32P labeled probe and washed using standard protocol. The membranes were then exposed to X-ray film at -70 °C for varying periods of time.
Western blot analysis Cells were treated with either AFP (20 mg·L-1), anti-AFP (40 mg·L-1) or AFP (20 mg·L-1) plus anti-AFP (40 mg·L-1) for 24 h. After three times wash, the cells in each reaction were lysed in 10 µl of lysis buffer containing 0.2% Triton X-100, 500 mmol·L-1 NaCl, 500 mmol·L-1 sucrose, 1 mmol·L-1 EDTA, 0.15 mmol·L-1 spermine, 0.5 mmol·L-1 spermidine, 10 mmol·L-1 HEPES (pH8.0), 200 µmol·L-1 phenylmethylsulfonyl fluoride, 2 mg leupeptin·L-1, 2 mg pepstatin·L-1, 24 IU aprotinin·ml-1 and 7 mmol·L-1γ-mercaptoethanol. 40 µg proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membrane for immunodetection. SDS-PAGE molecular weight markers (Bio-Rad) verified the correct location of the visualized bands. The membranes were blocked in 5% nonfat milk (w/v) in PBS-Tween, then probed with anti-p53 or anti-p21 and followed by secondary antibodies (goat anti-mouse Ig-alkaline phosphatase). Immunoreactive proteins were detected using color development system (NBT/BCIP).
Statistical analysis Data were analyzed by t test and expressed as mean ± SD based on 3 or 4 independent experiments.
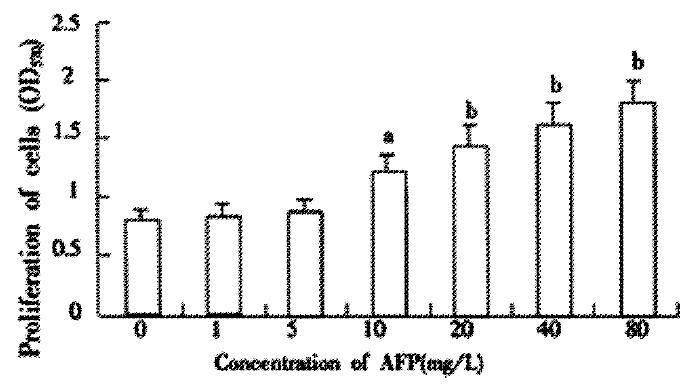
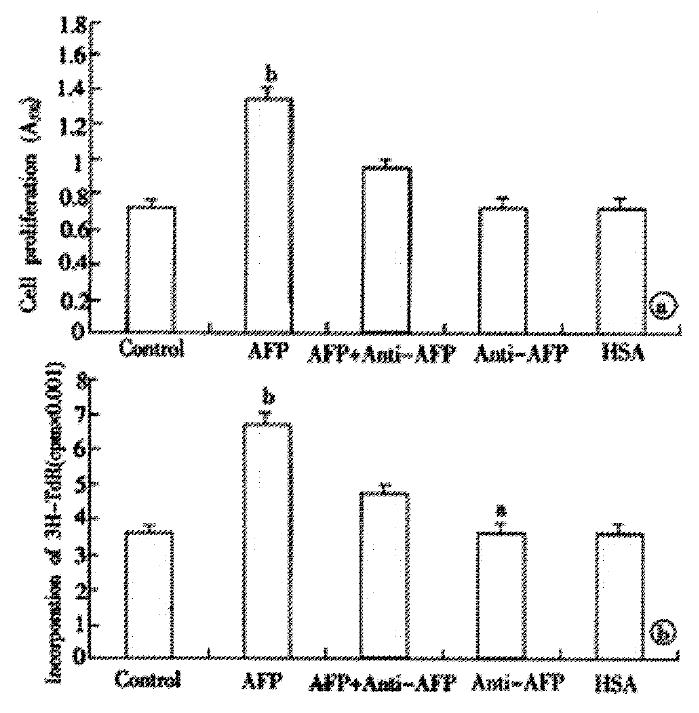
Pretreating Bel 7402 cells with AFP (1-80 mg·L-1) resulted in a dose-dependent increase in cell proliferation (Figure 1). The increase was about 2-fold at a dose of 80 mg·L-1as compared to the control (0 mg·L-1). The effects of AFP (20 mg·L-1) on cell proliferation could be blocked by anti-AFP (40 mg·L-1) which was observed both in MTT assay and 3H-Thymidine incorporation (Figure 2). The increase was not observed in the HSA-treated group and non-treatment control. Flow cytometric analysis showed that AFP (20 mg·L-1) pretreatment increased the S phase cell population by 59.3% of Bel 7402 cells (Table 1).
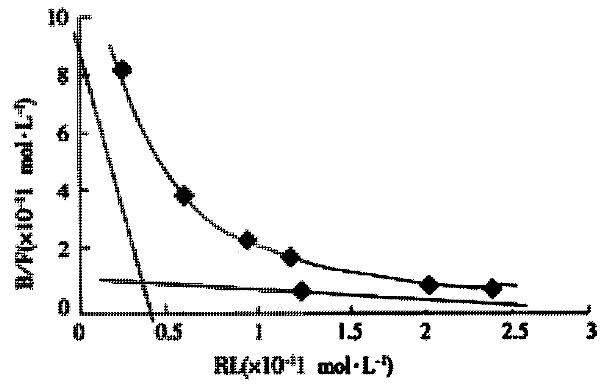
The binding sites of AFP on the surface of the cells and Kd values were calculated based on Scatchard plot analysis of 125I-AFP. Scatchard analysis showed that there were two classes of receptors with different affinities on Bel 7402 cells. As for Bel 7402 cells, KD1 with 89400 sites per cell was 1.3 × 10-9 mol·L-1 and KD2 with 582000 sites per cell was 9.9 × 10-8 mol·L-1 (Figure 3). To indicate a higher affinity of the binding sites for AFP, IC50 was calculated to achieve 50% inhibition. More than two fold of HSA were needed compared with AFP (data not shown), which indicated a higher affinity for the binding sites on the surface of cells to AFP.
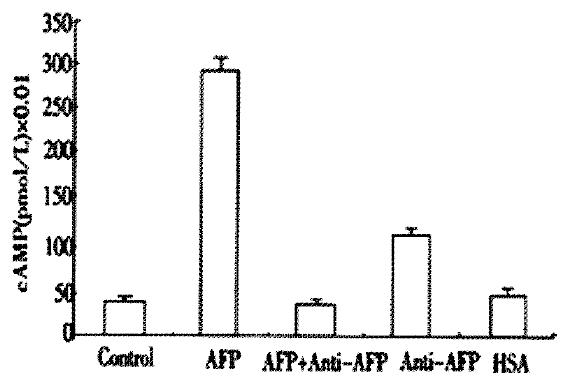
AFP markedly elevated the concentration of cAMP up to 625% in Bel 7402 cells (Figure 4). Anti-AFP could not alter the concentrations of cAMP when added alone, but it reversed the effect of AFP. As a control, HSA did not influence the content of cAMP.
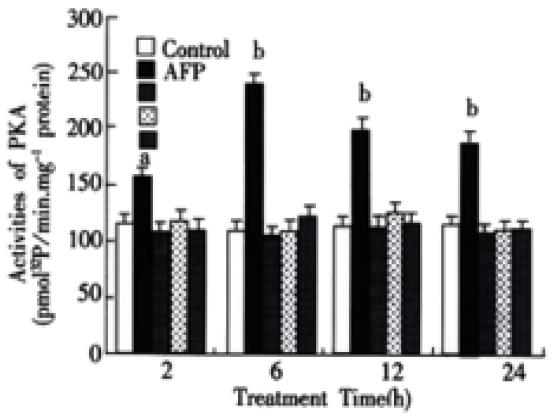
The activities of PKA in the cytosol of Bel 7402 cells were obviously elevated after being treated with AFP (20 mg·L-1) for 2, 6, 12 or 24 h (Figure 5). The activities of PKA were increased up to 37.5, 122.6, 73.7 and 61.2% in Bel 7402 cells at each time point. The peak value was achieved at 6 h and then declined gradually, but still maintained a higher activity for several hours. Anti-AFP or HSA alone did not affect the activity of PKA in Bel 7402 cells, but anti-AFP could block the effects of AFP on the activity of PKA.
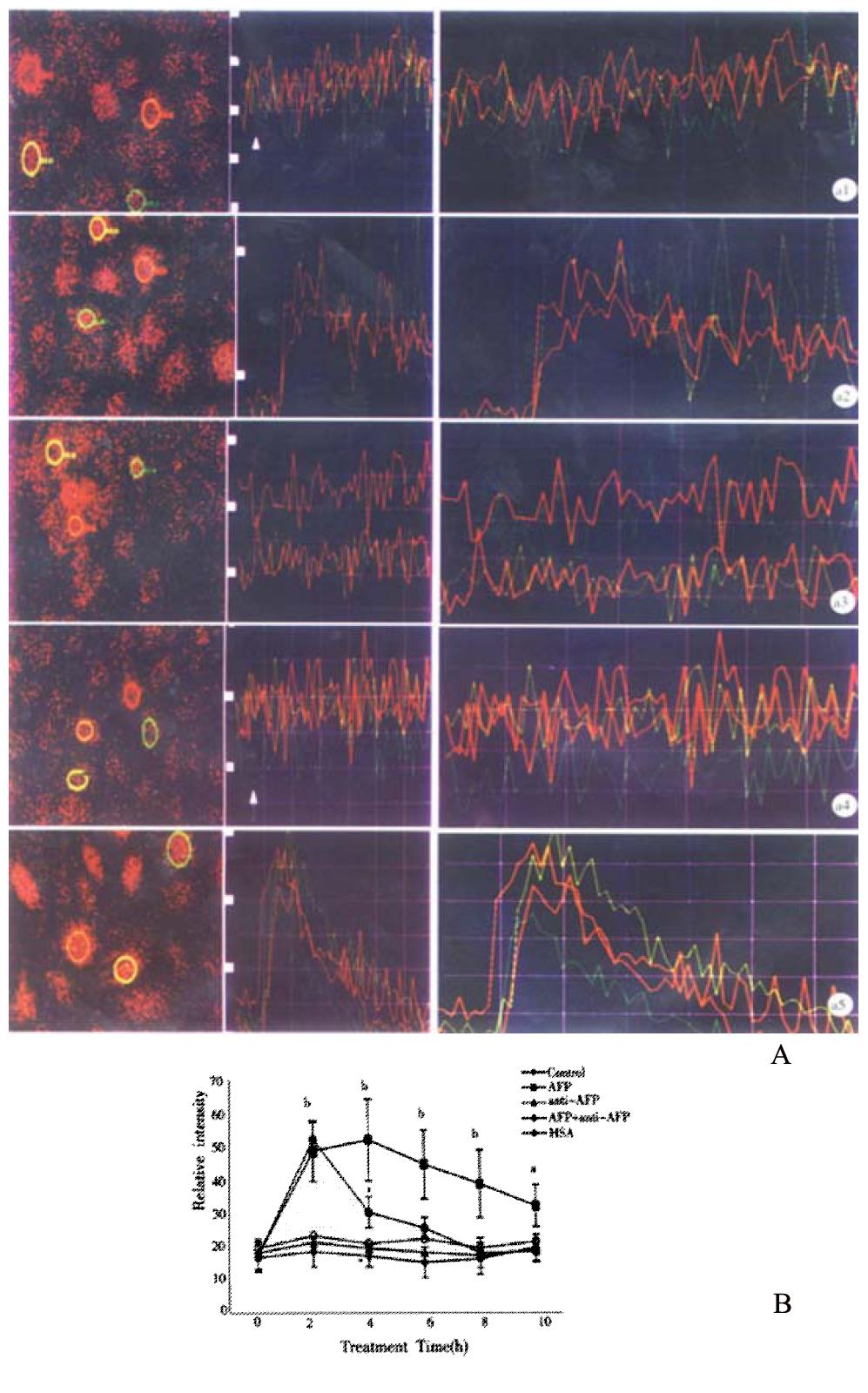
Figures 6A 1-5 and Figure 6B showed that AFP increased the intracellular [Ca2+]i after treatment of 2, 4, 6, 8 and 10 min in Bel 7402 cells. The peak was achieved at treatment time 4 min (increment 204.1% in Bel 7402 cells). Anti-AFP and HSA did not change the content of [Ca2+]i in either cell type. However, anti-AFP could reverse the effect of AFP.
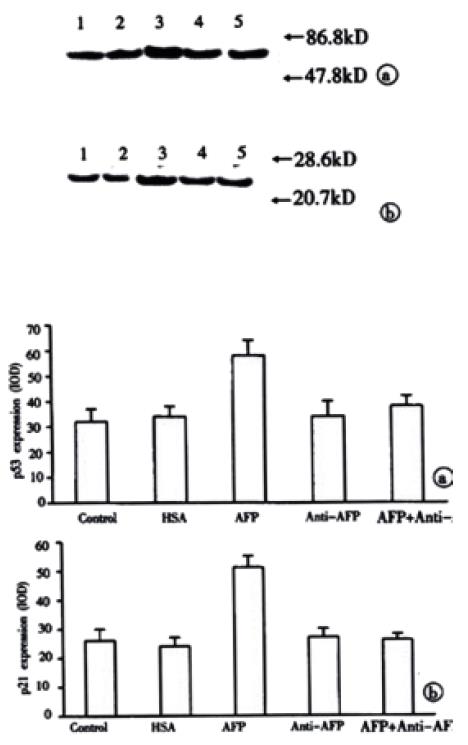
The results in Figure 7 demonstrated the overexpression of mutant p53 and p21 protein in AFP-treated group in Bel 7402 cells. Anti-AFP could reverse the upregulated effects of AFP on the expression of p53 and p21 genes. HSA could not influence the amount of these proteins. Each graph was selected from 3 similar results.
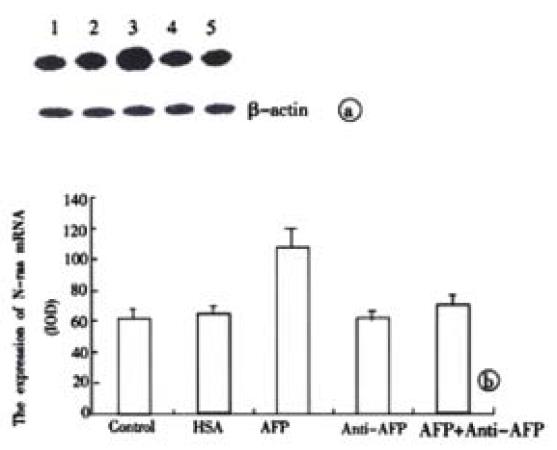
The results in Figure 8 demonstrated the overexpression of N-ras mRNA in AFP-treated group in the Bel 7402 cells. Anti-AFP could reverse the upregulated effects of AFP on the expression of N-ras mRNA. HSA could not influence the mRNA amount of the oncogene. Each graph was selected from 3 similar results.
AFP is an onco-developmental gene product. In the adult, AFP is highly expressed during liver regeneration and hepatocarcinogenesis and used as a maker for the diagnosis of hepatocellular carcinoma[14-16]. The regulation and activation on the expression of the AFP gene have been extensively investigated[17-21]. Although less data indicated AFP could causes apoptosis in tumor cells[22,23], the data from most current research demostrate that AFP is enhancer in tumor growth. The downregulation of expression of alpha-fetoprotein is able to induce the suppression of growth of malignant hepatocyte cell[24-26]. Although the biological role of AFP in cell growth has been reported[27-29], the properties of the AFP receptor as well as the subsequent events after AFP binding were still undefined. Our data indicate that a specific AFP receptor does exist in a human tumor cell line, Bel 7402 cells. There were two kinds of receptors with different affinities in Bel 7402 cells (KD:10-9 and 10-8 mol·L-1), which was consistent with similar experiments that characterized the KD of binding protein in monocytes in the range of 10-11-10-7 mol·L-1[6,30]. The AFP-binding protein possibly containing the AFP-receptor has been isolated from human embryos and human breast cancer tissue[31].
Based on the results of MTT assay, 3H-thymidine incorporation and flow cytometry analysis, as well as the enhanced expression of mutant p53 and p21 and expression of protooncogenes N-ras mRNA, AFP appears to be a potential growth promoting factor.
Since the albumin and AFP genes are similar in structure, they are believed to be derived from a common ancestral gene, even in the same albuminoid gene family. In all our AFP studies, none of the results showed that human serum albumin (HSA) as a control was able to alter the parameters of cell proliferation although it can non-specifically bind to the cell surface.
Little information on the effect of AFP on signal transduction was available. The present experiments demonstrated that intracellular cAMP was significantly elevated 7 fold in Bel 7402 cells. PKA activities were also increased. This indicates that a cAMP-dependent protein kinase pathway is involved in the effects of AFP on the tumor cells, even though some data from other laboratory indicated that the alteration of activity of PKC affected only liver gene expression rather than cell growth in fetal hepatocytes[32]. Other experiments for the relationship between AFP and message has been tested[33].
In addition, the results of intracellular calcium showed that AFP markedly increased intracellular [Ca2+]i. It has been reported that Bcl-2 suppresses apoptosis by inhibiting calcium activation of the permeability transition of mitochondria[34] and the inhibition of calcium influx was related to the suppression of lymphoma cell-line proliferation[35]. Furthermore, a reciprocal regulation between calcium signaling and hypertrophic growth has been identified[36]. According to these findings, a higher intracellular [Ca2+]i elicited by AFP may play a role in tumorigenesis.
Although growing evidence has confirmed the effects of AFP on the growth of tumor cells, little work has focused on the subsequent events in the nucleus. The impacts of overexpression of protooncogenes N-ras, mutant p53, p21 and other genes on tumor growth have been largely documented[37-41]. In the present experiment, mutant p53 and p21 protein were over produced under the treatment of AFP, which was consistent with similar work[42,43]. It suggested that the mechanism by which elevated levels of mutant p53 and p21 proteins might be involved in AFP-induced oncogenesis.
The pattern inducing the hepatocarcinogenesis is multimode[44,45], but the effect of AFP on the growth of tumor has been confirmed. Based on our experiments, the functional mechanism of AFP on the growth of tumors may be attributed, at least in part, to receptor-mediated cAMP pathway and/or calcium signaling resulting in overexpression of certain genes. The clarification of the mechanism will provide a possibility for the gene therapy of liver tumor[18,46,47]. Further investigations on the function of AFP may shed further light on the mechanism of AFP action.
This work was supported by National Natural Science Foundation of China (¹.39760077). We wish to thank Lei Hu, MD, PhD, Northwestern University, Chicago, USA, for critical reading of the manuscript.
| 1. | Mizejewski GJ, Warner AS. Alpha-fetoprotein can regulate growth in the uterus of the immature and adult ovariectomized mouse. J Reprod Fertil. 1989;85:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Keel BA, Eddy KB, Cho S, May JV. Synergistic action of purified alpha-fetoprotein and growth factors on the proliferation of porcine granulosa cells in monolayer culture. Endocrinology. 1991;129:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Wang W, Alpert E. Downregulation of phorbol 12-myristate 13-acetate-induced tumor necrosis factor-alpha and interleukin-1 beta production and gene expression in human monocytic cells by human alpha-fetoprotein. Hepatology. 1995;22:921-928. [PubMed] |
| 4. | Mizejewski GJ. alpha-fetoprotein as a biologic response modifier: relevance to domain and subdomain structure. Proc Soc Exp Biol Med. 1997;215:333-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Dudich E, Semenkova L, Gorbatova E, Dudich I, Khromykh L, Tatulov E, Grechko G, Sukhikh G. Growth-regulative activity of human alpha-fetoprotein for different types of tumor and normal cells. Tumour Biol. 1998;19:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Suzuki Y, Zeng CQ, Alpert E. Isolation and partial characterization of a specific alpha-fetoprotein receptor on human monocytes. J Clin Invest. 1992;90:1530-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Moro R, Tamaoki T, Wegmann TG, Longenecker BM, Laderoute MP. Monoclonal antibodies directed against a widespread oncofetal antigen: the alpha-fetoprotein receptor. Tumour Biol. 1993;14:116-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Nishi S, Hirai H. Purification of human, dog and rabbit -fetoprotein by immunoadsorbents of sepharose coupled with anti-human -fetoprotein. Biochim Biophys Acta. 1972;278:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Iwashita S, Mitsui K, Shoji-Kasai Y, Senshu-Miyaike M. cAMP-mediated modulation of signal transduction of epidermal growth factor (EGF) receptor systems in human epidermoid carcinoma A431 cells. Depression of EGF-dependent diacylglycerol production and EGF receptor phosphorylation. J Biol Chem. 1990;265:10702-10708. [PubMed] |
| 10. | Plet A, Evain-Brion D, Gerbaud P, Anderson WB. Retinoic acid-induced rapid loss of nuclear cyclic AMP-dependent protein kinase in teratocarcinoma cells. Cancer Res. 1987;47:5831-5834. [PubMed] |
| 11. | Tsugorka A, Ríos E, Blatter LA. Imaging elementary events of calcium release in skeletal muscle cells. Science. 1995;269:1723-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 229] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Pettit EJ, Hallett MB. Early Ca2+ signalling events in neutrophils detected by rapid confocal laser scanning. Biochem J. 1995;310:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual, 2nd edition. Cold spring harbor. 1989;18:60-75. |
| 14. | Tu DG, Wang ST, Chang TT, Chiu NT, Yao WJ. The value of serum tissue polypeptide specific antigen in the diagnosis of hepatocellular carcinoma. Cancer. 1999;85:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Sell S, Xu KL, Huff WE, Kabena LF, Harvery RB, Dunsford HA. Aflatoxin exposure produces serum alphafetoprotein elevations and marked oval cell proliferation in young male Pekin ducklings. Pathology. 1998;30:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Yuasa T, Yoshiki T, Ogawa O, Tanaka T, Isono T, Mishina M, Higuchi K, Okada Y, Yoshida O. Detection of alpha-fetoprotein mRNA in seminoma. J Androl. 1999;20:336-340. [PubMed] |
| 17. | Chen H, Egan JO, Chiu JF. Regulation and activities of alpha-fetoprotein. Crit Rev Eukaryot Gene Expr. 1997;7:11-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Ohguchi S, Nakatsukasa H, Higashi T, Ashida K, Nouso K, Ishizaki M, Hino N, Kobayashi Y, Uematsu S, Tsuji T. Expression of alpha-fetoprotein and albumin genes in human hepatocellular carcinomas: limitations in the application of the genes for targeting human hepatocellular carcinoma in gene therapy. Hepatology. 1998;27:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Ishikawa H, Nakata K, Tsuruta S, Nakao K, Kato Y, Tamaoki T, Eguchi K. Differential regulation of albumin gene expression by heparin-binding epidermal growth factor-like growth factor in alpha-fetoprotein-producing and -nonproducing human hepatoma cells. Tumour Biol. 1999;20:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Abelev GI, Eraiser TL. Cellular aspects of alpha-fetoprotein reexpression in tumors. Semin Cancer Biol. 1999;9:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Magee TR, Cai Y, El-Houseini ME, Locker J, Wan YJ. Retinoic acid mediates down-regulation of the alpha-fetoprotein gene through decreased expression of hepatocyte nuclear factors. J Biol Chem. 1998;273:30024-30032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Dudich I, Tokhtamysheva N, Semenkova L, Dudich E, Hellman J, Korpela T. Isolation and structural and functional characterization of two stable peptic fragments of human alpha-fetoprotein. Biochemistry. 1999;38:10406-10414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Dudich E, Semenkova L, Dudich I, Gorbatova E, Tochtamisheva N, Tatulov E, Nikolaeva M, Sukhikh G. alpha-fetoprotein causes apoptosis in tumor cells via a pathway independent of CD95, TNFR1 and TNFR2 through activation of caspase-3-like proteases. Eur J Biochem. 1999;266:750-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Zhang M, Gong Y, Assy N, Minuk GY. Increased GABAergic activity inhibits alpha-fetoprotein mRNA expression and the proliferative activity of the HepG2 human hepatocellular carcinoma cell line. J Hepatol. 2000;32:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Hamamoto R, Kamihira M, Iijima S. Growth and differentiation of cultured fetal hepatocytes isolated various developmental stages. Biosci Biotechnol Biochem. 1999;63:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Gruppuso PA, Bienieki TC, Faris RA. The relationship between differentiation and proliferation in late gestation fetal rat hepatocytes. Pediatr Res. 1999;46:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Wang XW, Xie H. Alpha-fetoprotein enhances the proliferation of human hepatoma cells in vitro. Life Sci. 1999;64:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Wang XW, Xu B. Stimulation of tumor-cell growth by alpha-fetoprotein. Int J Cancer. 1998;75:596-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Koide N, Nishio A, Igarashi J, Kajikawa S, Adachi W, Amano J. Alpha-fetoprotein-producing gastric cancer: histochemical analysis of cell proliferation, apoptosis, and angiogenesis. Am J Gastroenterol. 1999;94:1658-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Torres JM, Geuskens M, Uriel J. Activated human T lymphocytes express albumin binding proteins which cross-react with alpha-fetoprotein. Eur J Cell Biol. 1992;57:222-228. [PubMed] |
| 31. | Kanevsky VYu LP, Aksenova OA, Severin SE, Katukov VYu ES. Isolation and characterization of AFP-binding proteins from tumor and fetal human tissues. Biochem Mol Biol Int. 1997;41:1143-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Roncero C, Ventura JJ, Sánchez A, Bois-Joyeux B, Mesa ML, Thomassin H, Danan JL, Benito M, Fabregat I. Phorbol esters down-regulate alpha-fetoprotein gene expression without affecting growth in fetal hepatocytes in primary culture. Biochim Biophys Acta. 1998;1402:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Benassayag C, Rigourd V, Mignot TM, Hassid J, Leroy MJ, Robert B, Civel C, Grangé G, Dallot E, Tanguy J. Does high polyunsaturated free fatty acid level at the feto-maternal interface alter steroid hormone message during pregnancy? Prostaglandins Leukot Essent Fatty Acids. 1999;60:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Murphy RC, Schneider E, Kinnally KW. Overexpression of Bcl-2 suppresses the calcium activation of a mitochondrial megachannel. FEBS Lett. 2001;497:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Anesini C, Ferraro G, López P, Borda E. Different intracellular signals coupled to the antiproliferative action of aqueous crude extract from Larrea divaricata Cav. and nor-dihydroguaiaretic acid on a lymphoma cell line. Phytomedicine. 2001;8:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Kline R, Jiang T, Xu X, Rybin VO, Steinberg SF. Abnormal calcium and protein kinase C-epsilon signaling in hypertrophied atrial tumor myocytes (AT-1 cells). Am J Physiol Heart Circ Physiol. 2001;280:H2761-H2769. [PubMed] |
| 37. | Kirla R, Salminen E, Huhtala S, Nuutinen J, Talve L, Haapasalo H, Kalimo H. Prognostic value of the expression of tumor suppressor genes p53, p21, p16 and prb, and Ki-67 labelling in high grade astrocytomas treated with radiotherapy. J Neurooncol. 2000;46:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Yuen PW, Lam KY, Choy JT, Ho WK, Wei WI. Clinicopathological significance of p53 and p21 expression in the surgical treatment of laryngeal carcinoma. Anticancer Res. 2000;20:4863-4866. [PubMed] |
| 39. | Komarova EA, Christov K, Faerman AI, Gudkov AV. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene. 2000;19:3791-3798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Kumar Dhar D, Kubota H, Tabara H, Kotoh T, Monden N, Igarashi M, Kohno H, Nagasue N. nm23 in the primary and metastatic sites of gastric carcinoma. Relation to AFP-producing carcinoma. Oncology. 1999;56:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Yamamoto H, Fujimoto J, Okamoto E, Furuyama J, Tamaoki T, Hashimoto-Tamaoki T. Suppression of growth of hepatocellular carcinoma by sodium butyrate in vitro and in vivo. Int J Cancer. 1998;76:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Liu Z, Liu G, Zhang S. Reversing effect of dimethyl-4,4'-dimethoxy-5,6,5', 6'-dimethylenedioxybiphenyl-2,2'-dicarboxylate (DDB) on the phenotypes of human hepatocarcinoma cells line. Cancer Lett. 1996;108:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Mazume H, Nakata K, Hida D, Hamasaki K, Tsuruta S, Nakao K, Kato Y, Eguchi K. Effect of simvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, on alpha-fetoprotein gene expression through interaction with the ras-mediated pathway. J Hepatol. 1999;30:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Feng DY, Zheng H, Tan Y, Cheng RX. Effect of phosphorylation of MAPK and Stat3 and expression of c-fos and c-jun proteins on hepatocarcinogenesis and their clinical significance. World J Gastroenterol. 2001;7:33-36. [PubMed] |
| 45. | Sun BH, Zhang J, Wang BJ, Zhao XP, Wang YK, Yu ZQ, Yang DL, Hao LJ. Analysis of in vivo patterns of caspase 3 gene expression in primary hepatocellular carcinoma and its relationship to p21(WAF1) expression and hepatic apoptosis. World J Gastroenterol. 2000;6:356-360. [PubMed] |
| 46. | Ishikawa H, Nakata K, Mawatari F, Ueki T, Tsuruta S, Ido A, Nakao K, Kato Y, Ishii N, Eguchi K. Utilization of variant-type of human alpha-fetoprotein promoter in gene therapy targeting for hepatocellular carcinoma. Gene Ther. 1999;6:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Moskaleva EYu GA, Shmyrev II, Rodina AV, Muizhnek EL, Severin ES, Katukov VYu YM, Severin SE. Alpha-fetoprotein-mediated targeting--a new strategy to overcome multidrug resistance of tumour cells in vitro. Cell Biol Int. 1997;21:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Edited by Pagliarini R and Zhang JZ