Published online Jun 15, 2002. doi: 10.3748/wjg.v8.i3.455
Revised: October 2, 2001
Accepted: October 29, 2001
Published online: June 15, 2002
AIM: Epidermal growth factor (EGF) plays an important role in the regulation of gastrointestinal tissue growth and development, and it can stimulate epithelial proliferation, cell differentiation and growth. It has been established that the EGF can promote gastric cytoprotection and ulcer healing. But the potential ability of EGF to regulate the gastric cancer growth is unknown. This study is to investigate the influence of EGF on human gastric cancer cell and the implanted tumor growth of nude mice.
METHODS: The cell growth rates of human gastric adenocarinoma cell lines MKN-28, MKN-45, SGC-7901 and normal human gastric epithelial cells 3T3 were assessed when incubated with recombinant human EGF (rhEGF, 0.05, 0.1, 0.5, 1.0, 10, 50, 100 mg·L-1) using MTT method. The cells of MKN-28, MKN-45, SGC-7901 (gastric cancer tissue 1.5 mm3) were implanted in the BALB/cA nude mice for 10 days. The EGF was given intraperitoneally (15, 30, 60 μg·kg-1) for 3 weeks. The body weights of the tumor-bearing animals and their tumor mass were measured afterwards to assess the mitogenic effect of rhEGF in the nude mice.
RESULTS: Within the concentration range of 0.05-100 mg·L-1, rhEGF could increase the cell growth of normal 3T3 cells (cell growth rate 100% vs 102.8%, P < 0.05), but partially restrain the gastric cancer cell growth. The latter effect was related to cell differentiation. In 15-60 μg/kg rhEGF groups, the mean implanted tumor mass of MKN-28 cell were 1.75 g, 1.91 g, 2.08 g/NS group 1.97 g (P > 0.05), the mean tumor mass of SGC-7901 cell were 1.53 g, 1.07 g, 1.20 g/NS group 1.07 g (P > 0.05), and for MKN-45 cell, the tumor mass were respectively 1.92 g, 1.29 g, 1.77 g/NS group 1.82 g (P > 0.05). So rhEGF had no obvious effect on implanted MKN-28, SGC-7901 and MKN-45 tumor growth.
CONCLUSION: EGF has no stimulating effect on the human gastric cancer cell growth neither in vitro nor in vivo.
- Citation: Xia L, Yuan YZ, Xu CD, Zhang YP, Qiao MM, Xu JX. Effects of epidermal growth factor on the growth of human gastric cancer cell and the implanted tumor of nude mice. World J Gastroenterol 2002; 8(3): 455-458
- URL: https://www.wjgnet.com/1007-9327/full/v8/i3/455.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i3.455
Growth factors are found in a variety of adult and embryonic tissues. They are important regulators of cell differentiation and proliferation, and play an important role in maintaining the integrity of the epithelium. They have also been implicated in malignancy. Epidermal growth factor (EGF), a single-chain polypeptide of 53 amino acid residues, is found mainly in the submandibular glands and Brunner’s gland of the gastrointestinal tract. It can be combined with the specific receptor (EGF-R) of the target cell membrane[1]. Some studies suggested that the expression of EGF-R was increased in gastric cancer tissue. It was also reported that EGF can increase the mitosis in vitro[2]. Patients with EGF receptor-positive gastric cancer may have a poorer prognosis than those with EGF receptor-negative cancers. So, EGF has the function to influence the tumor cell growth. At present, the effect of EGF in this process has been unclear yet.
In this report, we seek to determine the effect of EGF on the growth of human gastric cancer cell (MKN-28, SGC-7901 and MKN-45) in vitro. In nude mice which underwent surgical implantation of the same gastric cancer cells, EGF was injected intraperitoneally to investigate the influence of EGF on tumor cell growth, so as to confirm the safety of EGF in the treatment of peptic ulcer[3-14].
Gastric cancer cell lines, MKN-28, SGC-7901 and MKN-45, are well-differentiated, moderate-differentiated and low-differentiated human adenocarcinoma cell lines respectively. 3T3 cell is normal human gastric epithlium. They are all established and characterized in our laboratory. rhEGF was obtained from Institute of Biochemistry and Cell Biology, Shanghai Institute for Biological Science, Chinese Academy of Science (100 μg/amp). 3-(4,5-dimethylathiazol-2-yl) and 5-diphenyltetrazolium bromide were the product of Fluda Chemie AG. Balb/cA nude mice: were obtained from Institute of Pharmatheutics, Shanghai Institute for Biological Science, Chinese Academy of Science. 35-40 day old, 18-20 g, female. Mitomycin C (MMC) was the product of Kyowa Hakko, Japan, 2 mg/Amp.
Cell cultures Human gastric cancer cells were propagated as adherent monolayers and removed from culture surfaces by treatment with trypsin, then seeded in microwells at 1 × 108·L-1 in complete medium composed of RPMI 1640 and 200 mL·L-1 fetal bovine serum (FBS). The cells were grown in 96-well microplates of RPMI1640 tissue culture medium supplemented with 200 mL·L-1 FBS at 37 °C in a humidified atmosphere of 50 mL·L-1 CO2 in air. After 24 h incubation, the cells were then added by rhEGF at the concentration of 0.05, 0.1, 0.5, 1.0, 10, 50, 100 mg·L-1 for further incubation of 72 hours. Uninoculated RPMI 1640 medium was used as a control under otherwise identical experimental procedures. At the end of cell incubation, cell numbers and their viability were determined by MTT method. Add MTT (1 g·L-1) in each microwell for 4 h in 37 °C air. After centrifugation, 100 μL dimethyl sulfoxode (DMSO) was added into each well for 30 minutes. Absorption rate of treated and control cells was measured at 570 nm (A value) for quantitative measurement of cell growth. Each test kit contained a positive control and an additional positive control. Experimental controls were treated with DMSO only.
Tumor implantation into nude mice Gastric cancer tissue (1.5 mm3) were implanted s.c. in the right dorsal area of 4-6 wk old male nude mice. Animals were fed with an autoclaved diet and tap water (acidified to pH2.5). After 10 d, the animals were assigned into the rhEGF treatment groups (15, 30, 60 ìg·kg-1, intraperitoneally, 5 times per week for 3 wk), negative control group (saline, 2 mL intraperitonium) and positive control group (MMC, 2 mg·kg-1, twice every week, 6 times altogether). The body mass of Balb/cA tumor-bearing animals and their tumor weights were measured using anesthesia with ether.
Inhibitory rate (IR) of tumor growth = m (tumor)C - m (tumor)T/m (tumor)C
(m (tumor)C: mean tumor weight of negetive control group; m (tumor)T: mean tumor weight of rhEGF treatment group).
Student’s t test was performed to assess potentially significant differences between individual groups of observations. The test statistics were then compared with values obtained from standard two-tailed tables. A P value of < 5% was accepted as indicating probable significance when comparing the various groups.
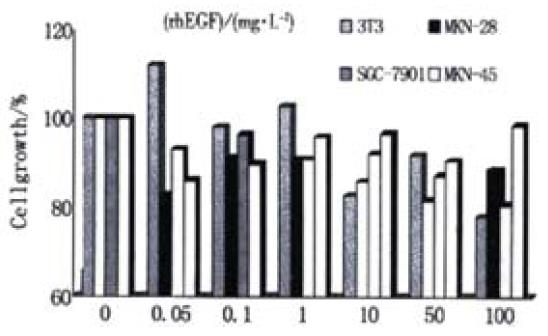
We found that EGF had no significant growth-stimulatory effects on gastric cancer cells in a dose-dependent manner (Figure 1). The lowest cell growth rates in MKN-28, S-7901 and MKN-45 cell lines were 81.7%, 80.7% and 86.1% respectively, compared with the control at the 0.05, 50, 100 mg·L-1 of rhEGF. EGF could inhibit the cancer cell growth within the level of 0.05 to 100 mg·L-1. But there was no probable significance within the same group. In contrast, for the normal 3T3 cells, EGF could increase the cell growth significantly after the coincubation (P = 0.0008). We also found that the influence of EGF on the gastric cancer cell growth was dependent on the differentiation of the cell. Under the same concentration, the inhibition was greater in well-differentiated cells.
The mean tumor weight of negative control group after the study was 1.97 g in MKN-28 nude mice. In MMC treatment group, the tumor weight was 0.47 g (P < 0.05). In rhEGF groups (15, 30, 60 µg·kg-1), the tumor weights were 1.75, 1.91 and 2.08 g respectively. The inhibitory rate were -5.3% to 11.1%, compared with negative control group. In rhEGF60 µg·kg-1 group, the positive data suggested that the weight was higher than control, but the difference was not significant. There were no significant difference compared with the negative control group (Table 1). In S-7901 and MKN-45 cell lines, the same results found indicated that intraperinoneal rhEGF treatment could not stimulate the tumor growth in nude mice within the concentration 15-30 µg·kg-1 (Table 2, Table 3).
| Group | Dosage | n | Body mass/g | Tumor mass -x±s/g | Inhibitary rate/% | P value | |
| Beginning | End | ||||||
| NS | 0.2 mL | 16 | 17.6 | 22.6 | 1.97 ± 0.94 | —— | —— |
| MMC | 2 μg·kg-1 | 8 | 17.8 | 20.0 | 0.47 ± 0.61 | 76.2 | < 0.05 |
| RhEGF | 15 μg·kg-1 | 8 | 17.4 | 22.0 | 1.75 ± 0.81 | 11.1 | < 0.05 |
| RhEGF | 30 μg·kg-1 | 8 | 17.9 | 23.8 | 1.91 ± 0.98 | 3.0 | < 0.05 |
| RhEGF | 60 μg·kg-1 | 8 | 17.9 | 22.9 | 2.08 ± 1.56 | -5.3 | < 0.05 |
| Group | Dosage | n | Body mass/g | Tumor mass -x±s/g | Inhibitary rate/% | P value | |
| Beginning | End | ||||||
| NS | 0.2 mL | 16 | 14.4 | 25.2 | 1.07 ± 0.60 | — | —— |
| MMC | 2 μg·kg-1 | 8 | 15.2 | 21.7 | 0.66 ± 0.29 | 38.6 | < 0.05 |
| RhEGF | 15 μg·kg-1 | 8 | 15.9 | 25.3 | 1.53 ± 0.29 | -43.8 | < 0.05 |
| RhEGF | 30 μg·kg-1 | 8 | 14.1 | 23.8 | 1.07 ± 0.63 | -0.7 | < 0.05 |
| RhEGF | 60 μg·kg-1 | 8 | 13.4 | 23.9 | 1.20 ± 0.47 | -12.5 | < 0.05 |
| Group | Dosage | n | Body mass/g | Tumor mass -x±s/g | Inhibitary rate/% | P value | |
| Beginning | End | ||||||
| NS | 0.2 mL | 20 | 16.1 | 19.8 | 1.82 ± 0.95 | — | —— |
| MMC | 2 μg·kg-1 | 10 | 16.2 | 19.7 | 1.07 ± 0.42 | 41.1 | < 0.05 |
| RhEGF | 15 μg·kg-1 | 10 | 16.0 | 20.3 | 1.92 ± 1.04 | -5.5 | < 0.05 |
| RhEGF | 30 μg·kg-1 | 10 | 16.5 | 19.8 | 1.29 ± 0.83 | 8.0 | < 0.05 |
| RhEGF | 60 μg·kg-1 | 8 | 16.1 | 20.4 | 1.77 ± 1.04 | 3.1 | < 0.05 |
We have examined the effect of EGF on the established cell line, MKN-28, SGC-7901 and MKN-45, derived from human gastric adenocarcinoma, both in vitro and in vivo. The results may be somewhat controversy to those formerly reported, that EGF had no obviously effect on the gastric cancer growth[15,16]. Growth factors are components of signal transduction pathways that have a considerable spectrum of biological activity, such as control of cell proliferation, differentiation, apoptosis and transformation[17,18]. Of these growth factors, EGF family are important agents for gastric mucosa. The EGF family include at least seven mammalian polypeptides: EGF, TGF-α, amphiregulin (AR), cripto heregulin, betacellulin and heparin-binding epidermal growth factor (HB-EGF). Except cripto and heregulin, all of these proteins have been shown to bind and activate the 170-kilodalton EGF receptor tyrosine kinase[19,20]. They share a similar spectrum of biological activities exerted through interaction with EGF-R. EGF-R is a transmembrane glycoprotein, which can stimulate cell proliferation mainly through induction of the proto-oncogenes c-fos and c-myc, and of molecules such as polyamines. The TGF can cause morphological transformation and promote anchorage independent growth in vitro. Although there is no evidence of TGF secretion from nonneoplastic adult tissue, it is synthesized during fetal development and produced by many tumor tissues[21,22]. TGF-α is frequently produced by malignant as well as normal cells and may stimulate their own proliferation. However, less is known about the role of EGF in oncogenesis[23-25]. The importance of growth factors in the healing and oncogenesis of gastrointestinal diseases has recently received much attention. In inflamed mucosa, EGF is found predominantly in the cytoplasm of the superficial epithelial and isthmus cells, as in the normal mucosa[26]. In addition to providing a mitogenic stimulus, EGF may also help the proliferating cells to migrate into the superficial epithelium during the process of "cytoprotective" epithelial repair[27].
The development of monoclonal and polyclonal antibodies against EGF has allowed studies of the localization of EGF in normal and neoplastic tissues to be performed[28-31]. Immunocytochemical staining has shown distribution of epidermal growth factor and transforming growth factor α (TGF-α) in the gastrointestinal tract with high levels[32-35]. Normal epithelial cells secrete such growth factors to regulate cell replacement by autocrine or paracrine mechanisms. It is speculated that these growth factors may regulate the transition rate between G2-phase and mitosis of the cell cycle[36]. It has reported that HB-EGF is mitogenic for some types of cells, such as fibroblasts, vascular smooth muscle cells, keratinocytes and rat hepatocytes, but not endothelial cells[37].
The mitogenic action of EGF and TGF-αin vitro has been reported in many gastrointestinal tissues, including esophagus, stomach and intestine, and there i s little information about the association between the mucosal expression of these peptides and indices of cellular proliferation in vivo[38]. It was reported that EGF immunoreactivity was present in 26%-37% of gastric cancers, and the presence of EGF in gastric cancer correlated with the degree of gastric wall invasion, lymph node metastasis and disease progression[39-42]. Although the epidermal growth factor/receptor system has been found abnormal in intestinal type gastric cancer, overexpression of EGF-R, erbB-2 and erbB-3 receptor genes was mainly found. There has been some controversy in the literature whether EGF-R overexpression related to tumor progression or to early stages of gastric carcinogenesis[25,43-45]. The study had shown that overexpression of the EGF-R gene was infrequent in the metaplastic gastric mucosa. A major problem in gastric carcinogenesis is to determine the changing point from benign pre-neoplastic lesions to malignancy. There is a general agreement that this process involves different steps in cellular changes, requiring both activation and inhibition of specific genes, but there is still no evidence to support EGF or EGF-R overexpression to be a reliable marker of increased cancer risk in patients[46-49]. The present study has sought to clarify their effect on the growth of gastric cancer cell in vitro and in vivo. In this study, we have found that there was no effect of EGF on the growth of established cell lines, MKN-28, SGC-7901, MKN-45, derived from human gastric adenocarcinoma, both in vitro and in vivo. Further study is headed to elucidate whether EGF could cause abnormal differentiation of the cells during the treatment of peptic ulcer for a long period.
| 1. | Playford RJ, Wright NA. Why is epidermal growth factor present in the gut lumen? Gut. 1996;38:303-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Maehiro K, Watanabe S, Hirose M, Iwazaki R, Miwa H, Sato N. Effects of epidermal growth factor and insulin on migration and proliferation of primary cultured rabbit gastric epithelial cells. J Gastroenterol. 1997;32:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Chen BW, Wang HT, Liu ZX, Jie BQ, Ma QJ. Effect of exogenous EGF on protooncogene expression in experimental gastric ulcer in rats. Shijie Huaren Xiaohua Zazhi. 1999;7:504-509. |
| 4. | Fiorucci S, Lanfrancone L, Santucci L, Calabro A, Orsini B, Federici B, Morelli A. Epidermal growth factor modulates pepsinogen secretion in guinea pig gastric chief cells. Gastroenterology. 1996;111:945-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Konturek PC, Brzozowski T, Konturek SJ, Ernst H, Drozdowicz D, Pajdo R, Hahn EG. Expression of epidermal growth factor and transforming growth factor alpha during ulcer healing. Time sequence study. Scand J Gastroenterol. 1997;32:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Konturek PC, Ernst H, Brzozowski T, Ihlm A, Hahn EG, Konturek SJ. Expression of epidermal growth factor and transforming growth factor-alpha after exposure of rat gastric mucosa to stress. Scand J Gastroenterol. 1996;31:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Hu YT, Zhen CE, Xing GZ, Zhang ML, Zhang JS, Wang DX, Lu YM. Relationship between transforming growth factor alpha, epidermal growth factor and prostagladin E2 in patients with peptic ulcer. Shijie Huaren Xiaohua Zazhi. 2002;10:43-47. |
| 8. | Konturek JW, Hengst K, Konturek SJ, Domschke W. Epidermal growth factor in gastric ulcer healing by nocloprost, a stable prostaglandin E2 derivative. Scand J Gastroenterol. 1997;32:980-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Kang JY, Teng CH, Chen FC. Effect of capsaicin and cimetidine on the healing of acetic acid induced gastric ulceration in the rat. Gut. 1996;38:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Furukawa O, Okabe S. Cytoprotective effect of epidermal growth factor on acid- and pepsin-induced damage to rat gastric epithelial cells: roles of Na+/H+ exchangers. J Gastroenterol Hepatol. 1997;12:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Arakawa T, Watanabe T, Fukuda T, Higuchi K, Takaishi O, Yamasaki K, Kobayashi K, Tarnawski A. Indomethacin treatment during initial period of acetic acid-induced rat gastric ulcer healing promotes persistent polymorphonuclear cell-infiltration and increases future ulcer recurrence. Possible mediation of prostaglandins. Dig Dis Sci. 1996;41:2055-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Qiu BS, Pfeiffer CJ, Wu W, Cho CH. Tungstic acid reduction of cold-resistant stress-induced ulceration in rats. J Gastroenterol Hepatol. 1997;12:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Blandizzi C, Gherardi G, Marveggio C, Lazzeri G, Natale G, Carignani D, Colucci R, Del Tacca M. Suramin enhances ethanol-induced injury to gastric mucosa in rats. Dig Dis Sci. 1997;42:1233-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Miyazaki Y, Hiraoka S, Tsutsui S, Kitamura S, Shinomura Y, Matsuzawa Y. Epidermal growth factor receptor mediates stress-induced expression of its ligands in rat gastric epithelial cells. Gastroenterology. 2001;120:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Wang L, Wilson EJ, Osburn J, DelValle J. Epidermal growth factor inhibits carbachol-stimulated canine parietal cell function via protein kinase C. Gastroenterology. 1996;110:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Wagner S, Beil W, Westermann J, Logan RP, Bock CT, Trautwein C, Bleck JS, Manns MP. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997;113:1836-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 212] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Messa C, Russo F, Pricci M, Di Leo A. Epidermal growth factor and 17beta-estradiol effects on proliferation of a human gastric cancer cell line (AGS). Scand J Gastroenterol. 2000;35:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Granelli P, Fichera G, Zennaro F, Siardi C, De Ruberto F, Fregoni F, Appierto V, Buffa R, Ferrero S, Biunno I. Expression of the epidermal growth factor receptor gene in human intestinal metaplasia: a preliminary report. Scand J Gastroenterol. 1997;32:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Naef M, Yokoyama M, Friess H, Büchler MW, Korc M. Co-expression of heparin-binding EGF-like growth factor and related peptides in human gastric carcinoma. Int J Cancer. 1996;66:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Chen DL, Wang WZ, Wang JY. Epidermal growth factor prevents gut atrophy and maintains intestinal integrity in rats with acute pancreatitis. World J Gastroenterol. 2000;6:762-765. [PubMed] |
| 22. | Seno M, Tada H, Kosaka M, Sasada R, Igarashi K, Shing Y, Folkman J, Ueda M, Yamada H. Human betacellulin, a member of the EGF family dominantly expressed in pancreas and small intestine, is fully active in a monomeric form. Growth Factors. 1996;13:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Becker KF, Keller G, Hoefler H. The use of molecular biology in diagnosis and prognosis of gastric cancer. Surg Oncol. 2000;9:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Werner M, Becker KF, Keller G, Höfler H. Gastric adenocarcinoma: pathomorphology and molecular pathology. J Cancer Res Clin Oncol. 2001;127:207-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Masaki T, Hatanaka Y, Nishioka M, Tokuda M, Shiratori Y, Reginfo W, Omata M. Activation of epidermal growth factor receptor kinase in gastric carcinoma: a preliminary study. Am J Gastroenterol. 2000;95:2135-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Sanz-Ortega J, Steinberg SM, Moro E, Saez M, Lopez JA, Sierra E, Sanz-Esponera J, Merino MJ. Comparative study of tumor angiogenesis and immunohistochemistry for p53, c-ErbB2, c-myc and EGFr as prognostic factors in gastric cancer. Histol Histopathol. 2000;15:455-462. [PubMed] |
| 27. | Ma L, Liu ES, Chow JY, Wang JY, Cho CH. Interactions of EGF and ornithine decarboxylase activity in the regulation of gastric mucus synthesis in cigarette smoke exposed rats. Chin J Physiol. 1999;42:137-143. [PubMed] |
| 28. | Peláez Buján Mdel C, Ruibal Morell A, Aza González J. Gastric carcinoma: expression of c-erbB-2/neu oncoprotein, epidermal growth factor receptor, cathepsin D, progesterone receptor and tumor associated glycoprotein-72 in different histological types. Rev Esp Enferm Dig. 1999;91:826-837. [PubMed] |
| 29. | Choi JH, Kim HC, Lim HY, Nam DK, Kim HS, Yi SY, Shim KS, Han WS. Detection of transforming growth factor-alpha in the serum of gastric carcinoma patients. Oncology. 1999;57:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Hirao T, Sawada H, Koyama F, Watanabe A, Yamada Y, Sakaguchi T, Tatsumi M, Fujimoto H, Emoto K, Narikiyo M. Antisense epidermal growth factor receptor delivered by adenoviral vector blocks tumor growth in human gastric cancer. Cancer Gene Ther. 1999;6:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Hosokawa N, Yamamoto S, Uehara Y, Hori M, Tsuchiya KS. Effect of thiazinotrienomycin B, an ansamycin antibiotic, on the function of epidermal growth factor receptor in human stomach tumor cells. J Antibiot (Tokyo). 1999;52:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Luan F, Wang M, You W. [The correlation of TGF-alpha, EGFR in precancerous lesions and carcinoma of stomach with PCNA expression]. Zhonghua Binglixue Zazhi. 1997;26:31-34. [PubMed] |
| 33. | Koyama S, Maruyama T, Adachi S. Expression of epidermal growth factor receptor and CD44 splicing variants sharing exons 6 and 9 on gastric and esophageal carcinomas: a two-color flow-cytometric analysis. J Cancer Res Clin Oncol. 1999;125:47-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Wang Q, Wu JS, Gao DM, Lai DN, Ma QJ. Expression significance of epidermal growth factor receptor and transforming growth factor α mRNA in human colorectal carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:590-592. |
| 35. | Hu X, Gao J, Li Y. [The amounts of inositol 1,4,5-triphosphate and it response to epidermal growth factor and laminin of carcinoma substrains with high or low metastatic potentials]. Zhonghua Yixue Zazhi. 1997;77:665-667. [PubMed] |
| 36. | Tsugawa K, Yonemura Y, Hirono Y, Fushida S, Kaji M, Miwa K, Miyazaki I, Yamamoto H. Amplification of the c-met, c-erbB-2 and epidermal growth factor receptor gene in human gastric cancers: correlation to clinical features. Oncology. 1998;55:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Slesak B, Harlozinska A, Porebska I, Bojarowski T, Lapinska J, Rzeszutko M, Wojnar A. Expression of epidermal growth factor receptor family proteins (EGFR, c-erbB-2 and c-erbB-3) in gastric cancer and chronic gastritis. Anticancer Res. 1998;18:2727-2732. [PubMed] |
| 38. | Murakami N, Fukuchi S, Takeuchi K, Hori T, Shibamoto S, Ito F. Antagonistic regulation of cell migration by epidermal growth factor and glucocorticoid in human gastric carcinoma cells. J Cell Physiol. 1998;176:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Romano M, Ricci V, Di Popolo A, Sommi P, Del Vecchio Blanco C, Bruni CB, Ventura U, Cover TL, Blaser MJ, Coffey RJ. Helicobacter pylori upregulates expression of epidermal growth factor-related peptides, but inhibits their proliferative effect in MKN 28 gastric mucosal cells. J Clin Invest. 1998;101:1604-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Ishikawa T, Ichikawa Y, Tarnawski A, Fujiwara Y, Fukuda T, Arakawa T, Mitsuhashi M, Shimada H. Indomethacin interferes with EGF-induced activation of ornithine decarboxylase in gastric cancer cells. Digestion. 1998;59:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Gao JH, Liang HJ, Liu WW, Fang DC, Wang ZH. Expression of C-myc gene protein and epidermal growth factor receptor in gastric mucosa pre-and post-Helicobacter pylori clearance. Shijie Huaren Xiaohua Zazhi. 1999;7:1018-1019. |
| 42. | Zarrilli R, Ricci V, Romano M. Molecular response of gastric epithelial cells to Helicobacter pylori-induced cell damage. Cell Microbiol. 1999;1:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | He Y, Zhou J, Wu JS, Dou KF. Inhibitory effects of EGFR antisense oligodeox ynucleotide in human colorectal cancer cell line. World J Gastroenterol. 2000;6:747-749. [PubMed] |
| 44. | Uribe JM, Barrett KE. Nonmitogenic actions of growth factors: an integrated view of their role in intestinal physiology and pathophysiology. Gastroenterology. 1997;112:255-268. [PubMed] |
| 45. | Murayama Y, Miyagawa J, Higashiyama S, Kondo S, Yabu M, Isozaki K, Kayanoki Y, Kanayama S, Shinomura Y, Taniguchi N. Localization of heparin-binding epidermal growth factor-like growth factor in human gastric mucosa. Gastroenterology. 1995;109:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Filipe MI, Osborn M, Linehan J, Sanidas E, Brito MJ, Jankowski J. Expression of transforming growth factor alpha, epidermal growth factor receptor and epidermal growth factor in precursor lesions to gastric carcinoma. Br J Cancer. 1995;71:30-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Yasui W, Oue N, Kuniyasu H, Ito R, Tahara E, Yokozaki H. Molecular diagnosis of gastric cancer: present and future. Gastric Cancer. 2001;4:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Saikawa Y, Kubota T, Otani Y, Kitajima M, Modlin IM. Cyclin D1 antisense oligonucleotide inhibits cell growth stimulated by epidermal growth factor and induces apoptosis of gastric cancer cells. Jpn J Cancer Res. 2001;92:1102-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | García I, Vizoso F, Andicoechea A, Raigoso P, Vérez P, Alexandre E, García-Muñiz JL, Allende MT. Clinical significance of epidermal growth factor receptor content in gastric cancer. Int J Biol Markers. 2001;16:183-188. [PubMed] |
Edited by Zhang JZ