Published online Apr 15, 2002. doi: 10.3748/wjg.v8.i2.367
Revised: December 1, 2001
Accepted: December 8, 2001
Published online: April 15, 2002
AIM: Artificial β-cell lines may offer an abundant source of cells for the treatment of type I diabetes, but insulin secretion in β-cells is tightly regulated in physiological conditions. The Tet-On system is a “gene switch” system, which can induce gene expression by administration of tetracycline (Tet) derivatives such as doxcycline (Dox). Using this system, we established 293 cells to an artificial cell line secreting insulin in response to stimulation by Dox.
METHODS: The mutated proinsulin cDNA was obtained from plasmid pcDNA3.1/C-mINS by the polymerase chain reaction (PCR), and was inserted downstream from the promoter on the expression vector pTRE2, to construct a recombined expression vector pTRE2mINS. The promoter on pTRE2 consists of the tetracycline-response element and the CMV minimal promoter and is thus activated by the reverse tetracycline-controlled transactivator (rtTA) when Dox is administrated. pTRE2mINS and plasmid pTK-Hyg encoding hygromycin were co-transfected in the tet293 cells, which express rtTA stably. Following hygromycin screening, the survived cells expressing insulin were selected and enriched. Dox was used to control the expression of insulin in these cells. At the levels of mRNA and protein, the regulating effect of Dox in culture medium on the expression of proinsulin gene was estimated respectively with Northern blot, RT-PCR, and radioimmunoassay.
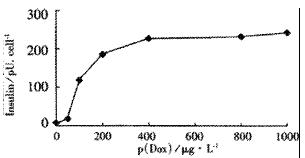
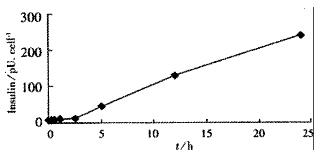
RESULTS: From the 28 hygromycin-resistant cell strains, we selected one cell strain (tet293/Ins6) secreting insulin not only automatically, but in response to stimulation by Dox. The amount on insulin secretion was dependent on the Dox dose (0, 10, 100, 200, 400, 800 and 1000 μg•L⁻¹), the level of insulin secreted by the cells treated with Dox (1000 μg·L-1) was 241.0 pU·d-1× cell-1, which was 25-fold that of 9.7 pU·d-1× cell-1 without Dox treatment. Northern blot analyses and RT-PCR further confirmed that the transcription of insulin gene had already been up-regulated after exposing tet293/Ins6 cells to Dox for 15 min, and was also induced in a dose-dependent manner. However, the concentration of insulin in the media did not increase significantly until 5 h following the addition of Dox.
CONCLUSION: Human proinsulin gene was transfected successfully and expressed efficiently in 293 cells, and the expression was modulated by tetracycline and its derivatives, improving the accuracy, safety, and reliability of gene therapy, suggesting that conditional establishment of artificial β-cells may be a useful approach to develop cellular therapy for diabetes mellitus.
- Citation: Qin XY, Shen KT, Zhang X, Cheng ZH, Xu XR, Han ZG. Establishment of an artificial β-cell line expressing insulin under the control of doxycycline. World J Gastroenterol 2002; 8(2): 367-370
- URL: https://www.wjgnet.com/1007-9327/full/v8/i2/367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i2.367
Owing to the steady improvement in methodology for islet and whole pancreas transplantation over the past three decades, this approach remains as the only means which is likely to substitute completely the function of the impaired β-cells in type Idiabetics patients[1].However, the limited availability of human donors, the immunological rejection, the low survival percentage of grafts, and the high expense limit the broad-scale applicability of islet transplantation[2-4]. As a result, in recent years much effort is being made to improve insulin secretion in the patient's non-beta cells to avoid the immune destruction[5-9]. To successfully mimic beta cell function, it is necessary to overcome three major obstacles: proinsulin synthesis, proinsulin processing, and mature insulin storage and regulated secretion. Our previous study (unpublished) and studies by other authors had demonstrated that correct proinsulin processing and insulin storage can be obtained in somatic cells transduced with the mutated proinsulin gene[10-13], so the major problem to ectopic insulin expression is the difficulty of reconstructing in non-beta cells the highly regulated insulin secretion of the normal beta cells[14-19].In 1992, Gossen and Bugard[20] developed the tetracycline (or doxycycline)-induced gene expression (Tet-on) system, which is useful for controlling the expression of targeted genes in quantitative manner and for determining the roles of the gene products in cellular functions[21-26]. In the present study, using this system, we generated the first non-beta cell line with deoxycycline-regulated insulin secretion. The strategy described here will contribute to the development of the use of insulin gene therapy for patients with type I diabetes.
pTet-on regulator plasmid (pTet-on), pTRE2 response plasmid (pTRE2) and pTK-Hyg plasmid, which contain hygromycin-resistance gene, were purchased from Clontech. Using the polymerase chain reaction, the proinsulin cDNA was amplified from expression vector pcDNA3.1/C-mINS, which had been reconstructed from plasmids pcDNA3.1/C and pN-PEPCK-INS (the genomic sequence of human!proinsulin, kindly provided by Professor LI Changchen, Dalian Medical University) in our lab. The primers used in the amplification created restriction sites BamH I and Hind III which allowed to clone the human proinsulin cDNA into the expression vector pTRE2. The sequences of the forward and reverse promers were: 5'-catggatcctgccatggccctgtggatg-3 and 5'-cagaagcttgcaggctgcgtctagttgc-3' respectively. After verification by restriction and sequencing, the vector, pTRE2.mINS, for expressing human insulin was generated.
To simplify the transfection procedure, the tet-293 cell line (human embryonal cell), which had been transfected with the plasmid pTet-on and stably expressed reverse tetracycline-controlled transactivator (rtTA), was purchased from Clontech. So the first step of transfection and clone selection was left out. The culture medium was DMEM supplemented with 100 mL•L⁻¹fetal bovine serum and G418 (100 mg•L⁻¹). The tet-293 cells were maintained at 37 °C under a 50 mL•L⁻¹ CO2 atmosphere until the cells were 80% confluent, then pTRE2.mINS was cotransfected with pTK-Hyg in a ratio of 20:1, using Lipofectin reagent (Gibco-Brl). After selection in the culture medium containing 30 mg•L⁻¹ hygromycin (Sigma) for more than eight weeks, one clone (tet-293/Ins6) expressing insulin under the control of Dox was selected by screening from 28 clones.
Tet293/Ins6 cells (1.0 × 106) were seeded in 35-mm-diameter dishes, and treated with Dox at 0, 10, 100, 200, 400, 800 and 1000 μg•L⁻¹ for 24 h, or at 1000 μg•L⁻¹ for 0, 15 and 30 min, 1, 2.5, 5, 12 and 24 h. The amounts of insulin secreted in the medium were measured with specific radioimmunoassay kit (Haikerei Co. Peking).
Total RNA was extracted from cells with TRIzol (Gibco), and the first chain of cDNA was prepared by RT with Superscript II reverse transcriptase (Gibco) using a oligo (dT)11 primer. The proinsulin gene was amplified with Taq polymerase (Progema). The PCR primers for proinsulin gene were: sense 5'-tgccatggccctgtggatg-3', and anti-sense 5'-gcaggctgcgtctagttgc-3'.
For each Northern blot, 10 μg total RNA was loaded per lane and separated, transferred onto nylon membrane (Schleicher and Schuell, Germany) and crossed-linked by UV irradiation. The hybridization probe specific for proinsulin, was labeled with (32P)dCTP using a DNA labeling kit (Takera). Following hybridization at 68 °C for 2 h, blots were washed twice for 30 min at room temperature with solution 1 (2 × SSC, 0.5 g•L⁻¹ SDS) and solution 2 (0.1 × SSC, 1 g•L⁻¹SDS), respectively, and exposed to a phosphorimaging screen overnight. The blots were then washed with 5 g•L⁻¹ SDS at 95 °C until complete removal of the probe and rehybridized with human β-actin cDNA (Clontech).
After screening in the culture medium containing hygromycin for more than eight weeks, a total of 28 independent Hyg-resistant cell lines were created from the tet-293 cells which had been cotransfected with pTR2.mINS and pTK-Hyg. Of the 28 clones, one (tet-293/Ins6) was obviously regulated to secrete insulin by Dox. The amount of insulin in the medium secreted by the cells treated with Dox (1 mg•L⁻¹) was 241.0 pU·d-1× cell-1, which was 25-fold that of 9.7 pU·d-1× cell-1 released by the cells without Dox treatment.
Tet-293/Ins6 cells were amplified and passaged (1.0 × 106 cells) into 16 35-mm dishes, and the concentrations of insulin in the culture media were measured with RIA. As shown in Figure 1, the level of insulin secretion increased from 18.2 to 241.0 pU·d-1× cell-1 over a range of Dox concentrations from 10 μg•L⁻¹ to 1000 μg•L⁻¹. Only a slightly increasing tendency of insulin concentration was observed at 2.5 h following the addition of 1 mg•L⁻¹ Dox to the culture medium. However, the amount of insulin increased significantly after exposing the cells to Dox for more than 5 h (Figure 2).
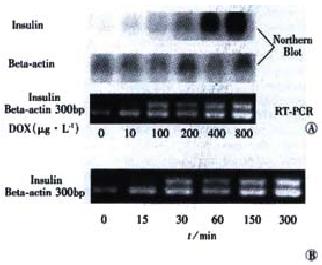
Theoretically, the Tet-on system exerts its regulatory effect on target genes at the transcriptional level. So RT-PCR and Northern blot methods were used to further demonstrate the inducibility of insulin by Dox. After tet-293/Ins6 cells were cultured in the media containing different concentrations of Dox for 24 h, it was found that 10 μg•L⁻¹ Dox could induce insulin expression obviously, and that the insulin mRNA level was up-regulated dramatically by Dox in a dose-dependent manner (Figure 3A). As shown in Figure 3B, the result of RT-PCR indicated that the transcription of insulin gene ha d already been up-regulated significantly after exposing tet-293/Ins6 cells to 1 mg•L⁻¹ of Dox for 15 min.
Insulin-dependent diabetes mellitus (IDDM) results from the autoimmune destruction of the insulin-producing beta cells of the pancreas. As a consequence, diabetic patients experience profound metabolic derangements (hyperglycemia, ketosis and hyperlipemia) and develop vascular and neurologic chronic complications[27-29]. These alterations can be only partially controlled by the exogenous administration of insulin, which brings patients suffering and inconvenience[30]. In recent years, engineering insulin-secreting cell lines has received a great deal of interest and some exciting advances have been made. But the application of gene therapy to diabetes presents formidable challenges, one of which is how the level of insulin is restricted within a very narrow limit. Otherwise, if the engineered artificial β-cells were transplanted into the body, the excessive insulin for metabolism would cause hypoglycaemia[8], and even death. Several inducible promoters originating from eukaryotic genes have been used to deliver gene in a regulated manner. The substances inducing the promoters include steroid hormones, oxygen, heavy metals, or physical stimulus (such as radiation)[31,32]. However, most of these promoters are not suitable for clinical application for various reasons: first, because these promoters are of mammalian origin, the exogenous regulation of transgene through such a promoter could at the same time affect the transcription of the host endogenous genes; and second, the inducers of these promoters are generally endogenous molecules (hormones, oxygen, etc.), the levels of which cannot be modulated significantly and safely. Other limitations include the potential toxicity or side effects of the inducer. Therefore, an inducer/promoter system, which regulates the transcription only of the transgene, without affecting endogenous genes, is acceptable for gene therapy in humans.
The Tet-on system, which utilizes an E. coli gene regulatory system[20], appears to fulfil the criteria for clinical application for several advantages[33-40]: (1)gene expression is easily regulated by administration of Dox; (2)Dox is minimally toxic; and (3)Dox acts specifically on the target gene, and does not activate other cellular genes. This expression system has two critical components, the regulatory plasmid and the response plasmid. The rtTA (reverse tetracycline-controlled transactivator) expressed by the regulated plasmid binds the TRE (tet-response element) in the response plasmid and activates the transcription of the target gene in the presence of Dox. Efrat et al[41] used the Tet-operon regulatory system to generate a β-cell line in transgenic mice, but there has been no report on the study of engineering non-β-cells for the treatment of diabetes in this regard. Efrat constructed a fusion protein, TETR-VP16 containing the tet repressor and the activating domain of the herpes simplex virus protein VP16, which converts the repressor into a transcriptional activator under the control of the insulin promoter. Then, the transgenic mice of the fusion protein were generated, whose β-cells could express the TETR-VP16 protein conditionally. In a separate lineage of transgenic mice, the simian virus 40 (SV40) large tumor (T) antigen (TAg) gene was introduced under the control of the tet operator sequences and a minimal promoter, which by itself is not sufficient for the expression of Tag gene. Mice from the two lineages were then crossed to generate double-transgenic mice. Expression of the TETR-VP16 protein in β-cells activated Tag transcription, resulting in the development of β-cells tumors. A stable β-cell line deriving from the tumor had the following characteristics: cell incubated in the absence of Tet proliferated normally; and cell incubation in the presence of Tet led to inhibition of proliferation. Thus, it is feasible that the expression of insulin gene can be regulated under the control of the proliferation of these β-cells. In the present study, utilizing the Tet-on system, we have established a cell line tet-293/Ins6 in which insulin gene can be expressed conditionally by Dox treatment. Ten μg•L⁻¹ Dox can activate the tet-response element and significantly enhance the expression of insulin gene in the cells in a dose-dependent manner. Additionally, the RT-PCR results showed that it is at 15 min that 1 mg•L⁻¹ Dox could activate the transcription of insulin gene in the tet-293/Ins6 cells following the addition of Dox, and that the amount of insulin in the media did not increase until 5 h after the treatment of Dox, suggesting that there is a periodic interval of time from the process of gene transcription, translation, and protein synthesis, processing to the final step, secreting the protein from the cells. The strategy used here, by which a stable cell line with low background and high Dox-induced expression of insulin was generated, is more efficient and rapid for regulating insulin gene expression as compared with Efrats' method[41], and will contribute not only to the development of artificial β-cells for the treatment of diabetes but to the generation of other condition-secreting cell lines for gene therapy of human diseases.
However, responding to the variation of blood glucose concentration, the expression level of insulin gene should be regulated strictly for gene therapy of diabetes[42-44]. The tet-293/Ins6 cells still have their imperfections for replacing islet transplantation in humans for the treatment of diabetes. Under normal conditions, increasing glucose concentration in blood stimulates β-cells to secrete insulin immediately; but the tet-293/Ins6 cells release insulin by control of Dox at the transcriptional level, the kinetics of feedback loops on transcriptional changes is much slower than that of the secretory response in β-cells. Therefore, based on the experiments we have carried out, we are making efforts to establish an artificial β-cell line in response to glucose stimulation with gene engineering.
| 1. | Newgard CB, Clark S, BeltrandelRio H, Hohmeier HE, Quaade C, Normington K. Engineered cell lines for insulin replacement in diabetes: current status and future prospects. Diabetologia. 1997;40 Suppl 2:S42-S47. [PubMed] |
| 2. | Yuan Z, Wu GY, He YS, Shao CM, Zhan Y. Islet separation and islet cell culture in vitro from human embryo pancreas. World J Gastroenterol. 1999;5:458-460. [PubMed] |
| 3. | Chen CQ, Lan P, Zhan WH. Studay on Fas-FasL system in langerhans islets transplantation. Shijie Huaren Xiaohua Zazhi. 1999;7:422-423. |
| 4. | Xu JT, Yan M, Yao YW, Wu R. Effect of pentagastrin on IL-1β inducede inhibition of insulin secretion in neonatal rat islets Langerhans. World J Gastroenterol. 2000;6:15. |
| 5. | Regazzi R, Verchere CB, Halban PA, Polonsky KS. Insulin production: from gene to granule. Diabetologia. 1997;40 Suppl 3:B33-B38. [PubMed] |
| 6. | Ferber S, Heimberg H, Brownlee M, Colton C. Surrogate beta cells. Diabetologia. 1997;40 Suppl 3:B39-B43. [PubMed] |
| 7. | Motoyoshi S, Shirotani T, Araki E, Sakai K, Kaneko K, Motoshima H, Yoshizato K, Shirakami A, Kishikawa H, Shichiri M. Cellular characterization of pituitary adenoma cell line (AtT20 cell) transfected with insulin, glucose transporter type 2 (GLUT2) and glucokinase genes: insulin secretion in response to physiological concentrations of glucose. Diabetologia. 1998;41:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Falqui L, Martinenghi S, Berra C, Monti L, Leone BE, Pozza G, Bordignon C. Human proinsulin production in primary rat hepatocytes after retroviral vector gene transfer. J Mol Med (. Berl). 1999;77:250-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Cheung AT, Dayanandan B, Lewis JT, Korbutt GS, Rajotte RV, Bryer-Ash M, Boylan MO, Wolfe MM, Kieffer TJ. Glucose-dependent insulin release from genetically engineered K cells. Science. 2000;290:1959-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Groskreutz DJ, Sliwkowski MX, Gorman CM. Genetically engineered proinsulin constitutively processed and secreted as mature, active insulin. J Biol Chem. 1994;269:6241-6245. [PubMed] |
| 11. | Gros L, Montoliu L, Riu E, Lebrigand L, Bosch F. Regulated production of mature insulin by non-beta-cells. Hum Gene Ther. 1997;8:2249-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Falqui L, Martinenghi S, Severini GM, Corbella P, Taglietti MV, Arcelloni C, Sarugeri E, Monti LD, Paroni R, Dozio N. Reversal of diabetes in mice by implantation of human fibroblasts genetically engineered to release mature human insulin. Hum Gene Ther. 1999;10:1753-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Yamasaki K, Sasaki T, Nemoto M, Eto Y, Tajima N. Differentiation-induced insulin secretion from nonendocrine cells with engineered human proinsulin cDNA. Biochem Biophys Res Commun. 1999;265:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Docherty K. Gene therapy for diabetes mellitus. Clin Sci (. Lond). 1997;92:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Efrat S. Prospects for gene therapy of insulin-dependent diabetes mellitus. Diabetologia. 1998;41:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Rivera VM, Wang X, Wardwell S, Courage NL, Volchuk A, Keenan T, Holt DA, Gilman M, Orci L, Cerasoli F. Regulation of protein secretion through controlled aggregation in the endoplasmic reticulum. Science. 2000;287:826-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 264] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Ye X, Rivera VM, Zoltick P, Cerasoli F, Schnell MA, Gao G, Hughes JV, Gilman M, Wilson JM. Regulated delivery of therapeutic proteins after in vivo somatic cell gene transfer. Science. 1999;283:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 231] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Lee HC, Kim SJ, Kim KS, Shin HC, Yoon JW. Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature. 2000;408:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 292] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547-5551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3634] [Cited by in RCA: 3829] [Article Influence: 112.6] [Reference Citation Analysis (1)] |
| 20. | Sonntag KC, Haller GW, Giauffret D, Germana S, Reeves SA, Levy J, Sachs DH, LeGuern C. Regulated expression of an MHC class II gene from a promoter-inducible retrovirus. Hum Gene Ther. 2000;11:1961-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 21. | Dressel R, Lübbers M, Walter L, Herr W, Günther E. Enhanced susceptibility to cytotoxic T lymphocytes without increase of MHC class I antigen expression after conditional overexpression of heat shock protein 70 in target cells. Eur J immunol. 1999;29:3925-3935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Hagihara Y, Saitoh Y, Arita N, Eguchi Y, Tsujimoto Y, Yoshimine T, Hayakawa T. Long-term functional assessment of encapsulated cells transfected with Tet-On system. Cell Transplant. 1999;8:431-434. [PubMed] |
| 23. | Krauthauser CM, Hall LA, Wexler RS, Slee AM, Mitra J, Enders GH, Kerr JS. Regulation of gene expression and cell growth in vivo by tetracycline using the hollow fiber assay. Anticancer Res. 2001;21:869-872. [PubMed] |
| 24. | Lottmann H, Vanselow J, Hessabi B, Walther R. The Tet-On system in transgenic mice: inhibition of the mouse pdx-1 gene activity by antisense RNA expression in pancreatic beta-cells. J Mol Med (. Berl). 2001;79:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Milo-Landesman D, Surana M, Berkovich I, Compagni A, Christofori G, Fleischer N, Efrat S. Correction of hyperglycemia in diabetic mice transplanted with reversibly immortalized pancreatic beta cells controlled by the tet-on regulatory system. Cell Transplant. 2001;10:645-650. [PubMed] |
| 26. | Lin L, Lu XZ, Zhao ZQ. Electrogastrography in patients with disordered gastric motility in diabetes and effect of cisapride. China Natl J New Gastroenterol. 1996;2:71-72. |
| 27. | Wang WM, Lu GX. Research on the treatment of diabetic gastroparesis by erythromycin. China Natl J New Gastroenterol. 1996;2:130. |
| 28. | Quigley EMM. The evaluation of gastrointestinal function in diabetic patients. World J Gastroenterol. 1999;5:277-282. |
| 29. | Gu YP, Gu JY, Li JS. Pancreaticoduodenal transplantation with portal venous and enteric drainage in rats. World J Gastroenterol. 2000;6:914-916. [PubMed] |
| 30. | Saitoh Y, Eguchi Y, Hagihara Y, Arita N, Watahiki M, Tsujimoto Y, Hayakawa T. Dose-dependent doxycycline-mediated adrenocorticotropic hormone secretion from encapsulated Tet-on proopiomelanocortin Neuro2A cells in the subarachnoid space. Hum Gene Ther. 1998;9:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Paillard F. "Tet-on": A gene switch for the exogenous regulation of transgene expression. Hum Gene Ther. 1998;9:983-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Bachl J, Carlson C, Gray-Schopfer V, Dessing M, Olsson C. Increased transcription levels induce higher mutation rates in a hypermutating cell line. J immunol. 2001;166:5051-5057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Molin M, Shoshan MC, Ohman-Forslund K, Linder S, Akusjärvi G. Two novel adenovirus vector systems permitting regulated protein expression in gene transfer experiments. J Virol. 1998;72:8358-8361. [PubMed] |
| 34. | Kashima Y, Miki T, Minami K, Seino S. Establishment of a tet-on gene expression system in glucose-responsive and -unresponsive MIN6 cells. Diabetes. 2001;50 Suppl 1:S133. [PubMed] |
| 35. | Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J Neurosci. 1998;18:9620-9628. [PubMed] |
| 36. | Zhu HJ, Iaria J, Sizeland AM. Smad7 differentially regulates transforming growth factor beta-mediated signaling pathways. J Biol Chem. 1999;274:32258-32264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Wang D, Grammer JR, Cobbs CS, Stewart JE, Liu Z, Rhoden R, Hecker TP, Ding Q, Gladson CL. p125 focal adhesion kinase promotes malignant astrocytoma cell proliferation in vivo. J Cell Sci. 2000;113 Pt 23:4221-4230. [PubMed] |
| 38. | Kobayashi T, Sawa H, Morikawa J, Zhang W, Shiku H. Bax induction activates apoptotic cascade via mitochondrial cytochrome c release and Bax overexpression enhances apoptosis induced by chemotherapeutic agents in DLD-1 colon cancer cells. Jpn J Cancer Res. 2000;91:1264-1268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Serguera C, Bohl D, Rolland E, Prevost P, Heard JM. Control of erythropoietin secretion by doxycycline or mifepristone in mice bearing polymer-encapsulated engineered cells. Hum Gene Ther. 1999;10:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Serguera C, Bohl D, Rolland E, Prevost P, Heard JM. Control of erythropoietin secretion by doxycycline or mifepristone in mice bearing polymer-encapsulated engineered cells. Hum Gene Ther. 1999;10:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Efrat S, Fusco-DeMane D, Lemberg H, al Emran O, Wang X. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc Natl Acad Sci USA. 1995;92:3576-3580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 179] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Mitanchez D, Doiron B, Chen R, Kahn A. Glucose-stimulated genes and prospects of gene therapy for type I diabetes. Endocr Rev. 1997;18:520-540. [PubMed] |
| 43. | Tiedge M, Elsner M, McClenaghan NH, Hedrich HJ, Grube D, Klempnauer J, Lenzen S. Engineering of a glucose-responsive surrogate cell for insulin replacement therapy of experimental insulin-dependent diabetes. Hum Gene Ther. 2000;11:403-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Barry SC, Ramesh N, Lejnieks D, Simonson WT, Kemper L, Lernmark A, Osborne WR. Glucose-regulated insulin expression in diabetic rats. Hum Gene Ther. 2001;12:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Edited by Ma JY