Published online Apr 15, 2002. doi: 10.3748/wjg.v8.i2.357
Revised: October 15, 2001
Accepted: November 1, 2001
Published online: April 15, 2002
AIM: To search for the biomarker of cellular immortalization, the telomere length, telomerase activity and its subunits in cultured epithelial cells of human fetal esophagus in the process of immortalization.
METHODS: The transgenic cell line of human fetal esophageal epithelium (SHEE) was established with E6E7 genes of human papillomavirus (HPV) type 18 in our laboratory. Morphological phenotype of cultured SHEE cells from the 6th to 30th passages, was examined by phase contrast microscopy, the telomere length was assayed by Southern blot method, and the activity of telomerase was analyzed by telomeric repeat amplification protocol (TRAP). Expressions of subunits of telomerase, hTR and hTERT, were assessed by RT-PCR. DNA content in cell cycle was detected by flow cytometry. The cell apoptosis was examined by electron microscopy (EM) and TUNEL label.
RESULTS: SHEE cells from the 6th to 10th passages showed cellular proliferation with a good differentiation. From the 12th to the 16th passages, many senescent and apoptotic cells appeared, and the telomere length sharply shortened from 23 kb to 17 kb without expression of hTERT and telomerase activity. At the 20th passage, SHEE cells overcame the senescence and apoptosis and restored their proliferative activity with expression of telomerase and hTERT at low levels, but the telomere length shortened continuously to the lowest of 3 kb. After the 30th passage cells proliferation was restored by increment of cells at S and G2M phase in the cell cycle and telomerase activity expressed at high levels and with maintenance of telomere length.
CONCLUSION: At the early stage of SHEE cells, telomeres are shortened without expression of telomerase and hTERT causing cellular senescence and cell death. From the 20th to the 30th passages, the activation of telomerase and maintenance of telomere length show a progressive process for immortalization of esophageal epithelial cells. The expression of telomerase may constitute a biomarker for detection of immortalization of cells.
- Citation: Shen ZY, Xu LY, Li EM, Cai WJ, Chen MH, Shen J, Zeng Y. Telomere and telomerase in the initial stage of immortalization of esophageal epithelial cell. World J Gastroenterol 2002; 8(2): 357-362
- URL: https://www.wjgnet.com/1007-9327/full/v8/i2/357.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i2.357
Telomerase activity was demonstrated in cancer of digestive tract[1-3], such as gastric[4-7], hepatic[8-10] colorectal[11-13] and esophageal cancers[14-19]. Inhibition of telomerase activity will be a new therapeutic for cancer[20-26]. Telomerase activity can be used as a diagnostic marker for cancer[27-30]. Normal mammalian cells grow in cultural medium with a limited number of passages before entering senescence and death[31], which are associated with shortening of telomere. Telomeres are specialized structures at chromosomal ends that are composed of TTAGGG DNA repeats[32]. Telomerase is a ribonuclear protein complex, which contains human telomerase reverse transcriptase (hTERT) as a catalytic domain, and human telomerase RNA component (hTR)[33]. Telemetries cap chromosomal ends perform the function of preventing abnormal chromosomal fusions and rearrangement[34]. However, each time a cell divides, the most distal part of the chromosome is incompletely duplicated and the telomere becomes shorter. Critically short telomeres enable the formation of aberrant chromosomal structures resulting in growth arrest or senescence[35]. With expression of telomerase or hTR and hTERT, the length of telomere extends to maintain the life span of the cells. There are other roles of telomerase in immortal and malignant lesion, such as proliferative potential[36], delaying senescence[37,38], promoting cell cycle, cell immortalization and carcinogenesis[39].
Recently, many papers have indicated that human papillomavirus (HPV) are the important etiological factor in esophageal carcinoma[40-42]. Induced by HPV 18 E6E7 genes, we established an immortalized cell line (SHEE) from the esophageal epithelium which underwent[43-46] malignant transformation[47,48]. Changes of telomere length and telomerase activity in the cell line are not clear at this early stage, nor is which criteria to use to detect immortalization of cells and what the relationship between telomerase and cell phenotype in SHEE cells is. The goal of this study is to explore when telomerase activity appears in the immortalized progressive process and study the relationship between telomerase and cellular phenotype.
The SHEE cell line was a kind of immortal embryonic esophageal epithelium induced by E6E7 genes of human papillomavirus (HPV) type 18 in our laboratory[43]. The continual growth cells from the 6th to 30th passages were routinely cultivated in flasks and the 24-well plates (Corning Co.) with culture medium 199 (Gibco), 100 mL•L⁻¹ bovine serum, 100 u penicillin and streptomycin in a humidified atmosphere of 50 mL•L⁻¹ CO2 and 950 mL•L⁻¹ air. The cell shape and size, anchorage-dependent growth and contact-inhibited growth were examined by phase contrast microscopy. For electron microscopic assessment, cells were spun to form a pellet and fixed with 25 g•L⁻¹ glutaraldehyde. They were dehydrated in graded ethanol and embedded in Araldite. Ultra-thin sections were cut with glass knifes and mounted on copper grids. They were contrasted for 15 min with uranyl acetate and for 3 min with lead citrate. The sections were examined by electron microscope (Hitachi, H-300).
Cells cultured in the flask were digested, washed twice with PBS, fixed by 70% alcohol, prepared as single-cell suspension and stored at 4 °C. Cells were stained with propidium iodide (Sigma) and analyzed with flow cytometry (FACSort, B-D Co.). The percentage of cells in various stages of the cell cycle, the apoptotic cell rate (AI) and proliferation index (PI = S + G2M/G0G1 + S + G2M) were calculated. These cells on the glass coverslips within the 24-well plate were incubated with 10 mg•L⁻¹ proteinase K for 15 min at room temperature. After the quenching of endogenous peroxidase, labeled nuclei with TUNEL (In-Situ Death Detection kit, Boehringer Mannhein Co.) were detected according to the instructions of the manufacturer. The brownish nucleus was considered positive apoptotic nucleus.
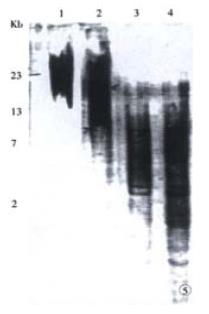
The genomic DNA of 106-108 cells was extracted. The telomeric restriction fragment (TRF) was measured by Southern blot. Briefly, 20 μg of genomic DNA was digested with Hinf I and run on 7 g•L⁻¹ agarose gel with marker DNA/Hind III. After electrophoresis the gel was blotted to nylon membrane (HybondTM N+, Amersham, Life Science) and hybridized to the Dig-labeled probes (CCCTAA) 3 at 50 °C in 5 × SSC, 1 g•L⁻¹ Sod. n-Lauroylsarcosine (SLS), 0.2 g•L⁻¹ SDS for 12-16 h and washed twice at room temperature in 2 × SSC, 1 g•L⁻¹ SDS for 5 min, once at 50 °C in 1 × SSC, 1 g•L⁻¹ SDS for 10 min, twice at 50 °C in 1 × SSC, 1 g•L⁻¹ SDS for 10 min, twice at 50 °C in 0.1 × SSC, 1 g•L⁻¹ SDS for 5 min, stained with NBT/BCIP and the median points were measured to obtain the mean telomere length[50].
Telomerase activity was measured using the telomeric repeat amplification protocol (TRAP). Frozen samples were homogenized in 10-50 μL of ice-cold lyses buffer (10 mmol•L⁻¹ Tris-HCl, pH7.5, 1 mmol•L⁻¹ EGTA, 0.1 mmol•L⁻¹ Benzamidine, 5 mmol•L⁻¹β-mercaptothanol, 5 g•L⁻¹ CHAPS, 100 mL•L⁻¹ glycerol). After 30 min of incubation on ice, the lysate was centrifuged at 12000 g for 20 min at 4 °C. TRAP-eze Telomerase Detection Kit (Oncor Inc.) reaction was performed using 1 μL lysate or 1/10 diluted lysate, 2.5 μL 10 × TRAP buffer (200 mmol•L⁻¹ Tris-HCl, pH8.3, 15 mmol•L⁻¹ MgCl2, 630 mmol•L⁻¹ KCl, 0.5% Tween 20, 10 mmol•L⁻¹ EGTA, 1 g•L⁻¹ BSA), 0.5 μL 2.5 mmol•L⁻¹ dNTP, 0.5 μL Ts primer, 0.5 μL TRAP primer mix, 19.5 μL water, 0.5 μL taq (2 × 106 U•L⁻¹). After incubation at 30 °C for 30 min, the reaction mix was immediately transferred to 94 °C and performed PCR (GeneAmp PCR System 2400, PE, USA) at 94 °C for 30 s, 55 °C for 30 s, for 30 cycles. PCR products were separated in a non-denaturing 120 g•L⁻¹ PAGE in 1 × TBE at 5V × cm-1. The gel was stained using AgNO3 and was photographed.
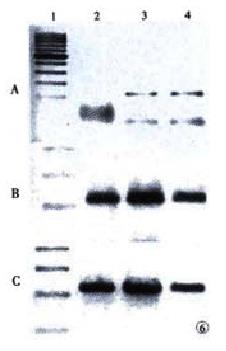
The activities of telomerase were performed by hTERT and hTR analysis. Analysis of expression of hTR and hTERT was determined by reverse transcription-PCR (RT-PCR) amplification in contrast with house-keeping gene GAPDH. Total RNA was isolated from the cell using GstractTM RNA Isolation Kit (Maxim Biotech, Inc.). cDNA was synthesized from 10 μg of total RNA using Ready-to-use First Strand cDNA Synthesis Kit (Maxim Biotech, Inc.). PCR reaction was performed using 2 μL aliquots of the reverse-transcribed cDNA, 2.5 μL 10 × PCR buffer, 1.5 μL 25 mmol•L⁻¹ MgCl2, 2.5 μL 1.0 mmol•L⁻¹dNTP, 2.0 μL specific primers, 14 μL water, 0.5 μL taq (2 × 106 U•L⁻¹). hTERT mRNA was amplified using the primer pair:5'-CGGAAGAGTGTCTG-GAGCAA-3' and 5'-GGATGAAGCGGAGTCTGGA-3' for 31 cycles (94 °C for 45 s, 55 °C for 45 s, 72 °C for 90 s). hTR was amplified using the primer pair: 5'-TCTAACCCTAACTGAGAAGGGCGTAG-3' and 5'-GTTTGCTCTAGAATGAACGGTGGAAG-3'. GAPDH was amplified using the primer pair:5'-GAAGGTGAAGGTCGGAGTC-3' and 5'-GAAGATGGTGATGGGATTTC-3' for 33 cycles (94 °C for 60 s, 55 °C for 60 s, 72 °C for 60 s). PCR products of each sample were subjected to electrophoresis in a 15 g•L⁻¹ agarose gel containing 0.5 mg•L⁻¹ ethidium bromide.
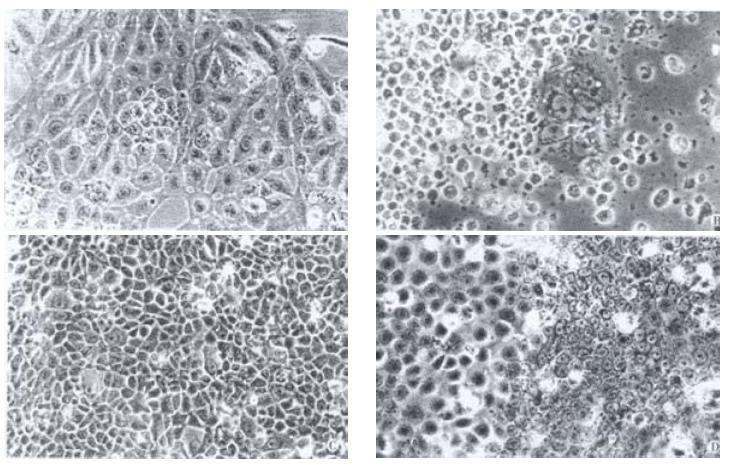
In the initiated passages, the cells in the 6th to the 10th passages were uniform in size and shape (Figure 1A), and grew as an even monolayer with characteristics of anchorage-dependent and attachment-inhibited growth. Cells continuously cultured in the 12th to the 16th passages exhibited morphologic changes in which cells were enlarged and flattened and exhibited differentiation and senescence. When many cells had shrunk, were round and floated freely, the majority underwent apoptosis and cell death with a few cells surviving (Figure 1B). Over coming senescence and apoptosis, the cells of the 20th passage restored their proliferation capacity (Figure 1C). After 30 passage the cells proliferated again and exhibited diphasic differentiation, a portion of cells displayed the undifferentiated basal epithelium and the other portion displayed differentiated squamous epithelium (Figure 1D).
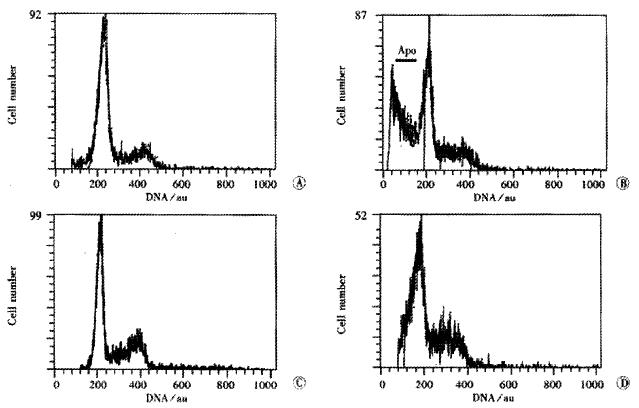
The PI of cell in 10, 16, 20, 30 the passage were 25.5%, 17.3%, 43.3%, and 43.0% respectively (Figure 2, A, B, C, D). Apoptotic cells index (AI) was in 10th passage, 7.3%; 16th, 57.5%; 20th, 5.7% and 30th, 7.5%. The cells in the 16th passage were at the stage of senescence and death, and the 10th, 20th and 30th were at their proliferated stages at various levels. Cells in G0G1 phase were identified as the differentiated cells containing some senescent cells.
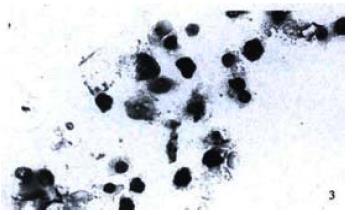
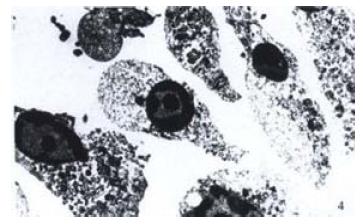
The TUNEL assay was also used to characterize the biological features of cells apoptosis. Many TUNEL-positive nuclei were observed in cells of passage 16th (Figure 3), and in a few cells in other passages. By EM examination, many apoptotic cells revealed rounded and shrunken nuclei with condensed chromatin stuck closely to the nuclear membrane (Figure 4).
Following continuous growth of the cells, the telomere length of the cells in the 6th to 10th passages exhibited shortening from a mean size of 23 kb in the normal esophageal mucosa to 17 kb at passage 10. At 20th passage telomere length was shortened continually to the shortest 3 kb, but maintained till the 30th passage (Figure 5).
Expression of hTR and hTERT in SHEE cells was determined by RT-PCR. The hTERT expression was positive in the 20th and 30th passages, but negative in the 10th passage (Figure 6A). Cells of the 10th, 20th and 30th passage showed positive expression of hTR (Figure 6B). House-keeping protein GAPDH was used as a control (Figure 6C). Comparing the variation of telomerase activity in SHEE from the 10th to the 30th passage, cells of the 10th passage were negative, while the 20th was weak and the 30th was apparent. The positive control of human esophageal squamous cell carcinoma expressed the highest telomerase activity (Figure 7).
At an early stage in the immortalization process, SHEE cells could be divided into three stages. At the primary stage, telomerase activity and expression of hTERT was absent and the telomere length shortened. The cells of SHEE were proliferated and differentiated and then their were senescent and apoptotic cells in the culture. At the early immortal stage, cells exhibited telomerase activity and its subunits hTERT at low levels with telomere length being continually shortened and proliferation restored. At the immortal stage, telomerase and hTERT were expressed in high level and telomere length maintained, accompanied by cell proliferation. From 6th to 30th passages the cells expressed hTR.
After transferring the HPV18E6E7 genes to the fetal esophageal epithelial cells, we established an immortal cell line designated SHEE. In order to focus on the process of immortalization, we monitored the dynamic changes of telomere length and telomerase activity of SHEE cells for extended periods of time (from the 6th passage to the 30th passage). At the primary stage the cells of passages 6-10 appeared as differentiated squamous epithelial cells with telomere length shortened without telomerase expression, after which the cells of passages 12-16 became senescent and underwent cell death. After overcoming the senescence and cell death, cells of the 20th passage expressed telomerase activity at low levels, where the length of shortened telomere was not maintained, but the proliferation of cells was restored. At the 30th passage, the cells exhibited a higher level of telomerase activity, hTR and hTERT, with maintained telomere length and continual proliferation of cells (immortalization). Therefore, one can suggest that telomerase activity and maintenance of telomere length might be necessary for immortalization of human esophageal epithelium in vitro.
To investigate the immortalization process, we used the SHEE cell model to define steps in aging of cells. After transduction of E6E7 genes from HPV type 18 normal cultured cells proliferate until they reach a discrete point (passage 12 times) in which the population growth ceases and develops to senescence. This period is termed the M1 stage of aging[51]. After M1, a large amount of cells dead to reach a “crisis” point with a few cells survival. This period is termed the M2 stage of aging[52]. Both M1 and M2 are therefore potential suppression pathways for tumorigenesis. The telomerase activity is sufficient to allow the cultured cells to escape from crisis[53]. In our experiment cells transfected with HPV, cell proliferated for an extended period of time, after that cells encountered senescence (M1) and apoptosis (M2). Overcome the M1 and M2, cells exhibited accelerant hyperplasia with telomerase activity.
Recent evidence suggests that viral oncogenes might directly up-regulate telomerase activity[54], such as HCV core protein, EBV-, HPV- and SV40 T antigen-expressing cell clones[55-58]. In our data the increase of hTR in the primary cells of SHEE might be directly or indirectly affected by the HPV viral oncogenes. Previous reports also have shown that telomerase activity can be achieved by the E6 and E7 protein of HPV[59]. HPV 16 E6 oncoprotein was capable of inducing telomerase activity in monolayer cultures of proliferating keratinocyte[60]. It was more likely that HPV E6/E7 transcription or other additional alterations, as chromosome instability[61,62] was prerequisite for induction of telomerase activity in proliferating cells. This hypothesis fits very well with our data on HPV-mediated immortalization of cells in vitro.
In summary, immortal cell line of the SHEE, up to 30th passage, may be divided into three stages. At the primary stage, telomerase activity and hTERT of cells were absent but hTR is positive with telomere length shortening and the cells became senescent and apoptotic. At the early-immortalized stage, the telomerase activity and hTERT expressed at low level and telomere length shortened continuously, but underwent cell hyperplasia occurred. At the immortalized stage, the telomerase activity and its two subunits expressed at a high level, telomere length was maintained and cell proliferation continued, in which the cells reached the stage of immortalization. The shortened telomere length and the activated telomerase activity display in a dynamic process. Our results prove that shortening of telomeres and absence of telomerase activity contribute to cellular senescence and cell death, and the activation of telomerase to maintain telomere length is necessary for immortalization. Therefore, the telomerase activity is the biomarker of immortalization of cell.
| 1. | Yakoob J, Hu GL, Fan XG, Zhang Z. Telomere, telomerase and digestive cancer. World J Gastroenterol. 1999;5:334-337. [PubMed] |
| 2. | He XX, Wang JL. Activity of telomerase and oncogenesis. Huaren Xiaohua Zazhi. 1998;6:1100-1101. |
| 3. | Yang SM, Fang DC, Luo YH, Lu R, Liu WW. Telomerase activity in gastroeintestind submucosal tumors and its clinical significance. Huaren Xiaohua Zazhi. 1998;6:765-767. |
| 4. | Zhan WH, Ma JP, Peng JS, Gao JS, Cai SR, Wang JP, Zheng ZQ, Wang L. Telomerase activity in gastric cancer and its clinical implications. World J Gastroenterol. 1999;5:316-319. [PubMed] |
| 5. | He XX, Wang JL, Wu JL, Yuan SY, Ai L. Telomerase expression, Hp infection and gastric mucosal carcinogenesis. Shijie Huaren Xiaohua Zazhi. 2000;8:505-508. |
| 6. | He XX, Wang JL, Wu JL, Yuan SY, Ai L. Telomere, cellular DNA content and gastric mucosal carcinogenesis. Shijie Huaren Xiaohua Zazhi. 2000;8:509-512. |
| 7. | Yao XX, Yin L, Zhang SY, Bai WY, Li YM, Sun ZC. hTERT expression and cellular immunity in gastric cancer and precancerosis. Shijie Huaren Xiaohua Zazhi. 2001;9:508-512. |
| 8. | Meng ZQ, Yu EX, Song MZ. Inhibition of telomerase activity of human liver cancer cell SMMC 7721 by chemotherapeutic drugs. Shijie Huaren Xiaohua Zazhi. 1999;7:252-254. |
| 9. | Fu JM, Yu XF, Shao YF. Telomerase and primary liver cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:461-463. |
| 10. | Qu B, Li BJ, Lu ZW, Pan HL. Clinical significance of telomerase activity detected in fine-needle aspiration speciments to liver cancer diagnosis. Shijie Huaren Xiaohua Zazhi. 2001;9:538-541. |
| 11. | Qiu SL, Huang JQ, Wang YF, Peng ZH. Analysis of telomerase activity in colorectal cancer, precancerous lesions and cancer washings. Shijie Huaren Xiaohua Zazhi. 1998;6:992-993. |
| 12. | Sobti RC, Kochar J, Singh K, Bhasin D, Capalash N. Telomerase activation and incidence of HPV in human gastrointestinal tumors in North Indian population. Mol Cell Biochem. 2001;217:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 13. | Jia L, Li YY. Telomerase activity of exfoliated cancer cells in colonic luminal washings. Huaren Xiaohua Zazhi. 1998;6:955-957. |
| 14. | Koyanagi K, Ozawa S, Ando N, Takeuchi H, Ueda M, Kitajima M. Clinical significance of telomerase activity in the non-cancerous epithelial region of oesophageal squamous cell carcinoma. Br J Surg. 1999;86:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Hiyama T, Yokozaki H, Kitadai Y, Haruma K, Yasui W, Kajiyama G, Tahara E. Overexpression of human telomerase RNA is an early event in oesophageal carcinogenesis. Virchows Arch. 1999;434:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Kiyozuka Y, Asai A, Yamamoto D, Senzaki H, Yoshioka S, Takahashi H, Hioki K, Tsubura A. Establishment of novel human esophageal cancer cell line in relation to telomere dynamics and telomerase activity. Dig Dis Sci. 2000;45:870-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Koyanagi K, Ozawa S, Ando N, Mukai M, Kitagawa Y, Ueda M, Kitajima M. Telomerase activity as an indicator of malignant potential in iodine-nonreactive lesions of the esophagus. Cancer. 2000;88:1524-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Xu LY, Shen ZY, Li EM, Cai WJ, Shen J, Li C, Chen JY, Zeng Y. Telomere length and telomerase activity in immortalized and malignantly transformed human embryonic esophageal epithelial cell lines by E6 amd E7 genes of HPV 18 type. Aibian Qibian Tubian. 2001;13:137-140. |
| 19. | Morales CP, Lee EL, Shay JW. In situ hybridization for the detection of telomerase RNA in the progression from Barrett's esophagus to esophageal adenocarcinoma. Cancer. 1998;83:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Xia ZS, Zhu ZH, He SG. Effects of ATRA and 5 Fu on growth and telomerase activity of xenografts of gastric cancer in nude mice. Shijie Huaren Xiaohua Zazhi. 2000;8:674-677. |
| 21. | Li ZS, Zhu ZG, Yin HR, Chen SS, Lin YZ. Diversity of telomerase activity in human and murine tumor cells transfected with cytokine genes. Shijie Huaren Xiaohua Zazhi. 1999;7:194-196. |
| 22. | Zhu ZH, Xia ZS, He SG. The effects of ATRA and 5 Fu on telomerase activity and cell growth of gastric cancer cells in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:669-673. |
| 23. | Sarvesvaran J, Going JJ, Milroy R, Kaye SB, Keith WN. Is small cell lung cancer the perfect target for anti-telomerase treatment. Carcinogenesis. 1999;20:1649-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Zhang FX, Zhang XY, Fan DM, Deng ZY, Yan Y, Wu HP, Fan JJ. Antisense telomerase RNA induced human gastric cancer cell apoptosis. World J Gastroenterol. 2000;6:430-432. [PubMed] |
| 25. | Bednarek A, Shilkaitis A, Green A, Lubet R, Kelloff G, Christov K, Aldaz CM. Suppression of cell proliferation and telomerase activity in 4- (hydroxyphenyl)retinamide-treated mammary tumors. Carcinogenesis. 1999;20:879-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Herbert BS, Wright AC, Passons CM, Wright WE, Ali IU, Kopelovich L, Shay JW. Effects of chemopreventive and antitelomerase agents on the spontaneous immortalization of breast epithelial cells. J Natl Cancer Inst. 2001;93:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Yeh TS, Cheng AJ, Chen TC, Jan YY, Hwang TL, Jeng LB, Chen MF, Wang TC. Telomerase activity is a useful marker to distinguish malignant pancreatic cystic tumors from benign neoplasms and pseudocysts. J Surg Res. 1999;87:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Shroyer KR, Thompson LC, Enomoto T, Eskens JL, Shroyer AL, McGregor JA. Telomerase expression in normal epithelium, reactive atypia, squamous dysplasia, and squamous cell carcinoma of the uterine cervix. Am J Clin Pathol. 1998;109:153-162. [PubMed] |
| 29. | Wisman GB, Hollema H, de Jong S, ter Schegget J, Tjong-A-Hung SP, Ruiters MH, Krans M, de Vries EG, van der Zee AG. Telomerase activity as a biomarker for (pre)neoplastic cervical disease in scrapings and frozen sections from patients with abnormal cervical smear. J Clin Oncol. 1998;16:2238-2245. [PubMed] |
| 30. | Rudolph P, Schubert C, Tamm S, Heidorn K, Hauschild A, Michalska I, Majewski S, Krupp G, Jablonska S, Parwaresch R. Telomerase activity in melanocytic lesions: A potential marker of tumor biology. Am J Pathol. 2000;156:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Jones CJ, Kipling D, Morris M, Hepburn P, Skinner J, Bounacer A, Wyllie FS, Ivan M, Bartek J, Wynford-Thomas D. Evidence for a telomere-independent "clock" limiting RAS oncogene-driven proliferation of human thyroid epithelial cells. Mol Cell Biol. 2000;20:5690-5699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1730] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 33. | Nakano K, Watney E, McDougall JK. Telomerase activity and expression of telomerase RNA component and telomerase catalytic subunit gene in cervical cancer. Am J Pathol. 1998;153:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Schwartz JL, Jordan R, Liber H, Murnane JP, Evans HH. TP53-dependent chromosome instability is associated with transient reductions in telomere length in immortal telomerase-positive cell lines. Genes Chromosomes Cancer. 2001;30:236-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 734] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 36. | Yang XY, Kimura M, Jeanclos E, Aviv A. Cellular proliferation and telomerase activity in CHRF-288-11 cells. Life Sci. 2000;66:1545-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Nickoloff BJ, Chaturvedi V, Bacon P, Qin JZ, Denning MF, Diaz MO. Id-1 delays senescence but does not immortalize keratinocytes. J Biol Chem. 2000;275:27501-27504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | MacKenzie KL, Franco S, May C, Sadelain M, Moore MA. Mass cultured human fibroblasts overexpressing hTERT encounter a growth crisis following an extended period of proliferation. Exp Cell Res. 2000;259:336-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Sugihara M, Ohshima K, Nakamura H, Suzumiya J, Nakayama Y, Kanda M, Haraoka S, Kikuchi M. Decreased expression of telomerase-associated RNAs in the proliferation of stem cells in comparison with continuous expression in malignant tumors. Int J Oncol. 1999;15:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Wang DX, Li W. Advances in esophageal neoplasms etiology. Shijie Huaren Xiaohua Zazhi. 2000;8:1029-1031. |
| 41. | Ma QF, Jiang H, Feng YQ, Wang XP, Zhou YA, Liu K, Jia ZL. Detection of human papillomavirus DNA in squamous cell carcinoma of the esophagus. Shijie Huaren Xiaohua Zazhi. 2000;8:1218-1224. |
| 42. | Zou SY, Liu XM, Tang XP, Wang P. Immunohistochemical and electron microscopic observation on positive HPV 16-E6 protein in esophageal cancer. Shijie Huaren Xiaohua Zazhi. 1998;6:47-48. |
| 43. | Shgn Z, Cen S, Zeng Y. [Immortalization of human fetal esophageal epithelial cells induced by E6 and E7 genes of human papilloma virus 18]. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 1999;13:121-123. [PubMed] |
| 44. | Shen Z, Shen J, Zeng Y. [Biological characteristics of human fetal esophageal epithelial cell line immortalized by the E6 and E7 gene of HPV type 18]. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 1999;13:209-212. [PubMed] |
| 45. | Shen ZY, Xu LY, Chen MH, Cai WJ, Shen J, Chen JY, Hon CQ, Zeng Y. Biphasic differentiation of immortalized esophageal epitheliums induced by HPW18E6E7. Bingdu Xuebao. 2001;17:210-214. |
| 46. | Shen ZY, Xu LY, Chen XH, Cai WJ, Shen J, Chen JY, Huang TH, Zeng Y. The genetic events of HPV-immortalized esophageal epithelium cells. Int J Mol Med. 2001;8:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Shen Z, Cen S, Shen J, Cai W, Xu J, Teng Z, Hu Z, Zeng Y. Study of immortalization and malignant transformation of human embryonic esophageal epithelial cells induced by HPV18 E6E7. J Cancer Res Clin Oncol. 2000;126:589-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Shen ZY, Shen J, Cai WJ, Cen S, Zeng Y. Biological characteristics of human fetal esophageal epithelial cell line immortalized by the E6 and E7 gene of HPV type 18. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 1999;13:209-212. |
| 49. | Xu LY, Li EM, Shen ZY, Cai WJ, Shen J. A nonradio-labelled method assays to measure the telomere length of human chromosome. Aibian Qibian Tubian. 2001;13:1-4. |
| 50. | Hou M, Xu D, Björkholm M, Gruber A. Real-time quantitative telomeric repeat amplification protocol assay for the detection of telomerase activity. Clin Chem. 2001;47:519-524. [PubMed] |
| 51. | Ouellette MM, Liao M, Herbert BS, Johnson M, Holt SE, Liss HS, Shay JW, Wright WE. Subsenescent telomere lengths in fibroblasts immortalized by limiting amounts of telomerase. J Biol Chem. 2000;275:10072-10076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 201] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 52. | Lustig AJ. Crisis intervention: the role of telomerase. Proc Natl Acad Sci USA. 1999;96:3339-3341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Halvorsen TL, Leibowitz G, Levine F. Telomerase activity is sufficient to allow transformed cells to escape from crisis. Mol Cell Biol. 1999;19:1864-1870. [PubMed] |
| 54. | Zhu J, Wang H, Bishop JM, Blackburn EH. Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening. Proc Natl Acad Sci USA. 1999;96:3723-3728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 271] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 55. | Nowak JA. Telomerase, cervical cancer, and human papillomavirus. Clin Lab Med. 2000;20:369-382. [PubMed] |
| 56. | Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology. 2000;271:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Guo W, Kang MK, Kim HJ, Park NH. Immortalization of human oral keratinocytes is associated with elevation of telomerase activity and shortening of telomere length. Oncol Rep. 1998;5:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 58. | Mutirangura A, Sriuranpong V, Termrunggraunglert W, Tresukosol D, Lertsaguansinchai P, Voravud N, Niruthisard S. Telomerase activity and human papillomavirus in malignant, premalignant and benign cervical lesions. Br J Cancer. 1998;78:933-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Nair P, Jayaprakash PG, Nair MK, Pillai MR. Telomerase, p53 and human papillomavirus infection in the uterine cervix. Acta Oncol. 2000;39:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Snijders PJ, van Duin M, Walboomers JM, Steenbergen RD, Risse EK, Helmerhorst TJ, Verheijen RH, Meijer CJ. Telomerase activity exclusively in cervical carcinomas and a subset of cervical intraepithelial neoplasia grade III lesions: strong association with elevated messenger RNA levels of its catalytic subunit and high-risk human papillomavirus DNA. Cancer Res. 1998;58:3812-3818. [PubMed] |
| 61. | Golubovskaya VM, Filatov LV, Behe CI, Presnell SC, Hooth MJ, Smith GJ, Kaufmann WK. Telomere shortening, telomerase expression, and chromosome instability in rat hepatic epithelial stem-like cells. Mol Carcinog. 1999;24:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Filatov L, Golubovskaya V, Hurt JC, Byrd LL, Phillips JM, Kaufmann WK. Chromosomal instability is correlated with telomere erosion and inactivation of G2 checkpoint function in human fibroblasts expressing human papillomavirus type 16 E6 oncoprotein. Oncogene. 1998;16:1825-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Edited by Wu XN