INTRODUCTION
The gastrointestinal (GI) tract is a hollow muscular tube serving as a digestive organ. The small intestine transports, digests and absorbs nutrients. It extends from the pylorus to the caecum and comprises the duodenum, jejunum and ileum which merges into the large intestine at the ileocaecal valve. Our knowledge of GI motility has increased considerably during the last decades. Most data dealt with motility patterns, flow rates, peristaltic reflexes, and tone in sphincter regions[1-5]. The GI tract, like other hollow organs such as the heart, blood vessels, urinary bladder and the urethra, is functionally subjected to dimensional changes. Hence, biomechanical properties are of particularly functional importance. Data in the literature pertaining to the passive mechanical properties of small intestine were concerned with the length-tension relationship in circular and longitudinal tissue strips[6-8], the compliance[9], and the stress-strain relationship of the intact wall[10,11]. However, a complete biomechanical analysis of GI physiology requires a full set of data describing the geometric properties and a valid reference state.
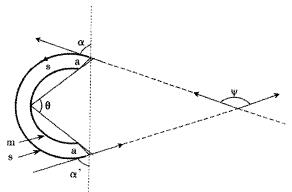
The zero-stress state of an organ is the state at which the organ is stress-free, meaning that all external and internal forces are removed. Thus, the morphometric properties are best described at the zero-stress state where no internal or external forces deform the tissue. Furthermore, knowing the zero-stress configuration is essential in any mechanical analysis since it serves as the reference state for computing stress and strain under physiological or pathophysiological conditions. Until 1983, it was believed that biological organs were free of stress when all external loads were removed (now known as the no-load state). Vaishnav and Vossoughi[12] and Fung[13] independently reported in 1983 that the no-load state of a blood vessel was not the zero-stress state. This was shown by an experiment where tissue rings that were cut radially opened up into sectors. The difference in stress and strain between the no-load and zero-stress state was called residual stress and strain, respectively, and it was found that the opening angle of the sector was a convenient way to quantitate the residual stress (Figure 1). Since then, a large number of studies have been published on the zero-stress state of different organ systems. The majority of these studies focused on the zero-stress state of systemic and pulmonary arteries, the heart and veins in normal animals and in various disease models such as experimental hypertension and diabetes and in venous grafts. Studies were also presented on the airway[14] and ureter[15].
Figure 1 Definition of the zero-stress state.
Traditionally, the zero-stress state is characterized by an opening angle defined as the angle subtended by two radii drawn from the mid-point of the inner wall to the inner tips of two ends of the sector in the zero-stress state. The opening angle is denoted by theta;. The labels m and s denote the mucosal and serosal surfaces and a is the line cutting. The angle between the tip tangents, which is described in the text, is denoted by Ψ. The angles between the tangents at the tip of the sector and the line joining the tips are designated as α and α', respectively. The mean value (α + α')/2 is referred to as the mean tangential angle.
The first data on residual strains and stresses in the gastrointestinal tract appeared in 1996[16]. Now we realize that the zero-stress configuration of the gastrointestinal tract is very different from that of the no-load condition and that the zero-stress state is sensitive to growth and remodeling by surgical resection (Dou et al, unpublished data). Gregersen et al[17] made an attempt to describe the geometry of the duodenum at the zero-stress state, and studied the strain distribution with reference to the zero-stress state in guinea pig duodenum using an elastomer casting method. It was found that the duodenum had very large opening angles and circumferential residual strains with axial variation. These studies opened up a whole new field of investigation in relation to gastrointestinal physiology and pathophysiology. The aims of the current study were to provide morphometric data at the no-load and zero-stress states of the entire small intestine in rats and the axial distribution of residual circumferential strains.
MATERIALS AND METHODS
Eleven three-month-old male SD rats weighing 293 ± 18 g were included in this study. The rats were anaesthetised with pentobarbital sodium (100 mg•kg⁻¹, ip). Following laparatomy, the calcium antagonist, papaverine (60 mg•kg⁻¹) was injected into the lower thoracic aorta through a cannula (22G/25 mm) in order to abolish contractile activity in the GI tract. After obtaining smooth muscle relaxation, the whole small intestine was harvested and 12 segments from various parts of the intestine were isolated. The most proximal segment was close to the pylorus whereas the most distal segment was close to the ileo-cacal valve. Within a short time, the residual contents in the lumen were gently cleared using saline. Then the segments were placed immediately into cold aerated Krebs solution containing 60 g•L⁻¹ dextran. Three rings, 1-2 mm in length, were cut from each segment for no-load and zero-stress state tests. The rings were photographed in the no-load state using a video camera (SONY CCD Camera, Japan) and then cut radially on the anti-mesenteric side to obtain their zero-stress state (Figure 1). A60-min-period was allowed for equilibration and the specimens were photographed again. The selection of this time period was based on pilot experiments.
Data Analysis
The morphometric data were obtained from the digitised images of the photographs of segments in the zero-stress and no-load states (Figure 2). Measurements were done using SigmaScan software (Jandel Scientific, Germany). The following data were measured from each specimen: the circumferential length (C), the wall thickness (h), the wall and lumen area, and the opening angle (α) at the zero-stress state. The subscripts i, o, n and z referred to the inner (mucosal) surface, outer (serosal) surface, no-load state and zero-stress state condition. The data from triplet of rings were averaged before further analysis.
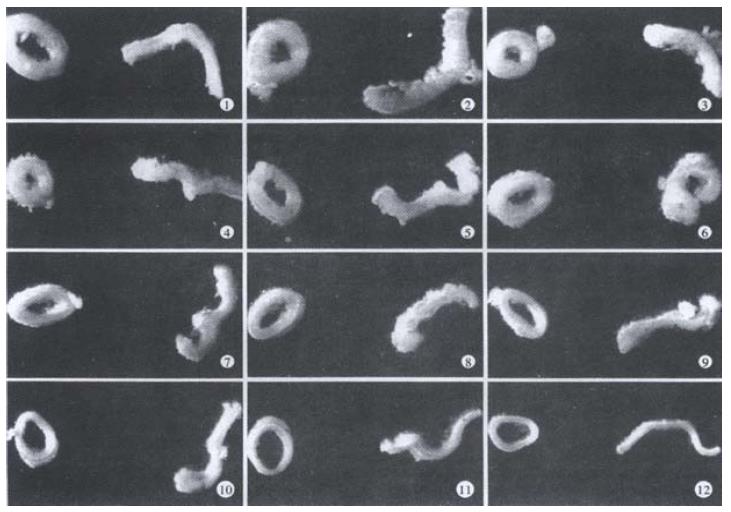
Figure 2 Photographs showing specimens obtained from different locations in the intestine in the no-load state (left, closed rings) and the zero-stress state (right, open sectors).
The rings of jejunum (site 5 to site 8) turned inside out when cut open.
Figure 2 shows a schematic of a tissue sector in the zero-stress state. The subtended angle is called the opening angle, theta;, and is defined as the subtended angle between the two radii from the midpoint of the arc of the inner wall of the specimen to the inner tips of the sector.
The opening angle was a convenient measure but might not always be useful in practice. First, it required that both the no-load and the zero-stress state to be circular. However, neither configuration might be exactly circular. The opening angle is a measure of the angle between the two radii and does not account for the shape of the curve. It provides no information on the curvature of the wall. The angles between the tangents at the tip of the sector and the line joining the tips are designated as α and α', respectively (Figure 1). The mean value (α + α')/2 was referred to as the mean tangential angle. If the sector is exactly circular, then theta; = (α + α')/2. The second difficulty with the opening angle is when its value exceeds 360° (i.e., the sector inverts inside out). Some organs such as the guinea-pig duodenum often have theta; greater than 360°[17]. The geometry of the duodenum at the zero-stress state can also be characterized by the angle between the two tangents of the tip, Ψ, as shown in Figure 1. Ψ refers to the angle between the tip tangents. The value of Ψ is relatively independent on where the cuts are made in the ring and does not require the no-load or zero-stress shape to be circular (see reference 18).
The measured data was used for computation of residual strains defined as:
Residual Green's strain at the mucosal surface: εi = (Ci-n/Ci-z)-1/2
Residual Green's strain at the serosal surface: εo = (Co-n/Co-z)-1/2
Thus, from the circumferential lengths at the no-load and zero-stress state, we can compute the circumferential residual strain, at the mucosal and serosal surfaces in the sense of green. Green strain is used when large deformations are encountered as in this study. Negative strain implies that the tissue is in compression whereas positive strain implies extension.
Statistical Analysis
The data were representative of a normal distribution and accordingly the results were expressed as ¯x ± s unless otherwise stated. Analysis of variance was used to detect possible variations in axial direction of the small intestine and between no-load and zero-stress state (Sigmastat 2.0TM). In case of significance, data were evaluated in pairs by a multiple comparison procedure Student-Newman-Keuls method. If the normality test or the equal variance test failed, Kruskal-Wallis one-way analysis of variance on ranks was used. The results were regarded as significant when P < 0.05.
RESULTS
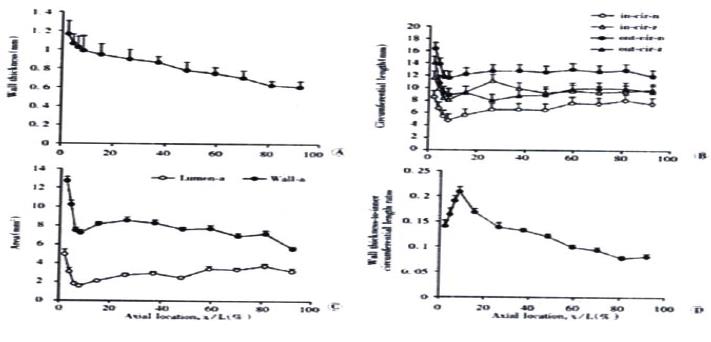
Representative images of rings in the no-load state and after cutting into the zero-stress state are illustrated in Figure 2. Figure 3 presented the morphometric data measured from the photographs of the segments in the no-load state and zero-stress state. In the no-load state (Figure 3A), the wall thickness was highest in the proximal duodenum (average value approximately 1.2 mm) and decreased in distal direction along the axis of the small intestine to a value close to 0.6 mm (f = 66.48, P < 0.001). The circumferential length and the inner and outer surfaces decreased rapidly along the length of duodenum by 30%-50% in both no-load state and zero-stress state (Figure 3B, inner, f = 22.46 and P < 0.001; outer, f = 37.68 and P < 0.001). The wall area and lumen area showed a similar pattern (Figure 3C, wall area, f = 43.43 and P < 0.001; Lumen area, f = 12.50 and P < 0.001). The wall thickness-to-inner circumferential length ratio increased along the length of duodenum after it decreased continuously towards the distal ileum (Figure 3D. f = 38.52 and P < 0.001). The highest average value was found close to the ligament of Treitz (0.21) and the lowest value of 0.08 was found close to the ileo-caecal valve.
Figure 3 Photographs showing morphometry of rat small intestine including wall thickness in the no-load state (A), inner and outer circuferential length in the no-load state and zero-stress state (B), the wall and lumen area in the no-load state (C) and wall thickness-to-inner circumferential length ratio (D).
Values are S. Variation of all morphometric parameters related to location along the axis of the small intestine was found (P < 0.001).
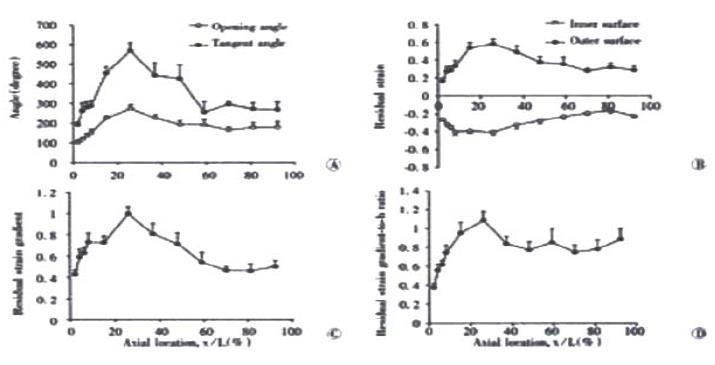
In the zero-stress state, the rings always opened up after making the cut. This resulted in larger inner circumferential length and smaller outer circumferential length when compared to the no-load state. Otherwise, the wall thickness and wall area did not differ between the no-load and zero-stress state (P > 0.5). The distributions of the opening angle and residual strains at the mucosal and serosal surfaces are shown in Figure 4. The opening angle and tangent rotation angle increased along the length of the duodenum and had its highest value 30% down the intestine. They decreased again further down the intestine (opening angle, f = 9.07 and P < 0.001; tangent rotation angle, f = 11.95 and P < 0.001). The opening angle attained values higher than 180 degrees in the jejunum and ileum, meaning that the rings turned themselves inside-out (Figure 4A). The serosal residual strain was tensile with the highest value close to the ligament of Treitz (approximately 0.6) and decreased to approximately 0.3 in the distal ileum (Figure 4B, f = 5.20 and P < 0.001). The mucosal residual strain was compressive in all segments of the small intestine with average values between -0.25 and -0.4. A significant variation was found along the small intestine with the lowest values close to the ligament of Treitz (f = 8.17 and P < 0.001). The curve for the residual strain gradient (i.e. the difference between mucosal and serosal residual strains) showed a pattern similar to that for the tangent rotation angle (Figure 4C, f = 7.11 and P < 0.001). Furthermore, the residual strain gradient normalised as the wall thickness increased rapidly along the length of the duodenum from average values of 0.4 to 1.1 and dropped to a constant level of approximately 0.8 in more distal parts of the small intestine (Figure 4D, f = 4.43 and P < 0.001).
Figure 4 Photographs showing (A) the distributions of the opening angle and tangent rotation angle, (B) residual strains at the mucosal and serosal surfaces, (C) the residual strain gradient and (D) residual strain gradient normalised with respect to the wall thickness (h).
Values are ¯x ± s. A significant variation was found along the small intestine for all variables (P < 0.001).
DISCUSSION
Mechanical properties are important for cardiovascular[18,19] and urogenital function[15,20]. The GI tract is similar to these organs in which it is functionally subjected to changes in wall stresses and strains. Therefore, during the last decade, acquiring biomechanical information in the digestive tract has increasingly called attention. Some studies have provided data on the small intestine[9-11,17]. However, many aspects are still left largely unexplored. With respect to biomechanics of the small intestine, we still lack a database on its geometry to fulfil a detailed biomechanical analysis of small intestinal physiology. Moreover, our knowledge about the small intestine at the zero-stress state is very limited. Gregersen H et al[17] provided the first data on the zero-stress state of the guinea pig duodenum. The current study aimed to describe the morphometry at the no-load and zero-stress states of the small intestine and to analyse the residual strain in the small intestine. The duodenal residual strains obtained in pigs[21] and rats in this study are less than that in the guinea-pig. In general, the opening angle in pigs and rats is not larger than 360 degrees.
Our results demonstrated large axial variations of all the morphometric parameters. The outer circumferential length and the luminal area increased whereas the wall thickness decreased in distal direction. A decrease in wall thickness is in line with findings in prior studies[22,23]. When radial cuts were made, the rings from the proximal small intestine were more likely to turn inside out resulting in a larger opening angle and larger residual strains than those in the jejunal and ileal rings. In accordance with observations in other hollow organs, the circumferential residual strain in the small intestine was compressive at the inner wall and tensile at the outer wall. From the mechanical point of view, a large residual strain may be nature's way to resist luminal pressures. The present study showed that the small intestinal mucosa at the no-load state was under compression and with axial variation from duodenum to ileum. This variation seems to correlate to morphometric variation along the small intestine[22]. In this sense we hypothesise that the compressed duodenal mucosa might be better protected against injury from frequent pressure changes produced by frequent contractions and the rush of luminal contents ejected at intervals from the stomach. By contrast, the less compressed ileal mucosa fulfils the protection in a region under relatively lower and stable pressure. From our studies, it is evident that a gradient in residual stress and strain exists and that the mucosal and submucosal layers are in compression. It still remains to be investigated whether mucosal growth is determined by the compression induced by residual stresses or whether the residual stress largely is a result of mucosal growth. It is of interest to notice that Rodriguez et al[24] predicted how volumetric tissue growth altered residual stress. Particularly, it was predicted that concentric hypertrophy (increase in wall thickness-to-radius ratio) increased residual stress and strain. The prediction is in accordance with the data in this study (comparison of Figure 3D with Figure 4).
Our previous data showed that the duodenal wall was stiffer than the jejunal and the ileal wall in circumferential direction. That is, the elastic properties differed among the small intestinal segments. The differences in stiffness may be associated with the specialised functions of the proximal and distal segments of the small intestine. The duodenum has a large influence on gastric emptying and has been proposed to act as a capacitative resistor[25], whereas the distal ileum acts as a reservoir[4]. The transit time is slower distally[1], which may be partly explained by the difference in the viscosity of the chyme. However, the passive elastic properties may also contribute to differences in intestinal flow patterns: A passing bolus will be slowed to a lesser degree in the duodenum where the wall stiffness is high whereas the compliant ileal walls bulges lead to pooling of luminal contents and decreased flow. Though we find the highest residual strains in the proximal small intestine, the association between the stiffness[9] and the residual strains found in this study does not seem that the mechanism is clear cut and further investigation is needed.
There are several important implications of the zero-stress state, some of which are, as yet, hypothetical. It is clear from the above discussion that using any state other than the zero-stress state will lead to error in the computed stresses and strains. Analysis of strain must refer to the zero-stress state in order to compare the specimens obtained from different locations of the GI tract in different species, and in remodeling induced by diseases, growth or degeneration. Furthermore, since the zero-stress state is not influenced by external or internal forces, it provides a standard state for describing tissue morphology.
All GI studies done thus far show that the rings open into sectors when cut radially. This implies that the mucosa is under compression in the no-load state (and at physiological conditions in the low pressure range) whereas the muscle layers are in tension. The residual tension in the muscle may provide a more optimum length for muscle contraction. It has been demonstrated that residual stress reduces the stress concentration at the inner wall of the GI tract at no-load and homeostatic states[17,21,26,27]. Thus, the compressed mucosa may be better protected against injury from the flow of luminal contents than uncompressed mucosa. These protection mechanisms could be important when unphysiologically high pressures are reached, such as in mechanical obstruction. Since peristaltic bolus transport bulges out the intestinal wall in the vicinity of the bolus[28,29], the residual strain would likely influence the resistance to bolus flow under normal conditions. Since residual strains exist, the mucosa will inevitably be under compressive stress and strain in the no-load condition, for instance, where the transmural pressure is zero. The GI muscles are usually passive since phasic contractions occur infrequently[30]. We may also ask if there are any other functions of the mucosa compression than those mentioned above It is well known that the mucosa is one of the tissues with the fastest turnover rate. The fast growth of the mucosal surface could easily cause mucosal compression and hence explain the presence of large residual strains. Likewise the large compressive stresses may affect mucosal growth. Studies on the intestine and other organs have shown that mechanical stresses are important factors in regulating gene expression and growth (Gregersen et al[31], unpublished data). Well-known examples are cardiac hypertrophy caused by hypertension and muscle atrophy in space flight. Theoretically, absorption of luminal contents may also be affected by the residual compression. It is well known that a gradient in the height of the mucosal villi exists along the small intestine, with the highest villi found in the proximal duodenum[23], and that small intestinal absorption depends on the luminal pressure[32]. Thus, there may exist a correlation between the residual strain gradient and the gradient in the height of villi. These interesting aspects of small intestinal function should be the focus of future studies.
Other important issues pertain to the function of the mechanoreceptors (endings of the sensory afferent nerves) in the gastrointestinal wall. Such receptors include noci-sensitive receptors and receptors in the peristaltic reflex pathway. It is well known that the receptors are located both in the submucosa and in the muscle layers. We have shown that a strain gradient exists from the mucosa to the serosa of the wall under physiological conditions. This suggests that the population of receptors from different locations in the wall may have different zero-settings and respond differently to the same stimuli. A difference in the responses of mucosal and muscle receptors to stimuli has in fact been observed[33]. The present results emphasize the importance of studying the receptor kinematics by using experimental models on the intact organ approximating the in vivo conditions. Data on receptor kinematics obtained from tissue strips or otherwise cut-open segments would likely give erroneous results.
Differences in morphometric properties and residual strains were found between rat duodenum, jejunum and ileum. Existence of a large residual circumferential strain indicates that the zero-stress state must be considered in future biomechanical studies. Residual stress may affect the tissue remodeling in response to disease and interventions by varying the stress distribution in the organ. The data on residual strains in the GI tract and the non-uniform remodeling may be used to improve surgery (for example, the procedures may be designed to prestress the tissue to make it more resistant to injury).