INTRODUCTION
Gene therapy is a novel approach that might lead to improved treatments of some types of cancer[1-9]. A suicide gene is a gene encoding a protein, which under appropriate conditions (i.e., exposure to prodrugs) is toxic to the genetically modified cell (i.e., tumor cell). Apart from the direct killing effects, further investigation led to the fundamental discovery, termed the “bystander effect”, which allowed a therapeutic application of suicide gene therapy. The bystander effect demonstrated that a tumor population could be killed if only a fraction of the tumor expressed the suicide gene, and thus was a seminal finding that overcame the limitations of gene therapy technology, i.e., the inability to genetically modify an entire tumor mass[10,11]. The gene for herpes simple virus-thymidine kinase (HSV-1 TK) has been the subject of many investigations in vitro and in vovo[12-19]. The Escherichia coli gene coding for the cytosine deaminase (CD) enzyme is another suicide gene. CD transforms nontoxic prodrug 5-fluorocytosine (5-FC) into an antitumor chemotherapeutic agent 5-fluorouracil (5-FU). This gene has already been tested in vitro as well as in several animal models; most of them being based on colorectal carcinoma cells[20-24].
If suicide gene wese delivered into cells, these cells, either target cells or normal cells, might be killed. The ability to direct suicide gene to transfer to particular cell will be important not only to achieve a therapeutic effect but also to limit potential adverse effects. But gene targeting continues to be a major obstacle to the overall success of gene therapy. For such strategies to have clinical applications the therapeutic gene should be expressed in the cells of interest, while avoiding expression in non-target cell population. In the case of cancer therapy, one such method would be uhe use of tumor cell-specific or tissue-specific promoters capable of directing expression of a heterologous gene in the cells[25-27]. Systemic delivery of a vector, which contains a tumor- or tissue-specific expression cassette, would then be feasible since expression of the transgene would be limited to these cells exclusively[28,29].
Previously we constructed a replication-deficient recombinant adenovirus vector containing CD gene controlled under cytomegalovirus (CMV) promoter, termed AdCMVCD, and confirmed that AdCMVCD/5-FC system presented direct killing and bystandes effect for many kinds of neoplasms in vitro and in vivo[30]. In the present study we constructed a new replication-deficient recombinant adenovirus vector, termed AdCEACD, containing CD gene under control of the carcino-embryonic antigen (CEA) promoter, and investigated its in vitro effects.
MATERIALS AND METHODS
Cells and ceml culture
Ad5-transformed human embryonic kidney (293) cell line, human colorectal carcinoma (Lovo) cell line and human cervix carcinoma (Hela) cell line were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco) supplemented with 100 mL•L⁻¹fetal bovine serum (FBS) (Gibco) in a 50 mL•L⁻¹ CO2 atmosphere at 37 °C.
Plasmid and virus
Expression plasmid pCEACD contained whole length CD gene sequence and CEA promoter sequence. Shuttle plasmid pAdE1CMV contained the E1A enhancer linked to the cytomegalovirus (CMV) promoter. The recombinant adenoviral vector AdCMVlacZ carried the E. coli lacZ gene for β-galactosidase under control of the CMV enhancer/promoter. We constructed a recombinant adenoviral vector, termed AdCMVCD, containing CD gene under CMV promoter.
Shuttle plasmid containing CEA promoter and CD gene
A fragment of CEA promoter (about 0.4 kb) and CD gene (about 1.3 kb) was obtained from pCEACD by digestion with Hind III and BamH I (Promega), then the terminal of BamH I was treated with Klenow fragment of E. coli DNA polymerase (Promega), finally the fragment was subcloned into pAdE1CMV with Xba I and Hind III (Promega). The resulting plasmid was named pAdCEACD.
Preparation of the “right arm” of adenovirus genome and recombination of adenovirus vector AdCEACD
Preparation of the “right arm” of adenovirus genome DNA and construction of recombinant adenovirus by homologous recombination AdCEACD were conducted according to methods we had reported[31].
Identification of AdCEACD
Adenovirus supernatant of each clone were degraded by boiling, ten minutes later the supernatant was laid on ice. CEA fragment, 0.3 kb, obtained from pCEACD by digestion with Hind III and EcoR I, was used to prepare [α-32P]-labeled probe according to product instruction (Amersham Pharmacia Biotech). The probe was used to dot blotting with DNA of all clones on nylon membrane (Amersham Life Science) in Rapid-hyb buffer (Amersham Pharmacia Biotech).
For positive clones with dot blotting, polymerase chain reaction (PCR) assay was applied to determine CD gene. The sense sequence of CD primer was 5'-TATGGATCCTCAACGTTTGTAATCGATGGCTT-3', while the antisense sequence was 5'-ATAGAATTAAGGCTAACAATGTCGAATAACGCTT-3'. Each 50 μL PCR system contained 3 μL DMSO, 1.5 μL dNTP (10 mmol•L⁻¹), upstream primer 20 pmol, downstream primer 20pmol, adenovirus supernatant 2 μL, 4 upfu DNA polymerase (Sangon, Shanghai), 5 μL 10 × pfu DNA polymerase buffer. PCR was performed for 30 cycles (94 °C 1 min, 60 °C 1.5 min, 72 °C 1.5 min) in an automated DNA Thermal Cycler (Perkin-Elmer Cetus).
Propagation, purification and titration of AdCEACD
AdCEACD was propagated in 293 monolayer cells. The purification of adenovirus was performed with ultra-concentration in CsCl step gradients for 2 h at 20 °C and 22000 r•min-1. The titration of AdCEACD was measured with plaque formation assay.
CEA secretions of Lovo and Hela cells
Lovo cells or Hela cells were subcultured in 60-mm cell culture dishes respectively. After determining the number of cells on a dish that was about over 90% confluent, we removed the medium, and added 3 mL fresh complete media and continue to incubate for 24 h, then collected the supernatant to test the concentration of CEA with radioimmunologic assay.
The sensitivity of cells infected by adenovirus to 5-FC
Lovo or Hela cells were seeded at a concentration of 2000 cells/well on 96-well, flat-bottom microplates in medium supplemented with 100 mL•L⁻¹ FBS. After 12 h, cells were infected by adenoviruses in different multiplicity of infection (M.O.I). After 12 h, cells were cultivated in fresh medium containing various concentrations of 5-FC (0, 0.15, 1.5, 15, 150, 1500 and 15000 μmol•L⁻¹; Sigma). After 48 h of incubation with the drug, cell viability was measured using MTT (Huamei Corporation) assay. The fifty percent inhibition concentration (IC50) of 5-FC was calculated using a curve-fitting parameter, and the results were represented as the means ± SD from three independent experiments.
The bystander effect of AdCEACD/5-FC
After tumor cells had been infected by adenovirus for 12 h in 1000 M.O.I, the bystander effect was measured by co-culturing different proportions of transfected cells and untransfected cells at a concentration of 2 × 105 cells/well on 6-well, flat-bottom plates in 5-FC-containing medium that was replaced every day. After 5 d of incubation with the drug, cell viability was measured by counting live cells with typan blue staining.
Statistical Analysis
Quantitative results were expressed as the mean ± SD of the mean. Statistical analysis was performed using ANOVA test. P < 0.05 was considered statistically significant.
RESULTS
Construction of shuttle plasmid pAdCEACD
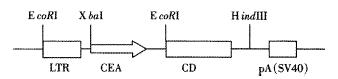
The structure of pAdCEACD is depicted in Figure 1. The map of pAdCEACD after treatment with restriction endonuclease is showed in Figure 2.
Figure 1 Schematic representation of the adenovirus shuttle plasmid, LTR, left inversed terminal repeats; pA, polyadenylation signal.
Figure 2 Map of pAdCEACD.
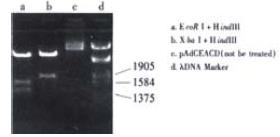
There was a fragment of 1.8 kb containing CEA and CD after being digested with Xba I and Hind III; As there were two corIrestriction sites, there were three fragments after treatment with EcoR I and Hind III, 0.9 kb for LTR and CEA, 1.3 kb for CD, 2.2 kb for LTR and CEA and CD.
Identification of AdCEACD
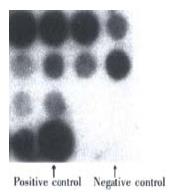
The result of dot blotting: There were 36 clones after recombination, 11 of which were hybridized. The result is showed in Figure 3.
Figure 3 The identification of the recombinant adenovirus AdCEACD by dot blotting.
During dot blotting the expression plasmid CEACD was used as positive control while virus AdCMVLacZ was used as negative control. The three well around negative controls were vacancy. The result showed that 6 of 11 clones were positive (over 50 percent). The left upper clone was to be further identified. The result of PCR Figure 4.
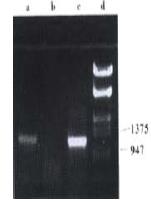
Figure 4 The identification of the recombinant adenovirus AdCEACD by PCR.
a, PCR product using AdCEACD as templet; b, PCR product using pAdE1CMV as templet; c, PCR product using pAdCMVCD as templet; d, λDNA Marker.
During identification by PCR, pAdCMVCD was used as positive control while pAdE1CMV as negative control. The result showed that the recombinant adenovirus (left upper clone) contained CD gene in its genome.
The titration of AdCEACD
After purification the titration of the recombinant adenovirus AdCEACD was 5.0 × 1014 pfu/L-1.
CEA secretions of Lovo or Hela
The concentration of CEA in the supernatant of Lovo cells was estimated to be 100 μg•L⁻¹, while lower 0.5 μg•L⁻¹ (beyond test) in the supernatant of Hela cells. So we consider that Lovo cell is CEA-producing and Hela cell CEA-nonproducing.
Sensitivity of the cells infected with AdCMVCD to 5-FC (Figure
5)
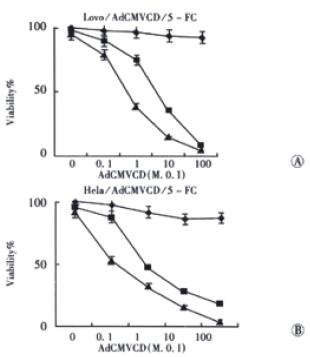
Figure 5 Sensitivity of cells infected with AdCMVCD to 5-FC.
◆: 5-FC 0; ■: 5-FC, 15 μmol•L⁻¹; ▲: 5-FC, 150 μmol•L⁻¹.
CMV promoter is ubiquitously strong, and effective in a variety of cells and tissues. As showed in Figure 5, after infected with AdCMVCD, the sensitivity of both Lovo cells (CEA-positive) and Hela cells (CEA-negative) to 5-FC had been enhanced, the IC50values of 5-FC for Lovo cells and Hela cells infected with AdCMVCD at 10 M.O.I were 128.8 ± 25.4 and 214.5 ± 31.3 μmol•L⁻¹, respectively, while the value of IC50 for parent cells of these two cell lines was more than 15000 μmol•L⁻¹ (P < 0.001, respectively). The cytotoxicity of 5-FC increased accordingly when the M.O.I of adenovirus was enhanced, the value of IC50 was reduced to 24.8 ± 7.1 and 23.9 ± 5.2 μmol•L⁻¹, respectively, when the adenovirus concentration was 100 M.O.I (P < 0.01, respectively).
Sensitivity of the cells infected with AdCEACD to 5-FC (Figure
6)
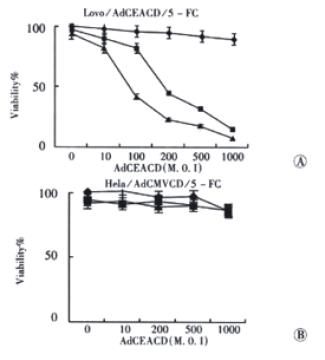
Figure 6 Sensitivity of cells infected with AdCEACD to 5-FC.
◆: 5-FC 0; ■: 5-FC, 15 μmol•L⁻¹; ▲:5-FC, 150 μmol•L⁻¹.
CEA promoter is cell- or tissue- specific, so it makes CD gene express only in CEA-producing cells. Figure 6 shows that, human colorectal carcinoma cell strain Lovo cells (CEA-positive) became sensitive to 5-FC significantly when they were infected with AdCEACD, the IC50 of 5-FC for Lovo cells infected with AdCEACD at 100 M.O.I was 216.5 ± 38.1 μmol•L⁻¹ (P < 0.001, compared with the IC50 of 5-FC in parent Lovo cells). The cytotoxicity of 5-FC increased accordingly when the M.O.I of adenovirus was enhanced, the value of IC50 of 5-FC was reduced to 27.9 ± 4.2 μmol•L⁻¹ in Lovo cells infected with 1000 M.O.I AdCEACD (P < 0.05). However, the sensitivity of Hela cells (CEA-negative) infected with AdCEACD to 5-FC was not enhanced (the IC50 of 5-FC in 100 M.O.I AdCEACD infected Hela cells and 1000 M.O.I AdCEACD infected Hela cells was more than 15000 μmol•L⁻¹ too, P > 0.05, compared with the IC50 of 5-FC in parent Hela cells, respectively), indicating that AdCEACD/5-FC system can result in specific killing of CEA-producing cells.
The bystander effect of AdCEACD/5-FC system (Figure
7)
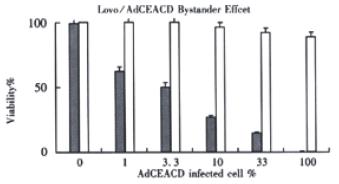
Figure 7 The bystander effect of AdCEACD/5-FC system.
□: without 5-FC; ■: 5-FC 150 μmol•L⁻¹
After tumor cells had been infected by AdCEACD for 12 h in 1000 M.O.I, the bystander effect was measured by co-culturing different proportions of transfected cells and untransfected cells at a concentration of 2 × 105 cells/well on 6-well, flat-bottom plates in 5-FC-containing medium that was replaced every day. After 5 d of incubation with the drug, cell viability decreased significantly. The viability was 24.7% ± 3.4% when the proportion of transfected cells was only 10 percent, while the value of according control was 98.7% ± 8.9% (P < 0.05), indicating that the AdCEACD/5-FC system had excellent bystander effect.
DISCUSSION
Gene therapy has made rapid progress during the past few years and appears to be approaching clinical application in medical practice[32-35]. Cancer is one of the most common causes of death in many countries and conventional treatments have not been able to cope with cancer adequately. Advances in our knowledge of molecular biology of cancer have brought a better understanding of the pathomechanism, as well as the development of molecular diagnostics and the basics of gene therapy. Cancer protocols (both marker and therapy) constitute about 70% of all current gene therapy protocols. Suicide gene therapy is one of these protocols and has a promising perspective. The purpose of this study is to apply CD gene, one of suicide genes, to treat colorectal carcinoma. One of the most important techniques of gene therapy is the choice of certain vector for gene transfer. Vectors are carriers or delivery vehicles for therapeutic genetic materials. There are two kinds of vectors available, non-viral vectors and viral vectors, the former contains liposomes, ligand-polylysine-DNA-complexes, dendrimers, cochleates, synthetic peptide complexes, electroporation and microinjection, etc., while the latter is composed of retroviral vectors, adenoviral vectors and adeno-associated virus vector.
Adenovirus vector system has many advantages. Adenovirus vectors can be prepared at much higher titres than retroviral vectors and have a high efficiency gene transfer regardless of the proliferative state of the tissues whereas retroviral vectors insert their genes only into dividing cells. Adenovirus genomes usually do not integrate into the host cell chromosome[36-42]. Although the duration of in vivo gene expression with adenovirus vector is short, the level of therapeutic gene expression is much higher. The purpose of suicide gene therapy is to kill transfected cells, so we choose adenoviral vector for suicide gene transfer. Suicide gene with efficient expression disappears rapidly after transfected tumor cells are killed, resulting in minimizing the underlying side effects of suicide gene. The adenovirus can infect a wide range of different cells, making it impossible to deliver genes to special target cells by intravenous administration. CD gene encodes cytosine deaminase which can transform 5-FC to cytotoxic 5-FU. 5-FU is often used for the treatment of colorectal carcinoma in combination with another chemotherapeutic agent. It causes cell death by inhibiting both RNA and DNA syntheses. If CD gene is delivered into normal cells, it maykill them when 5-FC is administrated.
The ability to target gene transfer to a particular class of cells is an important aspect of improving efficacy of gene therapy and limiting undesirable side effects. Ideal gene therapy vectors would be delivered intravenously to transfect only specific cells. Exiting vectors only transfect cells in vivo in a manner determined by blood flow and the site of introduction. Various strategies for targeted gene therapy have been designed, one of which is transcriptional targeting[43-46]. For certain procedures it may be necessary to deliver genes under the control of appropriate tissue- or cell-specific promoters, perhaps for the lifetime of the individual[47-50]. Transcriptional targeting of vectors is important for gene therapy of cancer because of the requirement to limit toxic therapies to the malignant target cells. It may be possible to identify and use transcriptional control elements that drive expression of proteins unique to, or overexpressed in malignant cells. These genes would reduce collateral expression of the transgene, and hence toxicity to healthy cells. For colorectal carcinoma, carcino-embryonic antigen (CEA) is one of such elements. CEA is a tumor-associated marker for human colorectal carcinoma and CEA is transcriptionally silent in adult normal tissues but over-expressed in approximate 60% to 80% human colorectal carcinomas[51-54]. Therefore we have used CEA as a transcriptional element to construct a new replication-deficient recombinant adenoviral vector containing CD gene controlled under CEA promoter in this study, and we have constructed the desired adenovirus vector, AdCEACD, successfully. We have applied dot bloting and PCR to identify CEA gene and CD gene in the vector respectively. In vitro experiments have showed the vector delivers CD gene only to CEA-producing cells, and only CEA-producing cells can be killed by 5-FC.
Currently available in vivo gene transfer vectors are not capable of transferring a gene to all tumor cells; however, successful application of suicide gene therapy in vivo relies on the bystander effect, where the active chemotherapeutic agent diffuses in the tumor cells and neighboring malignant cells in sufficient concentrations to suppress growth. In this study we also demonstrated that the AdCEACD/5-FC system presented bystander effect, as did AdCMVCD. The correction between CEA secretion and induction of CD activity and 5-FC/5-FU sensitivity is not straightforward. It is presumed that transcriptuional targeting is due to transcriptional activation. There are transcriptional activators in CEA-producing cells, which can activate CEA promoter, hence can drive the expression of CD gene, whereas CEA-nonproducing cells lack certain activators and CD gene can not express. The current results suggest that the recombinant adenovirus transfer of the CD gene under control of CEA promoter could theoretically be a useful vector for the CEA-producing tumors, such as colorectal carcinoma. It is not perfect that Hela cells had been used as CEA-nonproducing cell control, but we lack cell lines of normal intestinal mucosa or normal bone marrow. We will continue this research in vivo to further investigate the specificity of the recombinant adenoviral vector AdCEACD.