Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.44
Revised: October 10, 2001
Accepted: October 23, 2001
Published online: February 15, 2002
AIM: To investigate the effect of antisense RNA to vascular endothelial growth factor165 (VEGF165) on human esophageal squamous cell carcinoma cell line EC109 and the feasibility of gene therapy for esophageal carcinoma.
METHODS: By using subclone technique, the full length of VEGF165 amino acid cDNA, which was cut from pGEM-3Zf(+), was cloned inversely into the eukaryotic expression vector pCEP4.The recombinant plasmid pCEP-AVEGF165 was transfected into EC109 cell with lipofectamine. After a stable transfection, dot blot, enzyme-linked immunosorbent assay (ELISA), laser confocal imaging system analysis, transmission electron microscopy and flow cytometry were performed to determine the biological characteristics of EC109 cell line before and after transfection in vitro and whether there was a reversion in the tumorigenic properties of the EC109 cell in vivo.
RESULTS: The eukaryotic expression vector pCEP-AVEGF165 was successfully constructed and transfected into EC109 cells. The expression of VEGF165 was significantly decreased in the transfected cells while the biological characteristics of the cells were not influenced by the expression of antisense gene. The tumorigenic and angiogenic capabilities were greatly reduced in nude mice, as demonstrated by reduced tumor end volume (820 ± 112.5) mm3vs (7930 ± 1035) mm3 and (7850 ± 950) mm3,P£¼0.01£½ and microvessel density(8.5 ± 1.2) mm-2vs (44.3 ± 9.4) mm-2 and (46.4 ± 12.6) mm-2,P < 0.01) in comparison between experimental groups empty vector transfected group and control group.
CONCLUSION: The angiogenesis and tumorigenicity of human esophageal squamous cell carcinoma were effectively inhibited by VEGF165 antisense RNA. Antisense RNA to VEGF165 can potentially be used as an adjuvant therapy for solid tumors.
- Citation: Gu ZP, Wang YJ, Li JG, Zhou YA. VEGF165 antisense RNA suppresses oncogenic properties of human esophageal squamous cell carcinoma. World J Gastroenterol 2002; 8(1): 44-48
- URL: https://www.wjgnet.com/1007-9327/full/v8/i1/44.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i1.44
Angiogenesis, which is defined as the formation of new blood vessel from the pre-existing vascular bed, is essential for solid tumor growth, for the entrance of tumor cell into the circulation, and for the subsequent establishment and growth of metastasis. Many studies demonstrated that tumor angiogensis is associated with patient outcome and is an independent prognostic marker in almost all solid tumors, including esophageal carcinoma[1-10]. Tumor angiogenesis is a complex process, involving growth factors and extracellular matrix enzymes. Among the many known triggers of tumor angiogenesis, vascular endothelial growth factor (VEGF), also known as vascular permeability factor, is an endothelial cell-specific mitogen and an angiogenesis inducer released by a variety of tumor cells and expressed in human tumors in situ. VEGF165 is the most effective angiogenic factor in the VEGF family. Tumor cells engineered to express VEGF constitutively exhibit enhanced tumor growth and angiogenic phenotypes[11-13]. Conversely, inhibition of the expression of VEGF165 was considered as a therapeutic strategy for the treatment of solid tumors[14-24].
In this report, we constructed antisense RNA to VEGF165 eukaryotic expression vector and applied gene transfer technology to modulate the expression in stably transfected human esophageal squamous cell carcinoma cells. We assessed the effects of down-regulation of VEGF expression on the biological characteristics in vitro, microvessel density and turmorigenic capability in nude mice.
The EC109 human esophageal squamous cell carcinoma cell line was generously provided by Dr. Sun (Department of Thoracic Surgery, Tangdu Hospital, Fourth Military Medical University). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), high glucose media (Life Technoligies) and supplemented with 100 mL·L-1 fetal calf serum (HyClone Laboratories), penicillin, streptomycin, and nonessential amino acids (Life Technoligies). The vector pGEM-3Zf (+) (carrying the full length aminoacids cDNA of VEGF165) was kindly provided by Dr. Abraham (Columbia University, USA) and vector pCEP4 was a gift from Dr. Li (Department of Infectious Disease, Tangdu Hospital, Fourth Military Medical University,China).
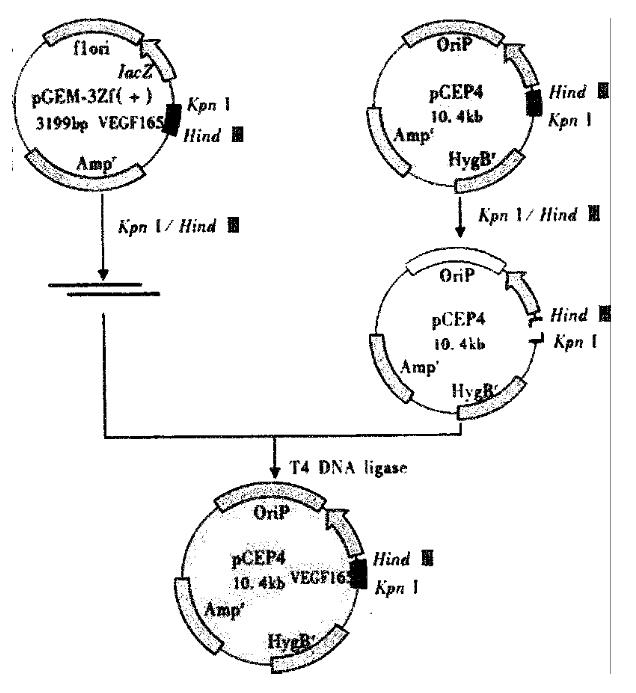
The expression vector for VEGF165 antisense RNA was constructed by subcloning cDNA fragment that code for VEGF165 into the eukaryotic expression vector pCEP4. pGEM-3Zf (+) was digested by Kpn I and Hind III. The fragment was purified by gel. The VEGF165 amino acids cDNA was cloned inversely in the Hind III/Kpn I site of pCEP4 to generate plasmid pCEP-AVEGF165 (Figure 1).
The transfection and selection of the EC109 cells were carried out in a 6-well plate. When the cells reached 70% confluence, the transfection process began. Briefly, solution A was prepared by diluting 10 μg of pCEP-AVEGF165 into 200 μL serum-free medium, and solution B was prepared by diluting 20 μL Lipofectimine 2000 (Life Technoligies) into 200 μL serum-free medium. The two solutions were combined for 20 min at room temperature, and then 0.6 mL serum-free medium was added to the tube containing the complex, and subsequently added to the rinsed cells. The medium was replaced with fresh and complete medium 18 h after the start of transfection. Seventy-two hours after transfection, it was replaced again with the selective medium containing 200 g·L-1 hygromycin B (Boehrringer Mannheim). Once stable transfections were obtained, the cells were maintained in 100 g·L-1 of hygromycin B. The EC109 cells were transfected with either the empty pCEP4 vector or pCEP-AVEGF165.
Total cellular RNA was extracted from the cultured cells using the Trizol isolation kit (Life Technoligies) according to the manufacturer’s instruction. The recovered total RNA was redissolved in diethyl pyrocarbonate-treated water and 20 μg was immobilized onto a gene screen plus membrane (DuPont) by gentle suction with a blotting manifold (Bethesad Research Laboratories). The membrane was then probed with a 5’-end-radiolabeled synthetic oligodeoxyribonucleotide complementary.
Approximately 5 × 10 6 centrifugal sedimentation cells were immediately fixed in 700 mL·L-1 ethanol and stored at 4 °C in PBS in preparation for fluorescent-activated cell sorting. Flow cytometry analysis was performed on a FACStar flow cytometer (Becton Dickinson). Histograms of cell number logarithmic fluorescence intensity were recorded for 10000 cells per sample.
The centrifugalized cells were placed in 40 g·L-1 glutaraldehyde and then post-fixed in osmium tetroxide and embedded in Epon. Routine thin sections were stained with uranyl acetate and lead citrate. Thin sections were mounted on grids and examined under a transmission electron microscope (JEM-2000EX) at 60 kV.
Indirect immunofluorescence techniques were applied in the transfected EC109 cells and the parental cells. VEGF165 protein was detected with mouse anti-human VEGF165 antibody and sheep anti-mouse IgG-FITC (Dako A/S Denmark). FITC was activated by light with a wavelength of 488nm. The data of laser scanning were 3%. The expression of VEGF165 was analyzed by confocal microscope system controlled by software obtained by Bio-Rad.
Athymic Balb/c nude mice were obtained from the Animal Center of Fourth Military Medical University. The mice were maintained in a laminar airflow cabinet under specific pathogen-free conditions and used at 8-12 weeks of age. Cells used for injection were grown to subconfluence, trypsinized, washed once, and resuspended in serum-free DMEM. The cell suspensions were examined microscopically to ensure that they were composed of single-cell suspensions. Mice were injected s.c. on the hind leg with 5 × 106 single cells in 0.1 mL. The mice were then separated into three groups, depending on whether they were injected with pCEP-AVEGF165 transfected cells, pCEP4 empty vector transfected cells, or control cells. Each group contained five mice. Calipers was used for the calculation of tumor size. Microvessel density was determined under light microscopy after immunostaining of sections with anti-CD34 monoclonal antibody according to the strepto ABC kit (Dako A/S Denmark) instruction.
The data were analyzed for significance by ANOVA.
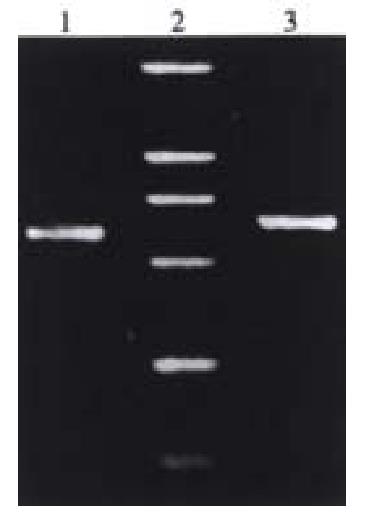
After ligation, transformation and selection, three clones were found likely to contain the desired recombinant. These clones were digested by restriction enzymes Kpn I/Hind III or Kpn I/Sfi I.The 640 bp or the 660 bp fragment was found by using polyacrylamide gel electrophoresis. These recombinant plasmids were the eukaryotic expression vectors of antisense RNA to VEGF165 (Figure 2).
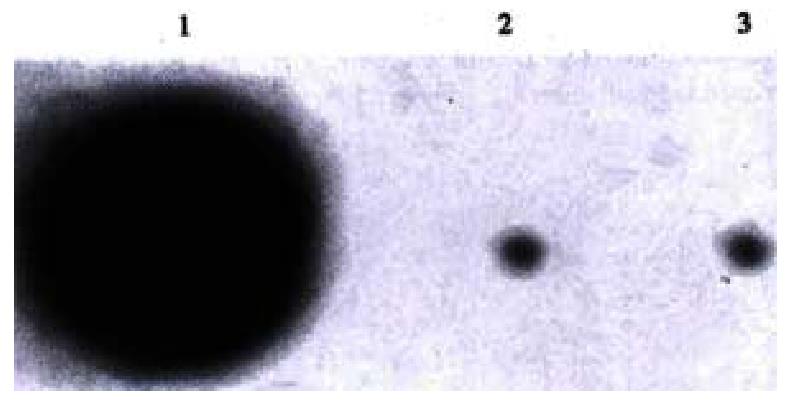
Two weeks after being transfected and selected by hygromycin B, the EC109 cells transfected by pCEP-AVEGF165 expressed antisense RNA to VEGF165 which was confirmed by dot blot analysis, whereas the cells transfected by pCEP4 empty vector and control group cells were negative (Figure 3).
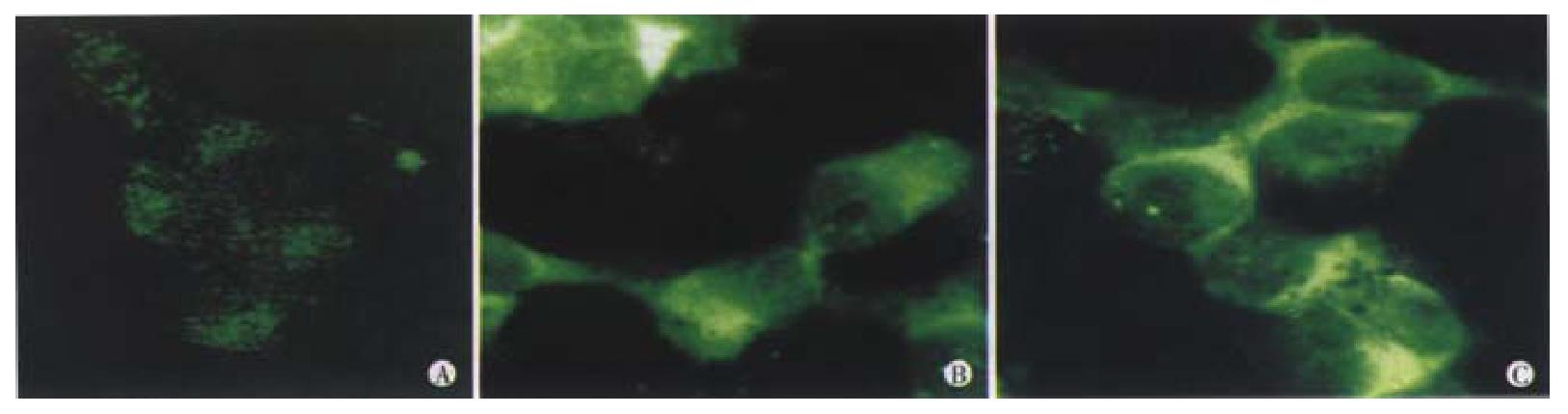
ELISA showed that a great number of VEGF165 accumulated in the pCEP4 empty vector transfected group and control group cells, whereas in the pCEP-AVEGF165 transfected group cells, the level of VEGF165 was very low. The level of VEGF165 expression was significantly lower in EC109 cells transfected by pCEP-AVEGF165 than that in the pCEP4 empty vector transfected group and control group cells (P < 0.01) determined under confocal microscope, as indicated in Figure 4.
There was no substantial change neither in the ultrastructure examined under transmission electron microscope nor in the cell cycle determined by flow cytometer.
The nude mice were sacrificed at week 5. Tumor volume was measured and morphological characteristics were assessed in HE stained sections. pCEP-AVEGF165 transfected xenografts grew very slowly, pCEP4 empty vector transfected group and nontransfected control xenografts were significantly larger than pCEP-AVEGF165transfected xenografts (P < 0.01), and the mean tumor volumes were (820 ± 112.5)mm3,(7930 ± 1035)mm3 and (7850 ± 950)mm3, respectively. pCEP-AVEGF165 transfected xenografts had a relatively large area of central necrosis. Immunohistochemical staining for CD34 was performed to evaluate tumor microvessel density. The microvessel density was expressed as the average number of the five highest areas identified within a single × 200 field, for the pCEP-AVEGF165 transfected mice, pCEP4 empty vector group and nontransfected controls were (8.5 ± 1.2)mm-2, (44.3 ± 9.4)mm-2 and (46.4 ± 12.6)mm-2, respectively (P < 0.01).
Mammalian cells require oxygen and nutrients for their survival and are therefore located within 100 μm-200 μm blood vessels-the diffusion limit for oxygen. For multicellular organisms which grow beyond this size, they must recruit new blood vessels by angiogenesis and vasculogenesis. This process is regulated by a balance between pro- and anti-angiogenic molecules, and is derailed in various diseases, especially cancer. Without blood vessels, tumor can not grow beyond a critical size or metastasize to another organ[25-29]. In 1971, Folkman[30] proposed that solid tumor growth and metastasis are critically dependent on angiogenesis, the formation of new blood vessels from pre-existing vasculature, and hence, blocking angiogenesis could be a strategy to arrest tumor growth. The induction of angiogenesis is mediated by several factors released by both tumor and host cells. One of the key mediators of angiogenesis is VEGF, a multifunctional growth factor that is overexpressed and secreted by a majority of human and animal tumors. VEGF was purified by Ferrara et al[31] from the conditioned medium of bovine pituitary folliculo stellate cells. VEGF is a homodimeric 46 ku heparin-binding glycoprotein with potent angiogenic, mitogenic, and vascular permeability-enhancing activities specific for endothelial cells. By alternative splicing of messenger RNA, VEGF may exist in at least four different homodimeric molecular species each monomer having 121, 165, 189 or 206 amino acids, respectively (VEGF121, VEGF165, VEGF189, VEGF206). Among this family, VEGF165 is the most important effector. Antiangiogenic therapy targeting VEGF has been proposed as a means of inhibiting VEGF-dependent tumor growth and metastasis[32-40].
It has been suggested that antisense RNA could block the translation progress of aim protein effectively and inhibit expression[41-45]. DeFatta et al[46] found that reducing eIF4E express on via antisense RNA suppressed both the tumorigenic and angiogenic properties of the head and neck squamous cell cancers, cell line FaDu, as demonstrated by lowered capacity to grow in soft agar, reduced expression of angiogenic factors, and loss of tumorigenicity in nude mice. Oku and associates[47] transfected human SK-MEL-2 melanoma cells with antisense VEGF which resulted in substantial inhibition of intracerebral tumor growth in nude mice, and a decrease in tumor vascularity, blood flow, and permeability.
The prognosis of human esophageal squamous cell carcinoma after curative resection is dismal. Radiotherapy and several conventional chemotherapeutic agents have been tried to improve the prognosis, but the results are generally disappointing. In this regard, antiangiogenic therapy could be a promising and hopeful strategy for esophageal cancer[48-51]. In this study, an antisense RNA to VEGF165 eukaryotic expression vector pCEP-AVEGF165 was constructed successfully. We transfected it into human esophageal squamous cell carcinoma cell line EC109. Under immunohistochemistry and confocal microscopy, it was found that the expression of VEGF165 decreased significantly in the cells transfected with VEGF165 antisense RNA compared with the empty vector transfected and control group. Under transmission electron microscopy and flow cytometry, we observed that the ultrastructure and cell cycle had no change among transfected and control groups. In the nude mice tumor model, the tumorigenicity, the rate of tumor growth, and microvessel density were significantly decreased for the tumors derived from antisense RNA transfected cells as compared with the empty vector transfected and parental cells. pCEP-AVEGF165 transfected tumors had a very low initial growth rate with central necrosis. These results suggested that inhibition of tumor growth might be achieved by VEGF165 antisense RNA’s down-regulation of endogenous VEGF expression in tumor tissues. In the meantime, we found that the VEGF165 antisense RNA therapy could slow the rate of tumor growth and not inhibit completely the tumorigenicity. This demonstrated that the process of angiogenesis and tumorigenicity is complex and involves multifactors. To the best of our knowledge, this is the first experimental report which shows that VEGF165 antisense RNA suppresses the growth of human esophageal squamous cell carcinoma in vivo in association with decreased vessel number in the treated tumors.
Esophageal carcinoma is still common in China[52-58], and the treatment remains a big problem up to date[59-65]. Our present study suggests that antisese RNA to VEGF165 can potentially be used as an adjuvant therapy for human esophageal squamous cell carcinoma. Further studies are needed to understand the details of the mechanisms for appropriate clinical application.
We especially thank Dr. Xiao Yan Sun for providing us the EC109 cell line, Dr. Abraham for providing the pGEM-3Zf (+) vector, we also thank Dr. Zhi Pei Zhang for his assistance with the animal experiments, and Prof. Bo Rong Pan for improving the paper.
| 1. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6572] [Article Influence: 252.8] [Reference Citation Analysis (0)] |
| 2. | Park JS, Qiao L, Su ZZ, Hinman D, Willoughby K, McKinstry R, Yacoub A, Duigou GJ, Young CS, Grant S. Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene. 2001;20:3266-3280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Millikan KW, Mall JW, Myers JA, Hollinger EF, Doolas A, Saclarides TJ. Do angiogenesis and growth factor expression predict prognosis of esophageal cancer. Am Surg. 2000;66:401-405; discussion 401-405;. [PubMed] |
| 4. | Köllermann J, Helpap B. Expression of vascular endothelial growth factor (VEGF) and VEGF receptor Flk-1 in benign, premalignant, and malignant prostate tissue. Am J Clin Pathol. 2001;116:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Volm M, Koomägi R, Mattern J. PD-ECGF, bFGF, and VEGF expression in non-small cell lung carcinomas and their association with lymph node metastasis. Anticancer Res. 1999;19:651-655. [PubMed] |
| 6. | Assy N, Paizi M, Gaitini D, Baruch Y, Spira G. Clinical implication of VEGF serum levels in cirrhotic patients with or without portal hypertension. World J Gastroenterol. 1999;5:296-300. [PubMed] |
| 7. | Xue JT, Wu J, Meng L, Dong ZW, Shou CC. Expression of VEGF(121) in gastric carcinoma MGC803 cell line. World J Gastroenterol. 2000;6:281-283. [PubMed] |
| 8. | Volm M, Mattern J, Koomägi R. Inverse correlation between apoptotic (Fas ligand, caspase-3) and angiogenic factors (VEGF, microvessel density) in squamous cell lung carcinomas. Anticancer Res. 1999;19:1669-1671. [PubMed] |
| 9. | Borre M, Nerstrøm B, Overgaard J. Association between immunohistochemical expression of vascular endothelial growth factor (VEGF), VEGF-expressing neuroendocrine-differentiated tumor cells, and outcome in prostate cancer patients subjected to watchful waiting. Clin Cancer Res. 2000;6:1882-1890. [PubMed] |
| 10. | Korshunov A, Golanov A. The prognostic significance of vascular endothelial growth factor (VEGF C-1) immunoexpression in oligodendroglioma. An analysis of 91 cases. J Neurooncol. 2000;48:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 2907] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 12. | Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 729] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 13. | Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 205] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Nishizaki M, Fujiwara T, Tanida T, Hizuta A, Nishimori H, Tokino T, Nakamura Y, Bouvet M, Roth JA, Tanaka N. Recombinant adenovirus expressing wild-type p53 is antiangiogenic: a proposed mechanism for bystander effect. Clin Cancer Res. 1999;5:1015-1023. [PubMed] |
| 15. | Kido Y. Vascular endothelial growth factor (VEGF) serum concentration changes during chemotherapy in patients with lung cancer. Kurume Med J. 2001;48:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Takayama K, Ueno H, Nakanishi Y, Sakamoto T, Inoue K, Shimizu K, Oohashi H, Hara N. Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res. 2000;60:2169-2177. [PubMed] |
| 17. | Morino F, Tokunaga T, Tsuchida T, Handa A, Nagata J, Tomii Y, Kijima H, Yamazaki H, Watanabe N, Matsuzaki S. Hammerhead ribozyme specifically inhibits vascular endothelial growth factor gene expression in a human hepatocellular carcinoma cell line. Int J Oncol. 2000;17:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Sandberg JA, Sproul CD, Blanchard KS, Bellon L, Sweedler D, Powell JA, Caputo FA, Kornbrust DJ, Parker VP, Parry TJ. Acute toxicology and pharmacokinetic assessment of a ribozyme (ANGIOZYME) targeting vascular endothelial growth factor receptor mRNA in the cynomolgus monkey. Antisense Nucleic Acid Drug Dev. 2000;10:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Oshika Y, Nakamura M, Tokunaga T, Ohnishi Y, Abe Y, Tsuchida T, Tomii Y, Kijima H, Yamazaki H, Ozeki Y. Ribozyme approach to downregulate vascular endothelial growth factor (VEGF) 189 expression in non-small cell lung cancer (NSCLC). Eur J Cancer. 2000;36:2390-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Nguyen JT, Wu P, Clouse ME, Hlatky L, Terwilliger EF. Adeno-associated virus-mediated delivery of antiangiogenic factors as an antitumor strategy. Cancer Res. 1998;58:5673-5677. [PubMed] |
| 21. | Brekken RA, Overholser JP, Stastny VA, Waltenberger J, Minna JD, Thorpe PE. Selective inhibition of vascular endothelial growth factor (VEGF) receptor 2 (KDR/Flk-1) activity by a monoclonal anti-VEGF antibody blocks tumor growth in mice. Cancer Res. 2000;60:5117-5124. [PubMed] |
| 22. | Kotoh T, Dhar DK, Masunaga R, Tabara H, Tachibana M, Kubota H, Kohno H, Nagasue N. Antiangiogenic therapy of human esophageal cancers with thalidomide in nude mice. Surgery. 1999;125:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Gaddipati JP, Mani H, Shefali K, Mathad VT, Bhaduri AP, Maheshwari RK. Inhibition of growth and regulation of IGFs and VEGF in human prostate cancer cell lines by shikonin analogue 93/637 (SA). Anticancer Res. 2000;20:2547-2552. [PubMed] |
| 24. | Zimmermann RC, Hartman T, Bohlen P, Sauer MV, Kitajewski J. Preovulatory treatment of mice with anti-VEGF receptor 2 antibody inhibits angiogenesis in corpora lutea. Microvasc Res. 2001;62:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4077] [Cited by in RCA: 3971] [Article Influence: 136.9] [Reference Citation Analysis (0)] |
| 26. | Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 353] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 27. | Rohan RM, Fernandez A, Udagawa T, Yuan J, D'Amato RJ. Genetic heterogeneity of angiogenesis in mice. FASEB J. 2000;14:871-876. [PubMed] |
| 28. | Ramanujan S, Koenig GC, Padera TP, Stoll BR, Jain RK. Local imbalance of proangiogenic and antiangiogenic factors: a potential mechanism of focal necrosis and dormancy in tumors. Cancer Res. 2000;60:1442-1448. [PubMed] |
| 29. | Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 568] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 30. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5115] [Cited by in RCA: 5985] [Article Influence: 108.8] [Reference Citation Analysis (1)] |
| 31. | Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1494] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 32. | Hyder SM, Murthy L, Stancel GM. Progestin regulation of vascular endothelial growth factor in human breast cancer cells. Cancer Res. 1998;58:392-395. [PubMed] |
| 33. | Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280:C1358-C1366. [PubMed] |
| 34. | Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9-22. [PubMed] |
| 35. | Salven P, Lymboussaki A, Heikkilä P, Jääskela-Saari H, Enholm B, Aase K, von Euler G, Eriksson U, Alitalo K, Joensuu H. Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am J Pathol. 1998;153:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 232] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336-30343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1473] [Cited by in RCA: 1518] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 37. | Bates DO, Heald RI, Curry FE, Williams B. Vascular endothelial growth factor increases Rana vascular permeability and compliance by different signalling pathways. J Physiol. 2001;533:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Rousseau S, Houle F, Huot J. Integrating the VEGF signals leading to actin-based motility in vascular endothelial cells. Trends Cardiovasc Med. 2000;10:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Pepper MS, Wasi S, Ferrara N, Orci L, Montesano R. In vitro angiogenic and proteolytic properties of bovine lymphatic endothelial cells. Exp Cell Res. 1994;210:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995-4004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 977] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 41. | Fu L, Mei Y, Li H. Effect of PAI-1 antisense RNA on vascular endot-helial growth factor expression in aorta smooth muscle cells cultured in vitro. Zhonghua Yixue Yi Chuanxue Zazhi. 2001;18:110-113. |
| 42. | Nguyen JT. Vascular endothelial growth factor as a target for cancer gene therapy. Adv Exp Med Biol. 2000;465:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Belletti B, Ferraro P, Arra C, Baldassarre G, Bruni P, Staibano S, De Rosa G, Salvatore G, Fusco A, Persico MG. Modulation of in vivo growth of thyroid tumor-derived cell lines by sense and antisense vascular endothelial growth factor gene. Oncogene. 1999;18:4860-4869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Cheng SY, Huang HJ, Nagane M, Ji XD, Wang D, Shih CC, Arap W, Huang CM, Cavenee WK. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc Natl Acad Sci USA. 1996;93:8502-8507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 233] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 45. | Inoue K, Slaton JW, Kim SJ, Perrotte P, Eve BY, Bar-Eli M, Radinsky R, Dinney CP. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res. 2000;60:2290-2299. [PubMed] |
| 46. | DeFatta RJ, Nathan CO, De Benedetti A. Antisense RNA to eIF4E suppresses oncogenic properties of a head and neck squamous cell carcinoma cell line. Laryngoscope. 2000;110:928-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Oku T, Tjuvajev JG, Miyagawa T, Sasajima T, Joshi A, Joshi R, Finn R, Claffey KP, Blasberg RG. Tumor growth modulation by sense and antisense vascular endothelial growth factor gene expression: effects on angiogenesis, vascular permeability, blood volume, blood flow, fluorodeoxyglucose uptake, and proliferation of human melanoma intracerebral xenografts. Cancer Res. 1998;58:4185-4192. [PubMed] |
| 48. | Wu XY, Zhang XF, Yin FS, Lu HS, Guan GX. Clinical study on surgical treatment of esophageal carcinoma in patients after subtotal gastrectomy. World J Gastroenterol. 1998;4:68-69. |
| 49. | Chen DF, Yang ZY, Yin WB. Radiotherapy of 180 cases of operable esophageal carcinoma. China Natl J New Gastroenterol. 1997;3:123-126. |
| 50. | Xiao ZF, Yang ZY, Zhou ZM, Yin WB, Gu XZ. Radiotherapy of double primary esophageal carcinoma. World J Gastroenterol. 2000;6:145-146. [PubMed] |
| 51. | Deng LY, Zhang YH, Xu P, Yang SM, Yuan XB. Expression of IL 1betaconverting enzyme in 5-FU induced apoptosis in esophageal carcinoma cells. World J Gastroenterol. 1999;5:50-52. [PubMed] |
| 52. | Zhang HX, Li XL, Zhao WX, Gao XP, Fu HM, Shang YQ. The study of trace elements in the hair of patients with esophageal carcinoma in high risk area. World J Gastroenterol. 2000;6:20. |
| 53. | Shen ZY, Shen WY, Chen MH, Hong CQ, Shen J. Quantitative detection of nitric oxide (NO) in apoptosis of esophageal carcinoma cell induced by arsenite. World J Gastroenterol. 2000;6:65. |
| 54. | Wu QM, Li SB, Wang Q, Wang DH, Li XB, Liu CZ. The expression of COX-2 in esophageal carcinoma and its relation to clinicopatho-logic characteristic. Shijie Huaren Xiaohua Zazhi. 2001;9:11-14. |
| 55. | Zhang J, Yan XJ, Yan QJ, Duan J, Hou Y, Su CZ. Cloning and expression of HPV16 L-2 DNA from esophageal carcinoma in E. coli. Shijie Huaren Xiaohua Zazhi. 2001;9:273-278. |
| 56. | Zhang X, Geng M, Wang YJ, Cao YC. Expression of epidermal growth factor receptor and proliferating cell nuclear antigen in esoph-ageal carcinoma and pre cancerous lesions. Huaren Xiaohua Zazhi. 1998;6:229-230. |
| 57. | Wang D, Su CQ, Wang Y, Ye YK. Deletion of p16 gene at a high frequency in esophageal carcinoma. Huaren Xiaohua Zazhi. 1998;6:1052-1053. |
| 58. | Gu HP, Shang PZ, Su H, Li ZG. Association of CD15 antigen expres-sion with cathepsin D in esophageal carcinoma tissues. Shijie Huaren Xiaohua Zazhi. 2000;8:259-261. |
| 59. | Liu J, Chen SL, Zhang W, Su Q. P21WAF1 gene expression with P53 mutation in esophageal carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:1350-1353. |
| 60. | Wang D, Su CQ, Wang Y, Ye YK. Deletion of p16 gene at a high frequency in esophageal carcinoma. Huaren Xiaohua Zazhi. 1998;6:1052-1053. |
| 61. | Shen ZY, Shen WY, Chen MH, Hong CQ, Shen J. Alterations of nitric oxide in apoptosis of esophageal carcinoma cells induced by arsenite. Shijie Huaren Xiaohua Zazhi. 2000;8:1101-1104. |
| 62. | Shen ZY, Tan LJ, Cai WJ, Shen J, Chen CY, Tang XM. Morphologic study on apoptosis of esophageal carcinoma cell line induced by arsenic trioxide. Huaren Xiaohua Zazhi. 1998;6:226-229. |
| 63. | Chen J, Zhang ZY, Zhu JQ, Wang CW, Xie Y, Huang DQ. Expres-sion of CD44v6 in esophageal carcinoma and its clinical significance. Huaren Xiaohua Zazhi. 1998;6:534. |
| 64. | He FX, Fan SH, Ge LZ, Shen YF, Lu XK. Radioprotection of radiated auto blood transfusion on patients with esophageal carcinoma. Huaren Xiaohua Zazhi. 1998;6:867-868. |
| 65. | Fu JH, Rong TH, Huang ZF, Yang MT, Wu YL. Comparative assess-ment of three prosthesis types of palliative intubation for late stage esophageal carcinoma. Huaren Xiaohua Zazhi. 1998;6:984-986. |
Edited by Ma JY