Published online Feb 15, 2002. doi: 10.3748/wjg.v8.i1.36
Revised: September 10, 2001
Accepted: October 18, 2001
Published online: February 15, 2002
AIM: To explore the correlation of the inherent cellular ROS level with the susceptibility of the digestive tract tumor cells to apoptosis inducted by As2O3.
METHODS: Two gastric carcinoma cell lines, SGC7901 and MKN45, and two esophageal carcinoma cell lines, EC/CUHK1(alternatively named EC1.71) and EC1867 with low concentration(2 μmol·L-1)of As2O3 were cultured respectly, which confirmed the difference in apoptosis susceptibility between SGC7901 and MKN45, and between EC/CUHK1 and EC1867. The cells were incubated with dihydrogenrhodamine123 (DHR123), used as a ROS capture in absence of As2O3. The fluorescent intensity of rhodamine123, which was the product of cellular oxidation of DHR123, was detected by flow cytometry, and ROS was measured.
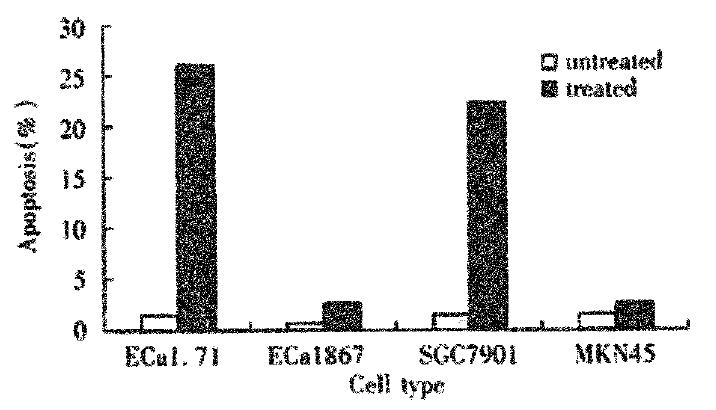
RESULTS: Apoptosis induced by a low concentration of As2O3 was more readily to occur in SGC7901 (22.4% ± 2.4%) and EC/CUHK1(27.0% ± 2.9%) than in MKN45(2.1% ± 0.5%) and EC1867(0.8% ± 0.5%). In other words, SGC7901 was more sensitive than MKN45 to As2O3, meanwhile EC/CUHK1 was more sensitive than EC1867 to As2O3. The level of inherent cellular ROS in SGC7901(650 ± 37) was higher than that in MKN45(507 ± 22)(P < 0.01), and the level of inherent cellular ROS in EC/CUHK1(462 ± 17) was higher than that in EC1867(187 ± 12) (P < 0.01).
CONCLUSIONS: The cellular sensitivity to apoptosis induced by As2O3 is associated with the difference in cellular ROS level. The inherent ROS level might determinate the apoptotic sensitivity of tumor cells to As2O3.
- Citation: Gao F, Yi J, Shi GY, Li H, Shi XG, Tang XM. The sensitivity of digestive tract tumor cells to As2O3 is associated with the inherent cellular level of reactive oxygen species. World J Gastroenterol 2002; 8(1): 36-39
- URL: https://www.wjgnet.com/1007-9327/full/v8/i1/36.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i1.36
Arsenic trioxide (As2O3) has proved to be effective in the treatment of acute promyelocytic leukemia (APL)[1-7]. While many researchers aimed at the effectiveness of As2O3-induced apoptosis on the other leukemic cells and some solid tumor cells, a lot of evidence showed that some types of tumor cells were sensitive while others were insensitive to apoptosis-inducing effect of As2O3[2,8-20]. Unraveling the causes of such sensitivity difference in the tumor cells will benefit not only the clinical selection of patients,to which As2O3 can be given, but also understanding the mechanisms underlying the apoptosis induced by As2O3.
Previously we investigated the sensitivity of a series of digestive tumor cell lines to As2O3. We identified that there were difference of sensitivity to apoptosis inductied by low concentration (2 μmol/L) of As2O3 between the gastric carcinoma cell line SGC7901 and MKN45, and between the esophageal carcinoma cell line EC/CUHK1(alternatively named EC1.71) and EC1867; SGC7901 was more sensitive than MKN45, and EC/CUHK1 was more sensitive than EC1867 to As2O3[15-16]. We found that As2O3 induced cell apoptosis via directly influencing mitochondrion , consequently causing decrease of transmembrane potential and increase of reactive oxygen species (ROS) level[17]. Recently it was evidenced that ROS participate the apoptosis induction of acute promylocytic leukemia[18,19,21,22]. But whether the difference of sensitivity of digestive tumor cells to apoptosis-inducing effect of As2O3 is associated with the inherent cellular ROS level is not clearly understood. In this study, we demonstrated the difference between SGC7901 versus MKN45, and EC/CUHK1 versus EC1867, thereby explored the ralation between the sensitivity of cell to apoptosis induction of As2O3 and the inherent cellular ROS level.
Gastric carcinoma cell line SGC7901 vs MKN45, and esophageal carcinoma cell line EC/CUHK1 vs EC1867(kindly provided by professor Shen, Shantou University) were cultured in DMEM medium supplemented with 100 kU·L-1 pennicillin,100 mg·L-1 streptomycin, and 100 mL·L-1 fetal bovine serum(Gibco) in a fully humidified atomosphere with 50 mL·L-1 CO2 at 37 °C. Cells were split when reached to 80% confluency.
About 5 × 105 tumor cells in logarithmic stage were treated with 2 μmol·L-1 concentration of As2O3 (Sigma) for 72 h and analyzed by flow cytometry and electron microscopy for apoptosis As2O3 powder was dissolved in small amounts of 1.0 mol·L-1 NaOH, then diluted to 10.0 mmol·L-1 with phosphate-buffered saline(PBS) as stock solutions.
The cells was incubated with 1 μmol·L-1dihydrorhodamine123 (DHR123,Sigma), as a ROS capture[23-25], for 1 or 24 h. Blank and positive controls were set, in which DHR123 was either omitted or plus 50 μmol·L-1 of hydrogen peroxide (H2O2). DHR123 could be oxidized intracellularly to form the fluorescent compound rhodamine123 (Rh123) by ROS, and be pumped into mitochondria and remained there. After incubated with DHR123, cells were trypsinized and harvested before an immediate detection of fluorescence intensity of Rh123 by flow cytometry FACscan (Becton Dickinson), and the cellular ROS level was thus measured.
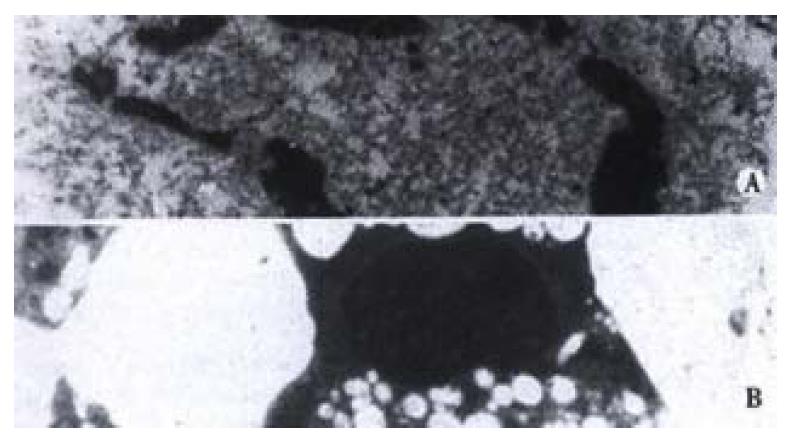
A significant apoptosis was observed in EC/CUHK1 and SGC7901 cells with 2 μmol/L of As2O3 for 3 days while no remarkable apoptosis could be seen in EC1867 and MKN45 cells with the equivalent As2O3. The characteristic morphological changes were displayed in the apoptotic cells, including the shrinkage of the nuclear membrane, condensation and margination of the chromatin, and nuclear breakage (Figure 1). DNA flow cytometry showed that the some cells with fractional DNA, as typical display of apoptosis, appeared, (27.0% ± 2.9%) and (22.4% ± 2.4%) (-x±s, n = 5)respectively in EC/CUHK1 and SGC7901 cells, but hardly visible in EC1867 (0.8% ± 0.5%) and MKN45 (2.1% ± 0.5%). (Figure 2).
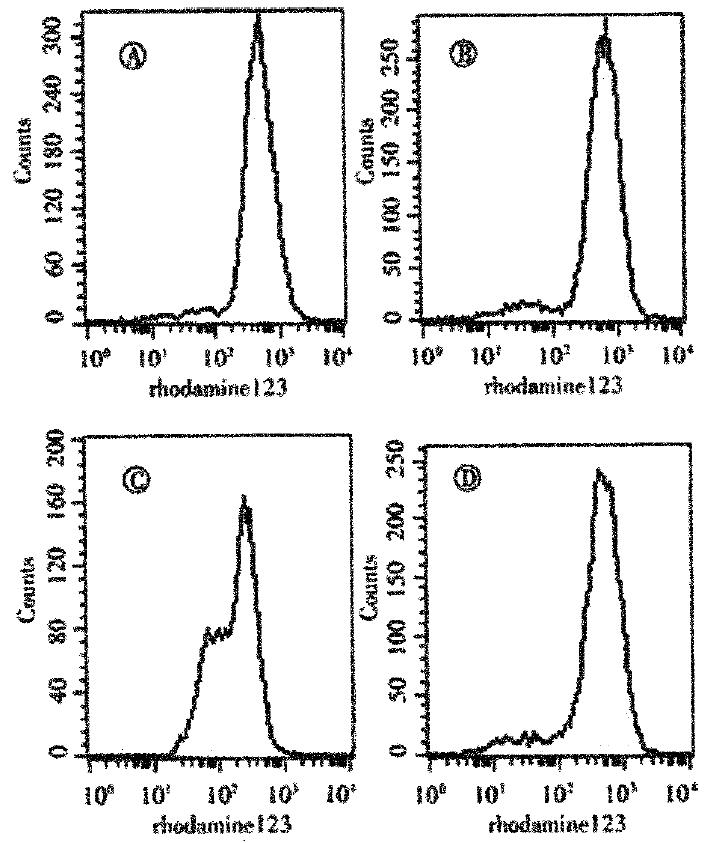
After incubation with DHR123 for 1 or 24 h in absence of As2O3, the values (-x±s, n = 3) of fluorescent intensity for Rh123 were 29 ± 4. 1 and 650 ± 37 in SGC7901 cells; 21 ± 1.4 and 507 ± 22 in MKN45 cells; 50 ± 3.9 and 462 ± 17 in EC/CUHK1; 46 ± 6.4 and 187 ± 12 in EC1867 cells. The fluorescent intensity in blank control was less than 3. The values for the positive controls (DHR123 plus hydrogen peroxide incubation for 1h) were 80 ± 4.9 in SGC7901; 27 ± 3.0 in MKN45; 72 ± 5.8 in EC/CUHK1; and 19 ± 2.1 in EC1867. Figure 3 displayed the fluorescence histograms for four types of cells after incubation with DHR123 for 24 h. The data showed that,in absence of As2O3, the cellular ROS level was higher in SGC7901 than in MKN45, and higher in EC/CUHK1 than in EC1867. Such differences were augmented in 24h incubation as shown above, where the value in SGC7901 was as 1.3 times as in MKN45, and in EC/CUHK1 was 2.5 times as in EC1867.
ROS, including superoxide anion (O2-), hydrogen peroxide(H2O2), hydroxyl free radical (OH) and singlet oxygen (1O2), continuously generated from mitochondrial respiratory chain, have powerfully oxidative potential. ROS is capable of attacking lipids, nuclear acids and proteins, resulting in certain degree of oxidative damages[26-35]. It has been thought recently to involve in apoptosis triggering and signaling[36-43]. Cell possesses an efficient antioxidant defense system, mainly composed of the enzymes such as superoxide dismutase, glutathione peroxidase, and catalase, which can scavenge the ROS excessive to cellular metabolism, and make ROS level relatively stable under physiological conditions[26-35]. Though it has been noticed that ROS were involved in As2O3-induced apoptosis[18,19,21,22], evaluation of ROS level differences directly by a flow cytometric detection of ROS, to our knowledge, has not been frequently reported. Instead, H2O2, a kind of ROS, was adopted to represent the total ROS level, usually judged from a decrease in activity of glutathione peroxidase or catalase, or a decrease in ratio of reductive/oxidative glutathione[18,19]. The total ROS level in the resting cells, however, was directly measured in the present study, by flow cytometric detection of Rh123. The comparative investigation on the inherent ROS levels in the cells showed that there were different apoptosis susceptibility to As2O3. In this study, inherent ROS level signified the basal cellular level of ROS in absence of any drug or exogenous ROS.
Detecting ROS level by flow cytometry has been a novel approach with characteristic of rapidness, convenience and reproducibility. DHR123, one of common ROS captures, is membrane permeable. It is oxidized by ROS intracellularly to become fluorescent Rh123, and is pumped into mitochondria and remain there, then is detectable by flow cytometry after a period of accumulation[23-25]. 6-carboxy-2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) is another agent used to capture ROS. It is cleaved by nonspecific esterases to form DCFH, which was further oxidized to form the fluorescent compound DCF and kept inside cells[19,44,45]. It proved important, as we realized in this study, to prolong the incubation time with the ROS capture in order to visualize the nuance in ROS, since the absolute quantity of ROS is scarce. We selected two time intervals to visualize the accumulation of Rh123 fluorescence, finding that difference began to display at 1 h and became much pronounced by 24 h. These parameters definitely represented the difference of ROS level inherently existed in the respective types of cells. A similar result was obtained by using DCFH-DA in our study. Recently it was evidenced that NB4 leukemia cell line, which is sensitive to low concentration of As2O3 (1-2 μmol/L), had higher H2O2 level than the U937 leukemia cell line which is insensitive to As2O3, and exposure of cells to low concentration of As2O3 elevated the level of H2O2 in NB4 but not in U937[19]. Though these studies indicated that a higher H2O2 level in NB4 might link to its higher sensitivity to As2O3 -induced apoptosis[19], whether there existed a difference in total ROS level between cell lines which possessed different susceptibility to As2O3 -induced apoptosis, prior to As2O3 treatment, has not been documented.
Based on our previous work, we selected two pairs of digistive tract cell lines EC/CUHK1 versus EC1867, SGC7901 versus MKN45 in which one type of cell was susceptible and the other type was unsusceptible to As2O3 -induced apoptsis in this study, and measured the inherent levels of total ROS in these cells. The data on both pairs showed that the inherent ROS level was higher in sensitive cells. These results indicated that difference in apoptosis susceptibility of tumor cells to low concentration of As2O3, was associated with the difference in the inherent cellular level of ROS, and what’s more, the inherent ROS level might be pivotal in determination of the cellular susceptibility to As2O3 -induced apoptosis. The difference of inherent ROS level between cells probably resulted from the differential expression of enzymes involved in ROS generation and elimination[46-51]. An interference to the expression of relevant enzymes or simply ROS is likely an approach by which an improved effect and expanded usage of arsenic trioxide can be achieved clinically.
| 1. | Cai X, Shen YL, Zhu Q, Jia PM, Yu Y, Zhou L, Huang Y, Zhang JW, Xiong SM, Chen SJ. Arsenic trioxide-induced apoptosis and differentiation are associated respectively with mitochondrial transmembrane potential collapse and retinoic acid signaling pathways in acute promyelocytic leukemia. Leukemia. 2000;14:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 188] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist. 2001;6 Suppl 2:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Kinjo K, Kizaki M, Muto A, Fukuchi Y, Umezawa A, Yamato K, Nishihara T, Hata J, Ito M, Ueyama Y. Arsenic trioxide (As2O3)-induced apoptosis and differentiation in retinoic acid-resistant acute promyelocytic leukemia model in hGM-CSF-producing transgenic SCID mice. Leukemia. 2000;14:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Ma DC, Sun YH, Chang KZ, Ma XF, Huang SL, Bai YH, Kang J, Liu YG, Chu JJ. Selective induction of apoptosis of NB4 cells from G2+M phase by sodium arsenite at lower doses. Eur J Haematol. 1998;61:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Munshi NC. Arsenic trioxide: an emerging therapy for multiple myeloma. Oncologist. 2001;6 Suppl 2:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 898] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 7. | Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, Tang W, Shi GY, Sun YP, Dai J. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 210] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532-539. [PubMed] |
| 9. | Shen ZY, Tan LJ, Cai WJ, Shen J, Chen CY, Tang XM. Morphologic study on apoptosis of esophageal carcinoma cell line induced by arsenic trioxide. Huaren Xiaohua Zazhi. 1998;6:226-229. |
| 10. | Tu SP, Jiang SH, Tan JH, Jiang XH, Qiao MM, Zhang YP, Wu YL, Wu YX. Proliferation inhibition and apoptosis induction by arsenic triox-ide on gastric cancer cell SGC-7901. Shijie Huaren Xiaohua Zazhi. 1999;7:18-21. |
| 11. | Chen HY, Liu WH, Qin SK. Induction of arsenic trioxide on apoptosis of hepatocarcinoma cell lines. Shijie Huaren Xiaohua Zazhi. 2000;8:532-535. |
| 12. | Maeda H, Hori S, Nishitoh H, Ichijo H, Ogawa O, Kakehi Y, Kakizuka A. Tumor growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res. 2001;61:5432-5440. [PubMed] |
| 13. | Xu HY, Yang YL, Gao YY, Wu QL, Gao GQ. Effect of arsenic trioxide on human hepatoma cell line BEL-7402 cultured in vitro. World J Gastroenterol. 2000;6:681-687. [PubMed] |
| 14. | Gu QL, Li NL, Zhu ZG, Yin HR, Lin YZ. A study on arsenic trioxide inducing in vitro apoptosis of gastric cancer cell lines. World J Gastroenterol. 2000;6:435-437. [PubMed] |
| 15. | Shi YH, Tan LJ, Li H, Shi GY, Shi XG, Tang XM. Study on arsenic trioxide induced apoptosis in tumor cell lines of digistive tract. Shang-hai Di-er Yike Daxue Xuebao. 1999;19:242-245. |
| 16. | Tan LJ, Shi GY, Shi XG, Tang XM. Induction of apoptosis of human esophageal cancer cell lines treated with arsenic trioxide. Zhongguo Aizheng Zazhi. 1999;9:85-87. |
| 17. | Tan LJ, Shi YH, Shi GY, Li H. Shi XG, Tang XM. Study on the mechanism of As2O3-induced apoptosis of human esophageal carci-noma cell lines. Shanghai Di-er Yike Daxue Xuebao. 2000;20:12-17. |
| 18. | Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268-277. [PubMed] |
| 19. | Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102-2111. [PubMed] |
| 20. | Shen ZY, Tan LJ, Cai WJ, Shen J, Chen C, Tang XM, Zheng MH. Arsenic trioxide induces apoptosis of oesophageal carcinoma in vitro. Int J Mol Med. 1999;4:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Gao F, Yi J, Shi GY, Li H, Jin HF, Shi XG, Tang XM. The susceptibil-ity of leukemia cells to arsenic trioxide-induced apoptosis is deter-mined by cellular reactive oxygen species level. Shengwu Huaxue Yu Shengwu Wuli Xuebao. 2001;33:109-113. |
| 22. | Gao F, Yi J, Shi GY, Jin HF, Shi XG, Tang XM. Cell cycle-related induction of apoptosis of NB4 cells by arsenic trioxide is associated with difference in reactive oxygen species level in cell cycle. Shanghai Di- er Yike Daxue Xuebao. 2001;21:296-299. |
| 23. | Shi GY, Gao F, Shi XG, Tang XM. Detection of cellular reactive oxygen species by flow cytometry. Shanghai Di-er Yike Daxue Xuebao. 2001;21:122-124. |
| 24. | Navarro-Antolín J, Hernández-Perera O, López-Ongil S, Rodríguez-Puyol M, Rodríguez-Puyol D, Lamas S. CsA and FK506 up-regulate eNOS expression: role of reactive oxygen species and AP-1. Kidney Int Suppl. 1998;68:S20-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | López-Ongil S, Hernández-Perera O, Navarro-Antolín J, Pérez de Lema G, Rodríguez-Puyol M, Lamas S, Rodríguez-Puyol D. Role of reactive oxygen species in the signalling cascade of cyclosporine A-mediated up-regulation of eNOS in vascular endothelial cells. Br J Pharmacol. 1998;124:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 867] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 27. | Yeldandi AV, Rao MS, Reddy JK. Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat Res. 2000;448:159-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Sun GY, Liu WW. Free radicals and digestive system neoplasms. Huaren Xiaohua Zazhi. 1998;6:272-273. |
| 29. | Greene EL, Velarde V, Jaffa AA. Role of reactive oxygen species in bradykinin-induced mitogen-activated protein kinase and c-fos induction in vascular cells. Hypertension. 2000;35:942-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Sun GY, Liu WW, Zhou ZQ, Fang DC, Men RP, Luo YH. Free radicals in development of experimental gastric carcinoma and pre-cancerous lesions induced by N-methyl N'nitro Nnitrosoguanidine in rats. Huaren Xiaohua Zazhi. 1998;6:219-221. |
| 31. | Sun GY, Liu WW, Zhou ZQ, Fang DC, Men RP, Luo YH. Free radi-cals in development of experimental gastric carcinoma and precan-cerous lesions induced by N-methyl-N'nitro N nitrosoguanidine in rats. World J Gastroenterol. 1998;4:124. |
| 32. | Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 507] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 33. | Chen DZ, Wei MX, Gu YC, Guan XZ. Oxygen free radical harm in Piyinxu and Shenyinxu patients. Huaren Xiaohua Zazhi. 1998;6:660-662. |
| 34. | Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 302] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 35. | Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005-L1028. [PubMed] |
| 36. | Perkins C, Kim CN, Fang G, Bhalla KN. Arsenic induces apoptosis of multidrug-resistant human myeloid leukemia cells that express Bcr-Abl or overexpress MDR, MRP, Bcl-2, or Bcl-x(L). Blood. 2000;95:1014-1022. [PubMed] |
| 37. | Carmody RJ, Cotter TG. Signalling apoptosis: a radical approach. Redox Rep. 2001;6:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 38. | Fadeel B, Ahlin A, Henter JI, Orrenius S, Hampton MB. Involvement of caspases in neutrophil apoptosis: regulation by reactive oxygen species. Blood. 1998;92:4808-4818. [PubMed] |
| 39. | Zhang C, Gong Y, Ma H, An C, Chen D, Chen ZL. Reactive oxygen species involved in trichosanthin-induced apoptosis of human choriocarcinoma cells. Biochem J. 2001;355:653-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Arai T, Endo N, Yamashita K, Sasada M, Mori H, Ishii H, Hirota K, Makino K, Fukuda K. 6-formylpterin, a xanthine oxidase inhibitor, intracellularly generates reactive oxygen species involved in apoptosis and cell proliferation. Free Radic Biol Med. 2001;30:248-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Jones DC, Gunasekar PG, Borowitz JL, Isom GE. Dopamine-induced apoptosis is mediated by oxidative stress and Is enhanced by cyanide in differentiated PC12 cells. J Neurochem. 2000;74:2296-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Moreno-Manzano V, Ishikawa Y, Lucio-Cazana J, Kitamura M. Selective involvement of superoxide anion, but not downstream compounds hydrogen peroxide and peroxynitrite, in tumor necrosis factor-alpha-induced apoptosis of rat mesangial cells. J Biol Chem. 2000;275:12684-12691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, Marrack PC. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 405] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 44. | Sawada M, Nakashima S, Kiyono T, Nakagawa M, Yamada J, Yamakawa H, Banno Y, Shinoda J, Nishimura Y, Nozawa Y. p53 regulates ceramide formation by neutral sphingomyelinase through reactive oxygen species in human glioma cells. Oncogene. 2001;20:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Sureda FX, Gabriel C, Comas J, Pallàs M, Escubedo E, Camarasa J, Camins A. Evaluation of free radical production, mitochondrial membrane potential and cytoplasmic calcium in mammalian neurons by flow cytometry. Brain Res Brain Res Protoc. 1999;4:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Atlante A, Calissano P, Bobba A, Giannattasio S, Marra E, Passarella S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 2001;497:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 264] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 47. | Faist V, König J, Höger H, Elmadfa I. Decreased mitochondrial oxygen consumption and antioxidant enzyme activities in skeletal muscle of dystrophic mice after low-intensity exercise. Ann Nutr Metab. 2001;45:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Caillaud C, Py G, Eydoux N, Legros P, Prefaut C, Mercier J. Antioxidants and mitochondrial respiration in lung, diaphragm, and locomotor muscles: effect of exercise. Free Radic Biol Med. 1999;26:1292-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp Clin Res. 1999;25:87-97. [PubMed] |
| 50. | Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci USA. 1999;96:4820-4825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 466] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 51. | Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. The effect of reactive oxygen species generated from the mitochondrial electron transport chain on the cytochrome c oxidase activity and on the cardiolipin content in bovine heart submitochondrial particles. FEBS Lett. 2000;466:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
Edited by Wang JH and Xu XQ