INTRODUCTION
Gastric cancer is common in China[1-42], and its early diagnosis and treatment in advanced stage are difficult[31-50]. In recent years, gene study in cancer is a hotspot, and great progress has been achieved[41-80]. Cancer gene therapy has shifted from the imagination into the laboratory and clinical trials. The “logic of how genes function” coupled with the connections of cell cycle processes to specific gene actions is creating a promise of treating tumors by gene therapy. There have been significant advances against both local and metastatic growths. The potential role of gene intervention extends from diseases caused by single gene defects, through severe viral infections, to polygenic disorders, such as diabetes mellitus and arteriosclerosis. However, gene therapy can be defined as the introduction and expression of an exogenous gene into human cells for therapeutic benefit, and is conventionally restricted to human diseases associated with single gene defects. The rapid progress in our understanding of some of the molecular mechanisms involved in the pathogenesis of cancer and metabolic disorders, with the development of gene delivery vector technology, has urged us to consider novel gene approaches to digestive diseases. There is no shortage of ideas and applications for gene intervention in human diseases, but there are great limitations not only with the efficiency and targeting of the present generation of gene transfer vectors but also with our incomplete understanding of transcription control[1,2].
The graduation of gene therapy from unfulfilled dreams to conventional therapy for gene and acquired disorders will require a mastery of multiple disparate components including gene delivery vectors, regulated tissue-specific gene expression, control of immunity and manipulation of cell viability. Improvement in suicide genes has opened up a whole new treatment modality for treating hyperproliferative disorders and for designing animal models for disease[3]. Along with herpes simplex virus-1 thymidine kinase, a host of additional suicide gene has been developed. A critical comparison of these will follow along with progress in utilizing these reagents for therapeutic benefits[81-90].
The current delineation of the molecular basis of cancer provides a strong rationale to consider gene therapy approaches for cancer as a complement to other cancer therapies. Phase III trials focused on adenoviral vector-mediated delivery of wild-type p53 to compliment p53 mutations were recently initiated for head and neck cancer and ovarian cancer. Clinical testing of the tumor inhibitory gene E1A, delivered by synthetic vectors is ongoing. Positive clinical data from these clinical studies will establish the use of gene therapy as a component of the multimodal treatment for certain cancers[4-6].
Although the rapid technological advances continue to sustain the field of cancer gene therapy, few individual patients have benefited from the revolution so far. The plethora of clinical trials described confirms that each malignancy has its own ideal strategy based on the associated molecular defects, and there has been rapid progress in this viewpoint. At the same time, there has been a renewed appreciation for the limitations to gene therapy, which include low efficiency of gene transfer, poor specificity of response and methods to accurately evaluate responses, and lack of truly tumor-specific targets at which to aim. With all new therapies, we are climbing a steep learning curve in encountering treatment-related toxicities, as well as profound ethical and regulatory issues[5-9].
GENE THERAPY THEORY
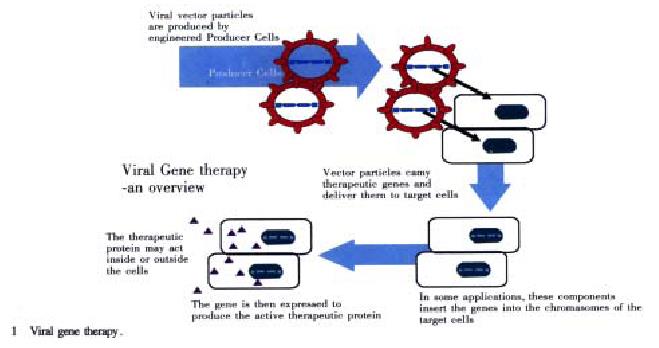
Recent advances in understanding and manipulating genes-the biological units of heredity-have set the stage for scientists to alter patients’ genetic material to fight or prevent diseases. One major goal of gene therapy is to supply cells with healthy copies of missing or flawed genes. This approach is revolutionary. Instead of giving a patient a drug to treat or control the symptoms of a gene disorder, physicians attempt to correct the basic problem by altering the gene makeup of some of the patient’s cells (http://www.chgb.org.cn/spec-topics/therapy/text00.htm). Hundreds of major health problems are influenced by gene functions. In the future, gene therapy could be used to treat many of these conditions. Theoretically, it could also be used to alter germ cells (egg or sperm) in order to prevent a gene defect from being transmitted to future generations. However, difficult ethical and social questions as well as technical obstacles will be set in the possibility of germ-line gene therapy (Figure 1, http://www.chgb.org.cn/spec-topics/hgp/medicine/med03.htm). Gene therapy could also be used as a drug delivery system. To accomplish this, a gene that produces a useful product would be inserted into the DNA of the patient’s cells[10-14]. For example, during blood vessel surgery, a gene that makes an ant-clotting factor could be inserted into the DNA of cells lining blood vessels to prevent dangerous blood clots from forming. Many other conditions might also lead themselves to treatment by using this general approach. As medicine operates increasingly at the molecular level, gene therapy for drug delivery could save much effort and expense. It could shortcut the lengthy and complicated process of collecting large amounts of a gene’s protein product, purifying the product, formulating it as a drug, and administering it to the patient. However, gene therapy is still extremely new and highly experimental. The number of approved trials is small, and relatively few patients have been treated to date[8,11-13].
Figure 1 Viral gene therapy.
BASIC STEPS INVOLVED IN CURRENT GENE THERAPY EXPERIMENTS
In some current experiments, cells from the blood or bone marrow are removed from the patient and grown in the laboratory under conditions that encourage them to multiply. Then the desired gene is inserted into the cells with the help of a disabled virus, and the successfully altered cells are selected out, encouraged to multiply, and returned to the patient’s body. In other cases, liposomes (lipid particles) or disabled viruses may be used to deliver the gene directly to cells within the patient’s body. Basic requirements for gene therapy are as follows[91-110].
Potential of gene therapy
Gene therapy offers a new treatment paradigm for curing human disease. Rather than altering the disease phenotype by using agents that interact with gene products, or are themselves gene products, gene therapy can theoretically modify specific genes resulting in disease cure following a single administration. Initially gene therapy was envisioned for the treatment of gene disorders, but is currently being studied in a wide range of diseases, including cancer, peripheral vascular disease, arthritis, neurodegenerative disorders and other acquired diseases[15-20].
Gene identification and cloning
Even though the range of gene therapy strategies is quite diverse, certain key elements are required for a successful gene therapy strategy. The most elementary of these is that the relevant gene must be identified and cloned. Upon completion of the Human Genome Project, gene availability will be unlimited, but until then the starting point for any gene therapy strategy remains gene identification and cloning for relevant genes related to the disease[3,16].
Gene transfer and expression
Once the gene has been identified and cloned, the next consideration must be expression. Questions pertaining to the efficiency of gene transfer and gene expression remain at the forefront of gene therapy research. Currently much debate in the field of gene therapy revolves around the transfer of desired genes to appropriate cells, and then obtaining sufficient levels of expression for disease treatment. Hopefully, future research on gene transfer and tissue-specific gene expression will resolve these issues in the majority of gene therapy protocols[21-23]. Other important considerations for a gene therapy strategy include: a sufficient understanding of the pathogenesis of the targeted disorder, potential side effects of the gene therapy treatment, and understanding of the target cells to receive the gene therapy[22].
Terminology
Like most fields, gene therapy has unique terminology. The list provided below will clarify the meaning of some of the most common terms[1-3,6,9,22-26].
Ex vivo In gene transfer, transfer of gene material to cells located outside the host. Following transfer of the gene material, the cells are then implanted back into the host. This term has also been called the indirect method of gene transfer.
In vivo In gene transfer, transfer of gene material to cells located within the host. This has also been termed the direct method of gene transfer.
Gene therapy The transfer of selected genes into a host with the hope of ameliorating or curing a disease state.
Cell therapy (genome therapy) The transfer of entire cells, that have not been genetically modified, into a host with the hope that the transferred cells will engraft into and improve host function. Somatic gene transfer Transfer of genes to non-germline tissues in the hope of correcting the disease state of a patient.
Germline gene Transfer of genes to germline (eggs or sperm) tissues in the hope of altering the genome of future generations.
Transgene The selected gene tested in a gene transfer experiment. For example, if you wished to treat a patient for phenylketonuria, you might plan to transfer a corrected version of the phenylalanine hydroxylase gene into the liver cells. In this example, the corrected version of the phenylalanine hydroxylase gene would be the transgene.
Reporter gene Genes that are used to test the efficiency of gene transfer. Examples include genes encoding luceriferase, -galactosidase, and chloramphenicol acetyltransferase.
Gene transfer vector The mechanism by which the gene is transferred into a cell.
Transfer efficiency The percentage of cells that are expressing the desired transgene.
APPROACHES TO CANCER THERAPY
The idealized approach to gene therapy is the replacement of a mutated gene with a correct copy that restores normal functioning and therapeutically alters the malignant phenotype. One meaning of the word “vector” is “carrier”. In the field of infectious diseases, the term has been used to describe an agent, such as an insect, that carries an infectious organism from one individual to another. By analogy, the genetically disabled viruses used in gene therapy are referred to as vectors because they carry genes to cells. Most often, these vectors are derived from mouse retroviruses.
Scientists are working on ways to genetically alter immune cells that are naturally or deliberately targeted to cancers. They are interested in arming such cells with cancer-fighting genes and returning them to the body, where they could more forcefully attack the cancer. Clinical trials along these lines are in progress for the treatment of melanoma. Alternatively, cancer cells can be taken from the body and altered genetically so that they elicit a strong immune response. These cells can then be returned to the body in the hope that they will act as a cancer vaccine. A variety of clinical trials using this approach are now under way. It is also possible to inject a tumor with a gene that renders the tumor cells vulnerable to an antibiotic or other drug. Subsequent treatment with the drug should kill only the cells that contain the foreign gene. Since other cells would be spared, the treatment should have few side effects. Two trials using this approach are in progress for treatment of brain tumors[17-19,24].
Tumor-suppressor gene therapy
Goals of tumor suppressor gene therapy are: cell death and changes in growth of the cell, behavior of the cell, invasiveness of the cell, and metastatic ability of the cell.
Because p53 is the most common mutated in cancer and influences transcription, cell cycle movement, apoptosis, and angiogenesis, it is a prime target for gene replacement. In model systems, transduction of cancer cells with p53 has been demonstrated to inhibit growth, inhibit angiogenesis, and induce apoptosis.
Early clinical trials using a p53 retrovirus have also been encouraging. Current limitations of tumor-suppressor gene therapy are: limited number of target genes known to induce or maintain malignancy; and difficulty of transducing enough cancer cells to result in a cure.
A bystander effect-the death of more cells than are actually transduced-has been proposed although how it occurs has not been yet understood. This effect may result from cell-cell contact, immune mediated responses, and/or other local actions.
Other therapeutic possibilities are: combining p53 transduction with radiation or apoptosis-inducing chemotherapy, systemic delivery of p53 using liposomes, systemic delivery of p53 using hepatic artery infusion, use of an adenovirus that replicates only in p53 mutant cells and results in cell death through lysis of the cell.
Suicide gene therapy
Suicide gene therapy is the transduction of a gene that transforms a non-toxic “pro-drug” into a toxic substance. Systems under investigation are as follows[22-26]:
Escherichia coli cytosine deaminase (CD) gene + 5-fluorocytosine (5-FC). CD converts 5-FC to 5-fluorouracil (5-FU), a chemotherapeutic agent. This combination produces a bystander effect and has been demonstrated to have some success in animals with hepatic metastasis of gastrointestinal tumors. Delivery of CD to specific sites and the use of tissue specific promotors are a focus of work with this strategy.
Herpes simplex virus thymidine kinase gene (HSV-tk) + ganciclovir (GCV). HSV-tk phosphorylates GCV causing it to inhibit the synthesis of DNA. Like the first system, this also causes a bystander effect. This strategy has been looked at for treatment of localized brain tumors, liver metastases, peritoneal-based metastases, and mesotheliomas. Thus far, the unpredictability of the bystander effect and difficulties in transduction has kept cure rates low. The use of tissue-specific vectors to deliver the genes and the combination of the strategy with radiation may improve the efficacy of the approach in time.
Immunomodulatory gene therapy
Immunomodulatory gene therapy is a method to induce cellular immune responses to metastatic lesions. The strategy involves injecting into the skin of a patient a suspension of irradiated tumor cells that have been transduced with a cytokine gene to stimulate a systemic immune response against tumor-specific antigens[1-4,27-29]. In preclinical cancer models vaccination with tumor cells have been demonstrated to generate a cellular immunological response against “challenge” tumors. There are several problems that still need to be addressed to make this strategy successful[20,30-32]: ① there are only a few tumor-specific antigens that act as recognition targets; ② antitumor activity has been active against a relatively low tumor burden in several studies; and ③ the financial and labor costs are high and efficiencies will need to be developed.
Future modifications to the basic strategy may include: a combination of cytokine and costimulatory molecule vaccination to increase tumor vaccine efficiency; the use of in situ strategies to invoke immunologic responses, including direct injection into a tumor, as has been done with an adenoviral vector containing the IL-2 gene; combining a suicide gene therapy approach (e.g., HSV-tk + GCV) with IL-2 transgene therapy or, more generally, using combinations of cytokines to induce antitumor activity both in situ within a tumor and systemically.
The future of cancer gene therapy appears to rest on increased competence with in situ transduction; further advances in techniques to induce expression of transduced genes; and further advances in inducing significant antitumor responses at the systemic level. These, in turn, will probably rest on advancing our understanding of tumor immunology, strategies to limit angiogenesis, and continued development of safe and effective vectors to carry genes to directed sites[27-31].
GENE TRANSFER TECHNOLOGY
The gene delivery technology is developing rapidly and there have been specific developments that could be translated into gene-based therapies for gastroenterological diseases. For example, ex vivo transfer methods are being studied extensively by using hepatocytes obtained through liver biopsy, partial hepatectomy, and from specimens harvested for liver transplantation. Adult liver cells transiently undergo active proliferation permitting in vitro gene transfer even with vectors that require active cell division for entry and expression. Gene transfer may then be facilitated by a number of methods, including viruses, liposome, calcium phosphate coprecipitation, particle bombardment, naked DNA injection, and electroporation. The transfected cells are reintroduced into the host by using, for example, a microcarrier system into the peritoneum, gel beads, hepatocyte coated cell support matrix implanted next to liver tissue, or into the spleen or portal circulation through direct injection[2-5,32-40].
The spectrum of delivery systems for ex vivo gene transfer is broadly applied also to the in vivo model. Although the transfer efficiency of liposomes is low, these lipids can be made comparatively easily to high chemical purity and have low immunogenicity, which may permit repeated administrations. They have been used successfully in an in vivo model, by topical administration to epithelial cells both in the airways and the intestinal tract and also by the intravascular route. A recent study showed high efficiency transfer of the APC tumor suppresser gene in liposome complexes delivered to normal mouse colonic epithelium by rectal catheter infusion. Almost 100% of epithelial cells expressed the gene for up to four days, which is consistent with the known rate of turnover of this tissue[41]. Intravenous injection of a rat insulin gene expression vector in liposome complexes results in uptake primarily by the liver and spleen. Improvement in hepatocyte uptake can be achieved by incorporating lactosyl ceramide into the phospholipid bilayer; this galactosyl terminal asialoganglioside is specifically recognized by a receptor highly selective for hepatocytes. Many different lipid agents are now being explored for efficacy of DNA transfer and it seems likely that the composition of the complex will have to be optimized for different targets and different routes of administration.
Of the available methods of gene delivery, viruses have been proved the most efficient so far. Achieving viral gene transfer to specific organs for clinical application will be difficult, however, particularly as viral titres 10 to 1000 times higher than those usually attained (typically 106 infectious units per milliliter) will be necessary for in vivo strategies. There is now extensive experience with retroviruses whose main advantages include their small size and easy manipulation, and with stable colinear integration with host genome. They are comparatively non-toxic and are efficient for gene transfer. Retroviruses persist in up to 5% of hepatocytes three months after injection of an infected hepatocyte cell suspension into the portal vein after partial hepatectomy. The small intestinal epithelium is an attractive target for gene therapy because of its large surface area, easy accessibility, and the presence of stem cells with known locations. Although few studies have yet targeted the intestinal system in vivo, marker genes have been transferred to the epithelial surface with retroviral vectors in animal models. Clearly, unless the therapeutic or marker gene is transferred to the stem cells, the rapid turn-over of this specialized epithelium would seriously limit potential benefits of delivered genes. Retroviruses have a number of disadvantages, notably the requirement for cells that are actively dividing to permit viral DNA integration, the ability to carry only small DNA sequences, and a small but finite risk of causing insertional mutagenesis as a result of random integration[42-47].
Currently alternative viral vectors with potential advantages over retroviruses in specific applications are under development. Adenoviruses can infect non-dividing cells, can be concentrated to high titres, and are comparatively highly efficient vectors. Adeno-associated viruses are ubiquitous and non-pathogenic in humans and can also infect non-replicating cells, but, like retroviruses and adenoviruses, are limited in the size of the foreign gene that can be inserted. This problem may be overcome by the use of herpes simplex group viruses and possibly even vectors based on hepatitis B virus, which has potential additional advantages of hepatotropism and an ability to integrate with host genome in vivo.
GENE THERAPY FOR GASTRIC CANCER
The generation of retroviral vectors that infect specific cell types through recognition of cell surface antigens is a promising and effective approach to targeted gene therapy of cancer. Carcinoembryonic antigen (CEA), a highly characterized, cell surface glycoprotein overexpressed by various tumor cells, provides a specific tool for tumor tissue-specific targeting by retroviral vectors. The conventional suicidal gene delivery systems need additional drugs other than their gene products. The inducible nitric oxide synthase (iNOS) gene product yields nitric oxide (NO), which directly induces autocytotoxicity and cytolysis of bystander cells. In the present study, we have developed a novel bifunctional Moloney murine leukemia virus-based recombinant retroviral vector that displays a chimeric envelope protein containing a single-chain variable fragmented (scFv) antibody to CEA and carries the iNOS gene in the genome. The resultant bifunctional retroviral vector showed a specific delivery of the iNOS gene to human CEA-expressing carcinoma cells, resulting in the direct and efficient killing of CEA-expressing carcinoma cells by induction of apoptosis. This is the first report of successful killing of CEA-expressing cells by specific targeting of the iNOS gene. This approach may offer a one-step procedure for effective gene therapy of CEA-expressing tumors[44,48].
This study used a recombinant antisense c-myc adenovirus (Ad-ASc-myc) to evaluate how alterations of c-myc expression in the SGC7901 human gastric carcinoma cells could influence the proliferation, apoptosis and the growth of human gastric tumors in nude mice[42,49]. The human gastric carcinoma cell line, SGC7901 cells, treated with Ad-ASc-myc or adenovirus recombinants carrying LacZ gene (Ad-LacZ) were analyzed by using X-gal stain, MTT, DNA ladder, TUNEL assay, flow cytometric analysis, polymerase chain reaction and western blot in vitro. The tumorigenicity and experimental therapy in nude mice models were assessed in vivo. The Ad-ASc-myc could strongly inhibit cell growth and induce apoptosis in SGC7901 cells. The proliferation of the Ad-ASc-myc-infected SGC7901 cells was reduced by 44.1%. The mechanism of killing gastric carcinoma cells by Ad-ASc-myc was found to be apoptosis, which was detected by the use of a DNA ladder, TUNEL and flow cytometric analysis.Infection of Ad-ASc-myc in nude mice showed that all three mice failed to form tumors from a 7-30 d period, as compared with injection of Ad-LacZ and parent SGC7901 cells. Experimental therapy on the nude mice bearing subcutaneous tumors of SGC7901 cells showed that intratumor instillation of Ad-ASc-myc inhibited the growth of the tumors. Recombinant antisense c-myc adenovirus-treated tumors were inhibited by 68.9%, as compared with tumors injected with Ad-LacZ and control (LacZ and phosphate-buffered saline). The expression of Ad-ASc-myc can inhibit growth and induce apoptosis of gastric cancer cells in vitro and in vivo, thus it is a potential clinical utility in gene therapy for the treatment of gastric carcinoma.
The oncofoetal antigen 5T4 is a 72 ku glycoprotein expressed at the cell surface. It is defined by a monoclonal antibody, mAb5T4, which recognises a conformational extracellular epitope in the molecule. Overexpression of 5T4 antigen by tumors of several types has been linked with disease progression and poor clinical outcome. Its restricted expression in non-malignant tissues makes 5T4 antigen a suitable target for the development of antibody directed therapies. The use of murine monoclonal antibodies for targeted therapy allows the tumor specific delivery of therapeutic agents. However, their use has several drawbacks, including a strong human anti-mouse immune (HAMA) response and limited tumor penetration due to the size of the molecules. The use of antibody fragments leads to improved targeting, pharmacokinetics and a reduced HAMA. A single chain antibody (scFv) comprising the variable regions of the mAb5T4 heavy and light a chain has been expressed in Escherichia coli. The addition of a eukaryotic leader sequence allowed production in mammalian cells. The two 5T4 single chain antibodies, scFv5T4WT19 and LscFv5T4, described the same pattern of 5T4 antigen expression as mAb5T4 in normal human placenta and by FACS. Construction of a 5T4 extracellular domain-IgGFc fusion protein and its expression in COS-7 cells allowed the relative affinities of the antibodies to be compared by ELISA and measured in real time using a biosensor based assay. The small size of this 5T4 specific scFv should allow construction of fusion proteins with a range of biological response modifiers to be prepared whilst retaining the improved pharmacokinetic properties of scFvs[50].
The assessment of the angiogenic profile of tumors may become an important tool as a guide for the inclusion of novel drugs and molecular therapies into the standard chemoradiotherapy policy. Several studies have shown the prognostic importance of microvessel density (MVD) and of angiogenic factor expression in operable gastric cancer. In the present study we investigated the MVD, with immunohistochemistry the expression of vascular endothelial growth factor (VEGF) and of thymidine phosphorylase (TP) expression as well as the nuclear expression of p53 protein, in a series of patients with locally advanced inoperable gastric cancer. A strong association of VEGF with TP expression was noted (P < 0.01), and tumors coexpressing these factors had a statistically higher MVD (P < 0.01). Nuclear p53 accumulation was also related to a high MVD (P < 0.01), and this was independent of VEGF or TP expression. Microvessel density showed a bell-shaped association with prognosis; cases with an intermediate MVD exhibit a favorable outcome (P < 0.05). A trend of nuclear TP expression to define a group of patients with poorer prognosis was noted (P > 0.05), while none of the remaining variables showed any significant association. The immunostaining results allowed the grouping of the angiogenic profile in four major categories: ① highly vascularized tumors with VEGF and/or TP expression (about 36%); ② highly angiogenic tumors with p53 nuclear accumulation and low VEGF/TP expression (7%); ③ poorly vascularized tumor with low VEGF/TP and negative nuclear p53 staining (32%) and poorly vascularized tumors with TP expression (7%). Specific therapies targeting hypoxia, VEGF, or TP expression as well as p53 gene therapy have entered clinical experimentation or are already available for clinical use. Using the suggested markers, more than 80% of locally advanced gastric carcinomas can be grouped in different categories according to their angiogenic profile. Such a categorization may be useful for phase III trials on novel therapies targeting the major angiogenesis-related features studied here[51].
Mammalian degenerin (MDEG) is a member of the amiloride-sensitive sodium ion channel family, and its site-directed active mutant (MDEG-G430F) induces massive Na+ influx into cells, leading to cell ballooning and cell bursting. We attempted a novel therapeutic approach for gastric cancers by transferring MDEG-G430F into cancer cells using tumor-specific promoters[52]. In carcinoembryonic antigen (CEA)-producing gastric cancer cells, the level of cell death observed when MDEG-G430F was used with a CEA promoter was similar to that observed when using a potent nonspecific promoter such as the cytomegalovirus promoter. In an in vivo study, fusogenic liposome complexes containing MDEG-G430F driven by the CEA promoter were injected intraperitoneally into CEA-producing gastric cancer cells in a mouse peritoneal dissemination model. Although all 15 of the control mice were dead by 50 d postinoculation, 13 of the 15 mice treated with MDEG-G430F survived. These results indicate that transferring MDEG-G430F into cancer tissues using tumor-specific promoters can achieve striking and selective cancer cell death irrespective of the transcriptional efficiency of the promoters used in vivo, and suggest that this approach is a promising new strategy for cancer gene therapy[38,41].
Lymph node metastasis is one of the prognostic factors in gastric cancer. Sunami et al[63] have previously reported that decreased intercellular adhesion molecule-1 (ICAM-1) expression on cancer cells is associated with lymph node metastasis using a gastric cancer cell. In this study, ICAM-1 gene into a gastric cancer cell line was transected, 2MLN, and analyzed the effect on lymph node metastasis in vitro and in vivo. A significantly greater amount of peripheral blood mononuclear cells (PBMC) adhered to ICAM-1 transfected 2MLN cells, 2MLN/ICAM cells, than to 2MLN/vector cells. The lysis of 2MLN/ICAM cells by PBMC was significantly increased as compared with that of 2MLN/vector cells. The tumor growth rate of 2MLN/ICAM cells was significantly decreased in vivo. Lymph node metastases caused by 2MLN/ICAM cells were found fewer in number and smaller in size, while many lymph node metastases were caused by 2MLN cells. Histologic findings showed that leukocytes were heavily infiltrated in both the 2MLN/ICAM tumors and metastasis lesions, while only a few leukocytes were observed in the lesions associated with 2MLN cells. The above findings indicate that ICAM-1 gene transduction could prove to be an effective gene therapy for lymph node metastasis of gastric cancer.
The elderly population has much to gain from the advances of molecular medicine, although at present genetic pharmacology remains mostly at the conceptual level. Cancer, in particular, is an increasing health burden and the majority (over 70%) of gene therapy trials aimed at tackling this problem. Available strategies employ both viral and synthetic vectors with the selective delivery and expression of therapeutic genes a pivotal requirement. Clinical trials are now in progress with a view to modulating disease at many different levels, including the direct replacement of abnormal genes. Suicide-gene formulations, and the delivery of “gain of function” genes, which seek to alter the malignant phenotype by indirect means, such as, immunopotentiation and stromal reorganisation. Early data from these studies is tantalising and we must remain optimistic that gene therapy will benefit the patients with cancer by both reducing morbidity and extending life[64].
The antitumoral effects of antisense RNA to K-ras were investigated in gastric cancer cell lines by examining the level of K-ras expression and the tumorigenicity in vitro and in vivo. Polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP), DNA sequencing, and immunoblotting analysis revealed that YCC-1 gastric cancer cells overexpressed wild type K-ras, whereas YCC-2 cells had a homozygous mutation in codon 12 from GGT (glycine) to AGT (serine), while SNU-1 cells had a heterozygous mutation to GAT (asparagine) in the identical position. Both YCC-1 and YCC-2 cells were transduced by LNC-AS/K-ras containing the antisense 2.2 kb genomic K-ras DNA fragment covering exon 2 and exon 3 specific for K-ras. The application of antisense K-ras significantly downregulated the expression of K-ras and had no influence on the expression of either H-ras or N-ras. The antisense-transduced YCC-2 cells grew considerably slower than the control group transduced by LNCX, whereas the growth inhibition of antisense-transduced YCC-1 cells was less prominent than that of transduced YCC-2 cells. In addition, the tumorigenicity of YCC-2 cells transduced by LNC-AS/K-ras was totally lost. Therefore, our results imply that the specific inhibition of K-ras p21 protein can be accomplished by introducing the antisense covering the K-ras-specific region to gastric cancer cells with aberrant K-ras expression, resulting in a reduction of the growth rate and suppression of tumorigenicity[65].Dysregulation of apoptosis may be closely related to the development of cancer and its chemoresistance. Overexpression of Bax, an inducer of apoptosis, has led to increased cell death in a variety of cancer cell lines. In this study, we investigated the effect of Bax overexpression in two gastric cancer cell lines, MKN-28 and MKN-45, using a Cre-loxP-mediated inducible expression system. After induction of bax, both cell lines showed decreased proliferation, partially due to increased cell death. Furthermore, Bax-expressing MKN-28 cells were more sensitive to cisplatin. These results indicate that up-regulation of the bax gene may provide a novel strategy for the treatment of gastric cancer[66].
In an attempt to obtain suitable in vivo models for optimizing new tumor therapy strategies for intestinal adenocarcinomas, carcinoembryonic antigen (CEA) promoter/SV40 T antigen gene constructs have been used to generate transgenic mice[67]. One transgenic line (L5496), which contains a 424-bp CEA promoter/SV40 T antigen transgene, exclusively developed multi-focal carcinomas in the pyloric region of the stomach in 100% of the offspring. Tumors were already observable in 37-day-old animals as dysplastic cell foci within the mucosal layer. In 50-day-old mice, the tumor mass was mainly restricted to the mucosa with invasive growth into the submucosal tissue. The animals became moribund at 100-130 d of age due to blockage of the pylorus. At this time, the tumor had penetrated into the duodenum and had invaded all tissue layers within the stomach. In contrast to most other stomach tumor models, this one perfectly matches the development of the most common stomach cancers found in humans. Furthermore, after crossing these mice with mice that are transgenic for the human CEA gene, the double transgenic offspring revealed expression of CEA in the resulting tumors. Thus, being a model for studying gastric carcinoma development and prevention, this system should provide a useful preclinical model for CEA-targeted gastric tumor therapy[31,37].
PROSPECT IN THE FUTURE
Recent scientific breakthroughs in the genomic field and our understanding of the important role of genes in disease has made gene therapy one of the most rapidly advancing fields of biotechnology with great promise for treating inherited and acquired diseases. Many human diseases are caused by the absence or inappropriate presence of a protein. The first promise of biotechnology was to isolate and produce these natural proteins through genetic engineering and recombinant technology. The protein could then be administered to patients in order to compensate for its absence. Because proteins are not orally available, biotech companies focused on innovative methods of protein delivery and sustained drug delivery. Today, gene therapy is the ultimate method of protein delivery, in which the delivered gene enters the body’s cells and turns them into small “factories” that produce a therapeutic protein for a specific disease over a prolonged period[82-93].
As gene therapy has shifted from the laboratory into the clinic, several issues have emerged as central to the development of this technology: gene identification, gene expression and gene delivery. Academic researchers supported by the government’s Human Genome Project and more recently through genomics companies originally tackled gene identification. A number of disease-related genes with direct clinical have already been identified, and this number is growing as the field rapidly advances. Some of these genes are in the public domain and some are proprietary. Genes with broader clinical application are also being utilized to make cells express immune activating agents locally at the disease site or to become susceptible to further drug treatment or to immune response recognition [76-84,100-112].
The control of gene transcription is extremely complicated. For the most intensely investigated systems such as the globin genes, our understanding is still fragmental. While most protocols presently use strong viral promoters to drive expression of recombinant cDNA copies of therapeutic genes, future work must be directed to defining the genomic elements that enable temporal and spatial control of expression through a lifetime. The identification of locus control regions that can insulate gene clusters from interference by surrounding genetic influences has been an important step, and many investigators are now working to understand how the promoter and enhancer/silencer elements of a gene interact with structures within the nucleus. Advances in this area will require parallel developments in the sophistication of vector design before they can be transferred into practice[95-104].