Published online Oct 15, 2001. doi: 10.3748/wjg.v7.i5.706
Revised: June 6, 2001
Accepted: June 20, 2001
Published online: October 15, 2001
AIM: To evaluate the efficacy and safety of the TECA-I bioartificial liver support system (BALSS) in treating canines with acute liver failure (ALF).
METHODS: Ten canines with ALF induced by 80% liver resection received BALSS treatment (BALSS group). Blood was perfused through a hollow fiber tube containing 1 × 1010 porcine hepatocytes. Four canines with ALF were treated with BALSS without porcine hepatocytes (control group), and five canines with ALF received drug treatment (drug group). Each treatment lasted 6 h.
RESULTS: BALSS treatment yielded beneficial effects for partial liver resection-induced ALF canines with survival and decreased plasma ammonia, ALT, AST and BIL. There was an obvious decrease in PT level and increase in PA level, and there were no changes in the count of lymphocytes, immunoglobulins (IgA, IgG and IgM) and complement (C3 and C4) levels after BALSS treatment. In contrast, for the canines with ALF in non-hepatocyte BALSS group (control group) and drug group, there were no significant changes in ammonia, ALT, AST, BIL, PT and PA levels. ALF canines in BALSS group, control group and drug group lived respectively an average time of 108.0 h ± 12.0 h, 24.0 h ± 6.0 h and 20.4 h ± 6.4 h, and three canines with ALF survived in BALSS group.
CONCLUSION: TECA-I BALSS is efficacious and safe for ALF canines induced by parcial liver resection.
- Citation: Chen XP, Xue YL, Li XJ, Zhang ZY, Li YL, Huang ZQ. Experimental research on TECA-I bioartificial liver support system to treat canines with acute liver failure. World J Gastroenterol 2001; 7(5): 706-709
- URL: https://www.wjgnet.com/1007-9327/full/v7/i5/706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i5.706
The incidence of acute liver failure (ALF) is high, and the survival rate of ALF patients is only 10%-15%[1,2]. The current comprehensive treatment for ALF is not satisfactory. Orthotopic liver transplantation (OLT) is an effective therapy for patients with ALF. However, because of the shortage of donor organs, many patients died before they could receive OLT. The recently developed bioartificial liver support system can temporarily replace the complex functions of liver such as synthesis, detoxication and “bridge” the patients with ALF to liver transplantation. To what extent can TECA-I BALSS replace normal liver functions In order to answer the question, we have introduced the basic techniques of a hollow fiber bioartificial liver system, used Chinese experimental miniature swines as the donor of xenogeneic hepatocytes, improved the culturing conditions of porcine hepatocytes in the BALSS, and identified the efficacy and safety of TECA-I BALSS in treating drug-induced ALF canines[3,4]. In the present study, we developed a partial liver resection-induced ALF canine model to assess the efficacy and feasibility of the TECA-I BALSS in treating ALF canines.
Nineteen healthy adult hybrid canines weighing 18 kg-22 kg were obtained from the Experimental Center of General Hospital of PLA, and fourteen adult Chinese experimental miniature swines weighing 8 kg-10 kg from the Breed Center of Chinese Agricultural University.
All chemicals were provided by Hong Kong TECA Ltd Co. and Sigma Chemical Co.
The canines received eighty percent liver resection. Left lateral, left anterior, right lateral and caudate lobe were removed.
Hepatocytes were isolated from Chinese experimental miniature swines by collagen ase digestion. Viability of the cells was assayed by trypan blue exclusion and AO/PI fluorescence staining.
Freshly isolated hepatocytes were cultured in the TECA-I BALSS provided by Hong Kong Ltd Co. The porcine hepatocytes circulated through the exterior space of capillary hollow fiber in the BALSS. Blood from femoral artery and vein on one side of the ALF canine circulated through the inner space of capillary hollow fiber and the substances exchanged through the membrane of hollow fibers between porcine hepatocytes and ALF blood.
Nineteen ALF canines were randomly divided into three groups: ① BALSS group (n = 10): perfused with hollow fiber tube of BALSS containing 1 × 1010 porcine hepatocytes. ② non-hepatocyte BALSS group (control group, n = 4): perfused with hollow fiber tube without porcine hepatocytes. ③ drug group (n = 5): intravenous injection with arginine, sodium glutamate and branch amino acid. Each treatment lasted six hours.
The plasma levels of ammonia, ALT, AST, bilirubin, PT and PA were measured before and 2, 4, 6 h, 1, 3, 5, 7, 14 and 30 d after treatment.
The count of lymphocytes, immunoglobulins (IgA, IgG, IgM) and complement (C3, C4) levels were measured before and 7, 14 and 30 d after BALSS treatment.
The volume of the remnant liver was observed, and the remnant liver, kidneys, heart and lungs were taken out when the canines died or survived for 30 d for paraffin section, and HE stained and examined under light microscope.
The values of blood biochemical and immunological parameters were expressed as means ± SD. Data of pre- and post-treatment among the three groups were compared using the Chi square test. The differences were considered significant at P < 0.05.
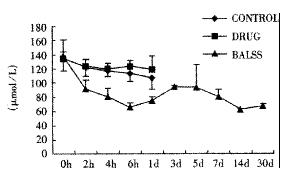
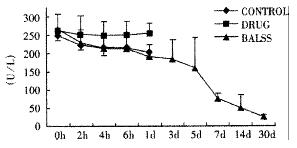
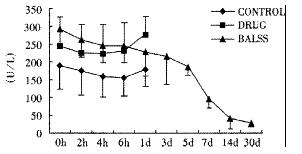
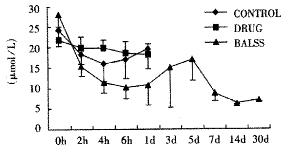
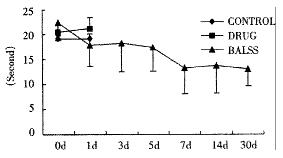
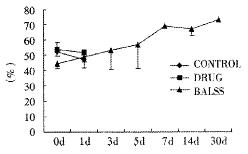
The canines demonstrated a significant increase in plasma ammonia, ALT, AST, bilirubin and PT levels, and decrease in PA level 24 h after operation: ammonia (64.52 ± 10.07 mol/L-128.33 ± 17.32 μmol/L, Figure 1), ALT (14.33 ± 5.12 IU/L- 277.04 ± 53 .35 IU/L, Figure 2), AST (16.56 ± 8.91 IU/L-236.06 ± 75.68 IU/L, Figure 3), BIL (5.07 ± 1.56 mmol/L-21.20 ± 3.22 mmol/L, Figure 4), PT (9.68 ± 0.76 s-19.91 ± 1.87 s, Figure 5) and PA (79.16% ± 4.31%-54.66% ± 4.84%, Figure 6). ICG excretion rate of post-operation was lower than that of pre-operation respectively at 5, 10 and 15 minutes (15%, 23% and 30% vs 20%, 55% and 76%).
Using the modified enzymatic digestive method, each liver yielded approximately 1-3 × 1010 hepatocytes. Viability of the final suspensions averaged 75%-90% by typan blue exclusion and AO/PI fluorescence staining.
BALSS treatment yielded beneficial effects with decreased plasma ammonia, bilirubin at 2 h of the treatment, and then returned to normal from 4-6 h of the treatment. There was an obvious decrease in PT, ALT and AST level and increase in PA level at 7 d after BALSS treatment. In contrast, there were no changes in ammonia, ALT, AST, bilirubin, PT and PA levels for the ALF canines in the control group and drug group. Among seven of ten canines in BALSS group which received one BALSS treatment, only one survived, and six lived an average time of 108.0 h ± 12.0 h (96 h-120 h). Three of ten canines received two BALSS treatments, two canines survived, and one died of lung infarction. There was no evidence that BALSS treatment had adverse effects on the morphology of kidneys, lungs and heart. In contrast, four ALF canines in the control group lived an average time of 24.0 h ± 6.0 h (18 h-50 h). Five ALF canines in the drug group lived an average time of 20.4 h ± 6.4 h (12 h-28 h).
There were no changes in count of lymphocytes, immunoglobulins (IgA, IgG, IgM) and complement (C3, C4) levels for 3 survived canines.
The volume of the remnant liver at 30 d after treatment became larger than that of ALF canines before treatment. BALSS treatment had no adverse effects on the morphology of kidneys, lungs and heart.
Acute liver failure is a kind of severe disease, and no effective clinical therapy is available currently. Since the liver has many complex physiological functions, such as synthesis, detoxication and biotransformation. The current non-bioartificial liver, for example, activated charcoal hemoperfusion and plasma exchange can not replace the liver function completely. It only can clear some middle and small molecule weight substance in circulation. Fortunately, encouraging success of currently developed bioartificial liver support system has been made in the experimental and clinical research. In BALSS, xenogeneic hepatocytes were used to exchange the substance with ALF blood so that it can temporarily replace the complex liver functions.
BALSS is made of biological and synthetic materials. The main biological material is hepatocytes which include human liver tumor cell lines[5,6], human embryo[7] and porcine hepatocytes[8]. There are possible dangers of the tumor cells to receptors and the scarce of the embryonic liver cells which hinders the application of BALSS, we therefore selected the pure strain Chinese experimental miniature swine as the source of hepatocytes in our experiment. Using modified enzymatic digesting and culturing method, we acquired a large quantity of porcine hepatocytes to meet the needs of BALSS. Porcine hepatocytes 1-3 × 1010 could be acquired from one swine liver, and the viability of hepatocytes reached 75%-90%. Hollow fiber tube is the main synthetic material in which hepatocytes are cultured and exchange substance with ALF blood through semipermeable membrane. A surgical model of acute liver failure is preferred to a toxic model, because it is more reproducible and induces only liver damage. In our study, we developed a subtotal hepatectomy induced ALF canine model. Twenty-four hours after operation, the blood hepatic biochemiscal parameters had obvious changes. The ALF canines died within 36-52 h without treatment. The ALF canine model had a good reproductivity and could suit the studies of BALSS.
One of the most striking observations from the present work is that the ammonial levels of the ALF canines in BALSS group were lowered dramatically at 2 h of the treatment, and then returned to normal from 4-6 h of the treatment. In contrast, there were no changes in the ammonial level for the ALF canines in the control group and drug group. The result indicated that ammonia could move through the semipermeable membrane of hollow fiber tube and was metabolized by porcine hepatocytes through urea cycle enzymes and glutamine synthetase. Significant decreases in plasma bilirubin levels after BALSS treatment were observed and demonstrated that porcine hepatocytes cultured in BALSS could accelerate metabolism of bilirubin in ALF blood. It was reported that hyperbilirubinemia in animals had been corrected by microencapsulated hepatocyte transplantation.
Results of a phase I clinical trial of BALSS treatment showed that more than two BALSS treatments could clear the toxins in ALF blood completely and provide sufficient metabolic, liver functional support[9,10]. Our experiment indicated that the survival rate of ALF canines that received two BALSS treatments was higher than that of ALF canines that received only one BALSS treatment. Certainly the ultimate goal of a liver support treatment is to treat patients early enough and repeatedly to allow them to regenerate their livers and recover normal liver function.
Although in the development of a bioartificial liver, the use of hepatocytes from xenogeneic origin is the most promising alternative for human hepatocytes due to the shortage of human donor material, potential immunological problems may arise. The central unit of TECA-I BALSS is the so-called bioreactor (hollow fiber tube) which is divided into two sets of compartment by semipermeable membrane: extracapillary and intracapillary compartment. The semipermeable membrane is set such that those substances which are metabolized, detoxified, or synthesized can readily cross the membrane. However, blood cells and hepatocytes should not cross the reactor membrane. In our experiment, in the 3 survived canines, there was neither change in the count of lymphocytes, immune proteins (IgA, IgG, IgM) and supplementary (C3, C4) levels and nor pathological change in kidneys, remnant liver, lungs and heart after BALSS treatment.
On one hand, porcine hepatocytes cultured in BALSS can provide directly liver functional support, and on the other hand, hepatocytes can produce some proteins such as growth factors, and stimulate liver regeneration. Hepatocytes transplanted into the spleen of rats could reverse the neurologic disorders associated with hepatic encephalopathy[11]. Thus, it is likely that both mechanisms direct action of hepatocytes cultured in BALSS and indirection stimulation of native liver are involved[12].
It is suggested that the TECA-I BALSS could temporarily replace the hepatic function of ALF canines effectively and safely, and it may provide a new approach for the treatment of ALF.
| 1. | Ascher NL, Lake JR, Emond JC, Roberts JP. Liver transplantation for fulminant hepatic failure. Arch Surg. 1993;128:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 112] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Pan BR, Yang SF, Ma LS. Acute liver failure: a progress report. China Natl J New Gastroenterol. 1995;1:4-8. |
| 3. | Xue YL, Zhao SF, Zhang ZY, Wang YF, Li XJ, Huang XQ, Luo Y, Zhang L, Huang YC, Liu CG. Effects of a hollow fiber bioartificial liver system to treat acute liver failure canines. Junyi Jinxiu Xueyuan Xuebao. 1998;19:83-86. |
| 4. | Xue YL, Zhao SF, Zhang ZY, Wang YF, Li XJ, Huang XQ, Luo Y, Huang YC, Liu CG. Effects of a bioartificial liver support system on acetaminophen induced acute liver failure canines. World J Gastroenterol. 1999;5:308-311. [PubMed] |
| 5. | Gao Y, Xu XP, Hu HZ, Yang JZ. Cultivation of human liver cell lines with microcarriers acting as biological materials of bioartificial liver. World J Gastroenterol. 1999;5:221-224. [PubMed] |
| 6. | Sussman NL, Chong MG, Koussayer T, He DE, Shang TA, Whisennand HH, Kelly JH. Reversal of fulminant hepatic failure using an extracorporeal liver assist device. Hepatology. 1992;16:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 215] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Wang YJ, Li MD, Wang YM, Chen GZ, Lu GD, Tan ZX. Effect of extracorporeal bioartificial liver support system on fulminant hepatic failure rabbits. World J Gastroenterol. 2000;6:252-254. [PubMed] |
| 8. | Lin C, Hou KY, Zhang WX, Lu JN, Zhou XS, Gong YD, Ding XL, Zhang XF, Zhao NM. Study on mechanism of treating acute hepatic failure with cryopreserved porcine hepatocyte xenotransplantation. China Natl J New Gastroenterol. 1996;2:212-214. |
| 9. | Rozga J, Podesta L, LePage E, Morsiani E, Moscioni AD, Hoffman A, Sher L, Villamil F, Woolf G, McGrath M. A bioartificial liver to treat severe acute liver failure. Ann Surg. 1994;219:538-44; discussion 544-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 156] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Watanabe FD, Mullon CJ, Hewitt WR, Arkadopoulos N, Kahaku E, Eguchi S, Khalili T, Arnaout W, Shackleton CR, Rozga J. Clinical experience with a bioartificial liver in the treatment of severe liver failure. A phase I clinical trial. Ann Surg. 1997;225:484-91; discussion 491-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 320] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Ribeiro J, Nordlinger B, Ballet F, Cynober L, Coudray-Lucas C, Baudrimont M, Legendre C, Delelo R, Panis Y. Intrasplenic hepatocellular transplantation corrects hepatic encephalopathy in portacaval-shunted rats. Hepatology. 1992;15:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Roger V, Balladur P, Honiger J, Baudrimont M, Delelo R, Robert A, Calmus Y, Capeau J, Nordlinger B. Internal bioartificial liver with xenogeneic hepatocytes prevents death from acute liver failure: an experimental study. Ann Surg. 1998;228:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Edited by Ma JY