INTRODUCTION
Lipopolysaccharide (LPS), a main component of gram-negative bacterial endotoxin [1], is the leading cause of sepsis or endotoxic shock (ES), and when administered experimentally to animals, mimics the same inflammatory response. The pathophysiological changes seen in sepsis are often not due to the infectious organism itself but to the uncontrolled production of proinflammatory cytokine s, including tumor necrosis factor (TNF)-α and interleukin (IL)-1β. Accumulation of these mediators leads to tissue damage, ultimately producing the lethality of sepsis[2]. Studies suggest that alterations in their circulating levels do not always reflect changes at the tissue level and this has led to the reassessment of tissue cytokine content as a more accurate reflection of the host response to stress[3]. Cholecystokinin (CCK), a component of the gastrin-CCK family and first isolated from hog intestine, shows a widespread distribution in different organs and tissues. The sulfated carboxy-terminal octapeptide (CCK-8), which has been isolated from the central nervous system and digestive tract, is the predominant active form. CCK-8 possessed both excitatory and inhibitory action on contractile activity of different regions of stomach in guinea pigs[4]. CCK-8 could antagonize the elimination of morphine on the potentiations of ACh to duodenal activities, and these effects were mediated by CCK-A receptor[5]. Besides the effects of CCK-8 on the digestive tract, other biological actions of this peptide have been observed, such as appetite inhibition[6,7]. In the spleen, CCK-8 is formed in high abundance in the white pulp where it appears to surround cell clusters. It seems that CCK -8 increases in vivo the secretion of immunoglobulins[8,9], while it causes in vitro an inhibition of Molt-4 lymphoblast proliferation[10] and modulates mitogen-induced lymphoproliferation and intracellular calcium mobilization[11-13]. CCK-8 is a chemoattractant for human monocytes and rat macrophages[14], enhances human eosinophil chemotaxis induced by PAF and LTB4 in allergic patients[15] and is a negative modulator of several murine macrophage and human neutrophil functions[16-18]. It was reported that CCK-8 reversed hemorrhagic shock[19,20]. Our previous studies demonstrated for the first time that CCK-8 could protect animals from LPS-induced ES[21]. However, whether this protecting effect of CCK-8 is related to its modulation of cytokines is still not clear. The AIM of this work is to study the effect of CCK-8 pretreatment on systemic hypotension and on production of cytokines such as TNF-α, IL- 1β and IL-6 in spleen, lung, heart and serum of ES rats.
MATERIALS AND METHODS
Materials
CCK-8 (sulfated), LPS (E. coli LPS, serotype 0111:B4), leupeptin, pepstatin A and Triton X-100 were all purchased from Sigma and aprotinin from Boehringer. ELISA kits were purchased from Endogen (USA) and Medsystem (Austria). All other reagents used were of analytic grade.
METHODS
Animal preparation Specific pathogen-free male Sprague-Daw ley rats (n = 48, weighing 150 g-200 g, obtained from Experimental Animal Center of Hebei) were housed in a controlled environment, exposed to 12 h:12 h light-dark cycle and fed standard rat diet. On the day of experiment, animals were randomly assigned to four groups injected different agents via tail vein. For group receiving LPS, a bolus dose (8 mg•kg¯¹, 5 g•L¯¹) of LPS was injected into the tail vein. For group of CCK-8+LPS, a bolus dose (40 μg•kg¯¹, 0.05 g•L¯¹) of CCK-8 was administered 10 min before injection of LPS. Negative control animals received saline, CCK-8 (40 μg•kg¯¹) was also administe red alone in the other group.
Mean arterial blood pressure (MABP) detection Catheter was inserted into arteriae femoralis before agents administration and MABP was detected using physiology record instrument (RM-6000, Japan). ES model was made by LPS administration, the effect of CCK-8 on MABP of ES rats was observed.
Enzyme linked immunoabsorbant assay (ELISA) Animals were sacrificed at 2 h or 6 h after treated with LPS, spleen, lung and heart were rapidly excised, rinsed of blood, and the blood was centrifuged for collection of serum. The samples were stored at -80 °C. The samples collected at 2 h were for the assay of TNF-α and at 6 h for the assay of IL-1β and IL-6. Serum TNF-α was measured using an ELISA kit (Bender MedSystem, Austria) specific for rat TNF-α with an inter-assay coefficient of variation to be < 10% and intra-assay coefficient of variation to be < 5%; the lowest limit of detection was 17 ng•L¯¹. Serum IL-1β was determined with a rat ELISA (Endogen Inc, USA). Intra- and inter-assay coefficients of variation were 5.3%-6.1% and 6.8%-8.8%, respectively. The limit of detection for this assay was < 12 ng•L¯¹. Serum IL-6 was determined with a rat ELISA (Endogen Inc, USA). The intra and inter-assay coefficients of variation were 8.3%-9.2% and 6.9%-7.8%, respectively. The lowest limit of detection was 16 ng•L¯¹.
Forzen tissue samples were weighed and placed in homogenization buffer (4 °C) at a ratio of 100 mg per milliliter of buffer. Buffer contained a protease-inhibitor cocktail including 1 mmol•L¯¹ phenylmethanesulfonyl fluoride (PMSF), 1 mg•L¯¹ pepstatin A, 1 mg•L¯¹ aprotinin, and 1 mg•L¯¹ leupeptin in phosphate-buffered saline solution, pH7.2, containing 5 g•L¯¹ Triton X-100. Samples were homogenized and centrifuged at 18000 r•min¯¹. Tissue supernatants were analyzed for TNF-α, IL-1β and IL-6 using the above described ELISAs.
Statistical analysis
Data were reported as ¯x ± s. Statistical differences between values from different groups were determined by one way ANOVA. Significance was set at P < 0.05.
RESULTS
Changes of MABP
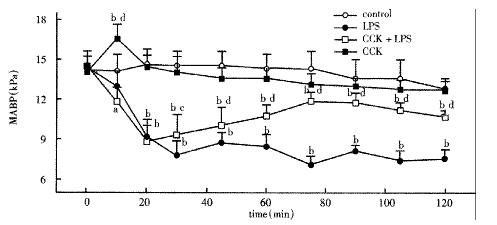
There was no significant difference among groups before treatment. LPS administration resulted in a significant sustained decrease in MABP during the period of 2 h, decreased to 7.82 ± 0.43 kPa 30 min after LPS administration and restored to 9.33 ± 0.63 kPa by pretreatment with CCK-8 (Figure 1).
Figure 1 Mean arterial blood pressure (MABP) of animals injected normal saline, LPS, CCK+LPS and CCK.
n = 6, aP < 0.05, bP < 0.01, vs Control; cP < 0.05, dP < 0.01, vs LPS.
Serum cytokine levels
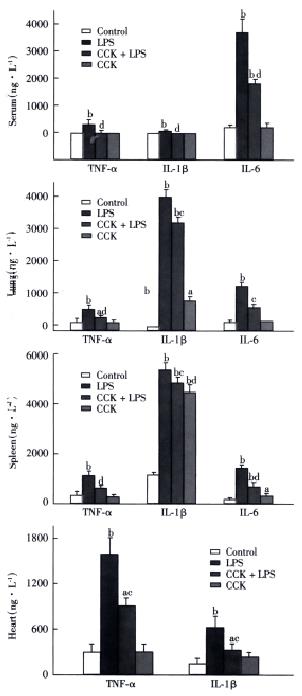
TNF-α and IL-1β were not detectable in the serum of saline or CCK-8 treated rats, while IL-6 concentration in serum of saline or CCK-8 treated rats were 128 ± 22 ng•L¯¹ and 84 ± 78 ng•L¯¹, respectively. Significant increase in serum level of TNF-α was observed in LPS group at 2 h to an average of 277 ± 86 ng•L¯¹, which was inhibited by CCK-8 to 12 ± 21 ng•L¯¹. Serum levels of IL-1β and IL-6 increased to 43 ± 9 ng•L¯¹ and 3567 ± 687 ng•L¯¹, respectively, 6 h after administration of LPS, while administration of CCK-8 prior to LPS significantly inhibited this LPS induced increase in IL-1β and IL-6 concentration (not detectable and to 1797 ± 69 ng•L¯¹, respectively. LPS elevated the serum level of IL-6 significantly, while serum levels of TNF-α and IL-1β rose significantly but less than IL-6 (Figure 2).
Figure 2 Effects of CCK on TNF-α, IL-1β and IL-6 2 h (TNF-α) or 6 h (IL-1β and IL-6) following LPS administration.
n = 6. aP < 0.05, bP < 0.01, vs Control; cP < 0.05, dP < 0.01, vs LPS.
Tissue cytokine contents
Spleen TNF-α content was significantly higher 2 h after administration of LPS than in control animals 941 ± 149 ng•L¯¹vs 282 ± 30 ng•L¯¹, P < 0.01, while CCK-8 significantly inhibited the LPS induced increase of TNF-α 462 ± 87 ng•L¯¹, P < 0.01. Similar results of TNF-α were noted in the lung and heart following 2 h post-LPS administration. CCK-8 pretreatment attenuated LPS-induced increases of IL-1β and IL-6 contents in spleen and lung 6 h after LPS administration. LPS elevated content of IL-1β in the spleen and lung significantly 5184 ± 85 ng•L¯¹vs 1047 ± 21 ng•L¯¹ and 4050 ± 614 ng•L¯¹ vs not detectable, respectively (Figure 2), while the levels of TNF-α and IL-6 rose but in less extent than IL-1β. CCK-8 pretreatment attenuated LPS-induced increases of IL-1β content in the heart 6 h after LPS administration from 621 ± 145 ng•L¯¹ to 282 ± 93 ng•L¯¹, P < 0.01. No significant changes were noted in contents of cytokines following CCK-8 administration compared with normal saline administration.
DISCUSSION
Interaction of the central nervous system (CNS) with immune system can occur via several endocrine pathways, the presence of various neuropeptide receptors on cells of the immune system suggests more direct communication pathways. Moreover, immune cells release cytokines, which can influence neuronal activity. Thus direct communication between the CNS and the immune system may well be bi-directional[22]. The present study show that administration of brain- gut peptide, CCK-8, prevents the LPS-induced decrease of MABP and attenuates LPS-induced increase of proinflammatory cytokines (TNF-α, IL-1β and IL-6) in vivo. Inhibition of these cytokines by CCK-8 may have contributed to the prevention of ES, such as restoring the blood pressure. The macrophages are the sources of proinflammatory cytokines (TNF-α, IL-1 and IL-6)[23]. These three important proinflammatory cytokines respond to the initial stimulation[24] and play predominant roles in the normal inflammatory response. Exaggerated endogenous production is likely responsible for the complications associated with sepsis such as tissue injury and ultimate organ failure[25-28], induce a wide range of pathophysiologic changes in LPS-induced ES. TNF is known to have cytotoxic and cytostatic effects on certain tumor cells, and with a pivotal role in inflammatory reactions and regulation of immunologic al response[29,30]. TNF can enhance the adhesion of PMN to endothelium[31]. Although the migration of leukocytes to and accumulation at the site of inflammation are important for killing micro-organisms, activation of recruited neutrophils coupled with excessive release of oxygen metabolites and proinflammatory mediators may induce tissue injury which can lead to organ dysfunction[32]. TNF-α was mainly produced in the early stage of endotoxemia, and decreased obviously from 6 h to 9 h after challenge[33]. The effect of TNF-α is influenced by other cytokines[34]. IL-1β and IL-6, produced mainly by activated phagocytes and lymphocytes, show a wide variety of biological functions. They can influence secretion of other cytokines and inflammatory mediators in an autocrine or paracrine fashion, induce expression of surface immune molecules of antigen-presenting cells to serve as an activation factor and differentiation factor on T cells and B cells, mediate immunoglobulin secretion, activate the complements, killer cells and phagocytes, and enhance tissue injury mediated by cellular and humoral immune reactions[35]. Most of our knowledge concerning the induction of these cytokines in response to LPS or gram-negative bacteria comes from studies performed either in vitro or in fluids obtained from animal models or patients with sepsis, whereas the major site of action of the cytokines are probably at the tissue and cell level[36]. Our study show that the increase in spleen and lung content of IL-6 following LPS administration was lesser than in serum. In contrast, the increase in spleen and lung content of IL-1β following LPS administration was more than in serum, while the content of IL-1β in heart was more than in serum and less than in spleen and lung. The results demonstrated a dissociation or poor correlation between intra -splenic, intra-pulmonic or intra-cardiac abundance of TNF-α, IL-1β and IL-6 and the magnitude of the increase in circulating cytokine levels. The production of cytokines varies from organ to organ in response to systemic administration of LPS. Activated macrophages in spleen and lung are known to be active producers of pro-inflammatory cytokines. This has led to the suggestion that spleen, lung and heart are important organs in the production of TNF-α and IL-1β following LPS administration, releasing the cytokines produced into the circulation, thereby contributing to the elevated serum levels of the cytokines. We found that CCK-8 significantly inhibits LPS-induced increase of pro-inflammatory cytokines in vivo. While Cunningham et al[37] reported that CCK-8 stimulated production of TNF-α, IL-1β and IL-6 by monocytes, but was considerably less than LPS response. Later studies[38] suggest that the increase of cytokines induced by CCK-8 may be due to the detection of endotoxin/LPS in medium.
Despite convincing data indicating the protective function of CCK-8 to organism in ES, the precise mechanism remains elusive. Our previous study demons trated that exogenous or endogenous CCK reversed pulmonary arterial hypertension (PAH) during endotoxic shock[39]. In vitro studies showed CCK can protect pulmonary artery endothelium against detrimental effects by LPS or TNF-α[40,41]. The anti-inflammatory effect of CCK- showed in this study may mediate the cell protective function of CCK-8 in ES. CCK-8 dose dependently attenuated gastric lesions induced by 75% ethanol, blockade of CCKA receptor with loxiglumide abolished the protective effects of CCK[42]. The effect of CCK may depend on its modulation of immunocyte function, which could occur through CCK receptors. There exists CCKB receptor in human lymphoid cells[43]. It was demonstrated recently that wild-type transcripts of both CCK receptor subtypes and splice variants of the CCK-B/gastrin receptor are expressed in nontransformed human mononuclear cells[44]. Endogenous opioids, such as β-endorphin, are produced within the immune system and are active regulators of the immune response. There were elevations in the circulating levels of β-endorphin in LPS-treated animals, which indicated the potential contribution to modulating the tissue cytokine response to LPS[45]. The hypotensive response was related to release of endorphin, while the mechanism of CCK-8 anti-ES may be related to its anti-endorphin effect[3]. CCK-8 decreased heart rate via CCK-A receptors located in the atrium of the rats[46]. Intravenous administration of CCK-8 to guinea pigs inhibited the motility of the left ventricle of heart[47]. CCK had hypertensive effect on rats and increased the cardiac contraction amplitude, decreased coronary outflow, while it had no effect on heart rate[48]. CCK-B agonist pentagastrin induced significant and very rapid, dose-dependent elevations in adrenocorticotropin and cortisol levels, and significant elevations in heart rate and blood pressure were seen[49]. The different effect of CCK-8 on cardiovascular system may due to the different doses and animals. However, additional mechanisms may be present in mediating the cytokine responses to LPS and CCK. Our study shows for the first time a previously unknown physiological function for CCK- 8 in a model of LPS-induced ES. CCK-8 may have modulatory effects on the immune functions of ES rats. CCK-8, therefore, might be used therapeutically to treat septic shock syndrome and other inflammatory disease states.