Published online Oct 15, 2001. doi: 10.3748/wjg.v7.i5.602
Revised: June 6, 2001
Accepted: June 15, 2001
Published online: October 15, 2001
INTRODUCTION: This paper provides a review of the practice of liver transplantation with the main emphasis on UK practice and indications for transplantation. Referral and Assessment: This section reviews the process of referral and assessment of patients with liver disease with reference to UK practice.
Donor Organs: The practice of brainstem death and cadaveric organ donation is peculiar to individual countries and rates of donation and potential areas of improvement are addressed.
Operative Technique: The technical innovations that have led to liver transplantation becoming a semi-elective procedure are reviewed. Specific emphasis is made to the role of liver reduction and splitting and living related liver transplantation and how this impacts on UK practice are reviewed. The complications of liver transplantation are also reviewed with reference to our own unit.
Immunosuppression: The evolution of immunosuppression and its impact on liver transplantation are reviewed with some reference to future protocols. Retransplantation: The role of retransplantation is reviewed.
Outcome and Survival: The results of liver transplantation are reviewed with specific emphasis on our own experience. Future: The future of liver transplantation is addressed.
- Citation: Bramhall S, Minford E, Gunson B, Buckels J. Liver transplantation in the UK. World J Gastroenterol 2001; 7(5): 602-611
- URL: https://www.wjgnet.com/1007-9327/full/v7/i5/602.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i5.602
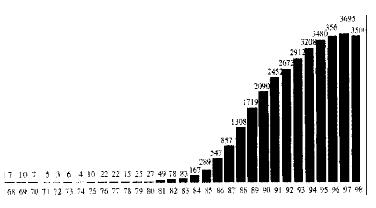
Recent years have seen dramatic changes in the practice of liver transplantation. In 1980 in Europe fewer than 30 liver grafts were performed compared to over 3000 in 1995 (Figure 1). During this period liver transplantation has evolved from a rare procedure in patients with end-stage liver disease, to a semi-elective operation with current predictable success rates of approximately 90% in patients with chronic disease. In the early period of liver transplantation it was reserved for patients with end stage chronic liver disease or unresectable primary liver malignancy, but in recent years there has been a considerable broadening of the accepted indications. Improving results have led to liver transplantation becoming a semi-elective procedure with both quantity and quality of life being of major concern. Patients who may not be in immediate risk of death from liver decompensation but have significantly impaired quality of life are now considered as candidates.
The current indications can be classified into four broad groups; chronic liver failure, acute liver failure, primary hepatic malignancy not treatable by conventional resection and inborn errors of metabolism due to a liver based enzyme defect but without parenchymal liver disease (Table 1).
| Birmingham series | |||
| n | % | ||
| Chronic liver failure | Primary biliary cirrhosis | 434 | 24.7 |
| Primary sclerosing cholangitis | 158 | 9 | |
| Autoimmune chronic active hepatitis | 96 | 5.5 | |
| Alcoholic cirrhosis | 122 | 6.9 | |
| Cryptogenic cirrhosis | 92 | 5.2 | |
| HBV or HBC cirrhosis | 165 | 9.4 | |
| Biliary atresia | 142 | 8 | |
| Alpha-1 anti-trypsin deficiency | 65 | 3.7 | |
| Budd-Chiari syndrome | 8 | 0.1 | |
| Acute liver failure | Viral hepatitis (non-A, non B, HBV, HAV) | 10 | 8 |
| Drugs (Paracetamol, anti-tuberculosis therapy, halothane) | 66 | 3.8 | |
| Toxins and solvents | 0 | ||
| Primary hepatic malignancy | Unresectable HCC | 20 | 0.4 |
| Small HCC in cirrhotic liver | 78 | 10.2 | |
| Inborn errors of metabolism | Crigler-Najjar type 1 | 1 | 0.05 |
| Proprionic acidaemia | 6 | 0.08 | |
| Primary oxalosis | 8 | 0.1 | |
| Urea cycle defects | 0 | ||
Chronic liver failure is the most common indication for liver replacement and can be caused by a wide variety of diseases including autoimmune, viral, congenital and alcohol induced liver disease. Primary biliary cirrhosis (PBC) is the commonest indication for liver transplantation in the UK. Several studies on survival in PBC have led to the development of a prognostic index that is helpful in planning the timing of liver replacement[1]. Primary sclerosing cholangitis (PSC) is a condition that is usually found in patients with inflammatory bowel disease. Progression of PSC is less predictable than PBC but approximately 30% of patients with PSC will develop cholangiocarcinoma that is usually incurable at diagnosis. Autoimmune hepatitis (AIH) is less common than PBC and PSC but immunosuppressive therapy can delay progression. Excess immunosuppression prior to transplantation however, may increase the morbidity and mortality associated with liver replacement and the optimal timing of liver replacement is a finely balanced decision.
An estimated 300 million people worldwide carry the hepatitis B virus (HBV). In Western Europe and North America the carrier rate is low (0.5%) and is mainly confined to high-risk groups including intravenous drug users, homosexuals and immigrants from high prevalence areas. HBV is a significant problem however, because of the risks of early recurrence after liver replacement. Patients who are HBV-DNA positive at time of transplant develop rapid recurrence with early death. The results of trials of antiviral therapy using agents such as lamivudine and HBV specific immunoglobulins prior to transplantation suggest that viral replication can be suppressed prior to and post liver replacement with encouraging early results[2-5]. Hepatitis C virus (HCV) is an increasing public health problem. Most patients seen in the UK have become infected following transfusion of blood products or from intravenous drug abuse. The development of cirrhosis following HCV infection is slow but with a significant risk of subsequent hepatocellular carcinoma development[6]. Recurrence of HCV after transplantation is common but not usually problematic in the early years[7,8]. The evolving strategies for anti-viral therapy in this group of patients are likely to have a significant impact on survival in this group of patients[9-11].
Alcoholic liver disease (ALD) is the commonest cause of cirrhosis in many parts of the western world although during the evolution of liver transplantation very few cases were accepted. Many transplant physicians were initially reluctant to consider liver replacement in these patients because of the risks of returning to alcohol and public attitudes[12]. The alcoholic who can prove abstinence prior to grafting has an equivalent survival to those transplanted for other chronic liver disease and recidivism is surprisingly uncommon[13]. There has therefore been an increasing pressure to accept reformed alcoholics and an increasing proportion are now being grafted[14].
The commonest cause of chronic liver failure in children is biliary atresia. If diagnosed early and treated surgically with a portoenterostomy (Kasai operation), the progression of liver disease is delayed and up to 40% of children will survive long term[15]. Many children however, will develop end stage liver disease and die within the first few years of life if not transplanted. Failure to thrive is a common sequelae of chronic liver disease in children and should be considered an indication for grafting.
The development of hepatic encephalopathy within eight weeks of onset of symptoms in a patient without previous liver disease is defined as acute fulminant hepatic failure (AFHF)[16]. Sub-acute or late onset hepatic failure has al so been recognised with encephalopathy developing between eight weeks and six months of onset of symptoms. The commonest causes of AFHF in the UK include drugs and toxins[17-20], viral hepatitis (Hepatitis A, B, and non-A non-B) [21,22] and miscellaneous causes including Wilson's disease, fatty liver of pregnancy and Budd-Chiari syndrome[23-26]. Specific prognostic factors for spontaneous recovery from AFHF have been published and are helpful in decision making about transplantation[27].
An increasing number of inborn errors of metabolism with a deficiency of a single hepatic enzyme are being treated by liver transplantation, even though the liver is otherwise structurally and functionally normal (Table 1). Timing is important and transplantation should be performed before irretrievable damage is done to other organs e.g. renal failure in primary oxalosis or cerebral damage in Crigler-Najjar syndrome[28,29].
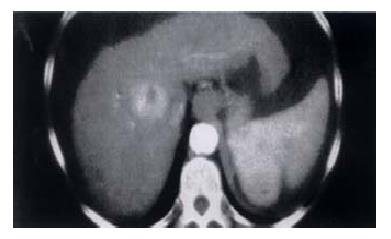
Hepatocellular carcinoma (HCC) is the commonest primary liver malignancy and although it is rare in the UK it is one of the commonest cancers worldwide[30]. The majority of cases occur in the background of liver cirrhosis with the presence of HCV and HBV being additional risk factors (Figure 2). It has been recommended that transplantation be restricted to patients with HCC who have lesions up to 3 cm and up to three in number[31-33]. There are other rare unresectable hepatic tumours that are occasionally conside red for transplantation. These include epithelioid haemangioendothelioma, sarcomata, cholangiocarcinoma and secondary neuroendocrine tumours[34-36]. Hilar cholangiocarcinomas almost invariably recur early after grafting and are no longer considered appropriate candidates[37].
In patients with acute or chronic liver failure timely referral is necessary if a successful outcome is to be achieved[38-40]. Many patients with chronic liver disease can remain stable for long periods and decompensation may occur secondary to a complication such as variceal bleeding, portal vein thrombosis, development of hepatic malignancy or spontaneous bacterial peritonitis. The ability to intervene before any major deterioration is dependent on the recognition of early indicators of disease progression; in cholestatic conditions (PBC and PSC) the level of bilirubin is an obvious indicator of the underlying disease severity and is likely to lead to an early referral for specialist opinion but for many liver conditions the appearance of jaundice is a late feature and other signs of a deterioration in liver synthetic function, such as a falling albumin or rising prothrombin time are a better indicator of the need for referral.
A multi-disciplinary team including hepatologists and transplant surgeons usually assess patients in the UK. A careful review is required to determine the diagnosis of the liver disease and this will include a specialist pathologist at the transplant centre reporting on the liver histology. Often patients who drink moderate amounts of alcohol are labelled as having alcoholic liver disease but an open mind for these cases is encouraged because modest alcohol intake may unmask an underlying liver condition such as alpha-1 anti-trypsin deficiency or haemochromatosis[41,42].
Assessment for transplantation includes both physical fitness for major surgery as well as psychological evaluation and counselling. A detailed evaluation of the cardio-respiratory system is often indicated and this may require ECG, echocardiogram, exercise ECG, coronary angiography and pulmonary artery catheter isation in those with evidence of ischaemic heart disease, pulmonary hypertension or suspected major pulmonary shunts as seen in the hepatopulmonary syndrome[43].
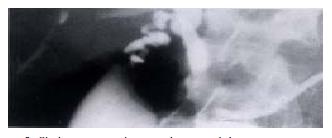
Technical considerations such as patency of the portal vein are also required and this can be determined by Doppler ultrasound, angiography, spiral computerised tomography (CT) or magnetic resonance imaging (MRI). An absent portal vein is not a contraindication to transplantation if a patent superior mesenteric vein or large coronary vein can be identified which would be suitable for anastomosis to the donor portal vein[44]. Patients with primary HCC require detailed investigation for evidence of disease outside the liver and this should include laparoscopy to detect peritoneal disease or transcapsular spread[45], isotope bone scanning and computerised tomographic studies of the abdomen and chest. Difficulty may occur in patients with PSC in trying to differentiate between malignant and benign hilar strictures (Figure 3). In our series malignancy was present in 25% of the patients with significant biliary dilatation but that pre-transplant diagnosis was difficult (unpublished data).
The number of liver transplants performed annually in the UK has remained largely stable over the last five years and this has been despite a slight fall in the number of cadaveric organ donors. The total number of liver transplants has been maintained in the UK by an increase in the number of split liver grafts performed and a wider use of more marginal liver donors. Over the last ten years the introduction of seatbelt laws and stricter drink driving legislation has reduced the number of cadaveric donors being derived from road traffic accident victims. Donor numbers have been largely maintained by utilising older donors who have usually died from cerebro-vascular disease and have concomitant co-morbidity. The use of such marginal donors does not seem to have been at the expense of worse outcomes. Successful outcome from liver transplantation is possible even in haemodynamically unstable donors and in those with abnormal liver function tests[46].
Assessment of the liver by an experienced transplant surgeon at time of retrieval is a useful guide to subsequent function but if there is evidence of fatty change, a frozen section histological assessment prior to implantation can be helpful[47-49]. A fit recipient can often cope with a marginal graft but a poor recipient will need a graft which functions well immediately for the best chance for survival.
Size matching of donor and recipient is attempted when selecting a patient for a particular liver. Attempting to place a large graft in a small recipient can cause major technical problems. Patients with cholestatic diseases such as PSC and particularly PBC often have large livers and will accept grafts from significantly larger donors, as can patients with marked ascites.
Approximately 60% of potentially suitable organ donors (approximately 1000 per year) are missed each year in the UK[50]. UK organ donation rates remain some of the lowest in Europe but a more aggressive approach to the identification and confirmation of brainstem death and improved family requesting could achieve significant improvements in organ donation in the UK[51]. A number of initiatives such as presumed donor consent and elective ventilation are currently being considered[50].
Many factors can be identified which have contributed to the improved early outcome after liver replacement. Semi-elective daytime operating ensures that the surgical and anaesthetic team produce the best technical results. The ability to store livers long enough to allow this came from the development of University of Wisconsin preservation fluid which allows satisfactory immediate graft function for storage periods of eighteen hours or more[52].
Meticulous attention to haemostasis has been aided by developments in surgical techniques and instruments (conventional diathermy, argon beam coagulator, fibrin glue, etc.). The monitoring of coagulation parameters in the operating room with the help of the thromboelastogram (TEG) means that blood coagulation is optimised and that predictable deteriorations in clotting which often occur on reperfusion can be anticipated and minimised[53]. The role of anti-fibrinolytic agents such as aprotinin (Trasyslol) and human recombinant factors (Novoseven) remains unclear but are the subject of clinical study[54,55].
The INTRODUCTION of venovenous bypass for the anhepatic phase produced a significant stabilisation of haemodynamic parameters during portal vein and caval clamping with a clear reduction in transfusion requirements and an improvement in renal function[56]. The alternative to venovenous bypass is to preserve the vena cava at the time of hepatectomy and anastomose the back of the donor vena cava to the front of the recipient cava (piggyback technique)[57]. Several techniques have been described but the piggyback technique is not without its complications[58-62]. Most units currently utilise a combination of techniques and a minority of units still perform liver replacement without either bypass or the piggyback technique.
Early techniques of biliary reconstruction involved utilising the donor gall bladder as a conduit between the donor and recipient duct. This technique has been abandoned because of the almost universal development of stones in the conduit. An end-to-end duct anastomosis is now the routine but this has been followed by stricture formation in up to 13% of cases[63-65] and techniques of anastomosing the ducts obliquely with the ends spatulated or by utilising a side- to-side anastomosis are gaining wider acceptance [66,67]. The use of a T-tube has been abandoned by most units[63-65,68].
The shortfall in size matched grafts for small children led to the development of reduced grafts in the mid 1980′s[69-71]. The most commonly used technique is to transplant the left lateral lobe segments II and III with venous outflow based on the left hepatic vein which is anastomosed to the retained recipient vena cava[72]. Weight ratios as high as ten-to-one between donor and recipient have been reported[73] the ideal weight ratio however, is four, five or six to one. Reduced liver grafts are not without their comp lications[74] but there appears to be a low incidence of hepatic artery thrombosis[75,76]. The INTRODUCTION of this technique has led to a significant reduction in mortality from liver disease in children[77]. The techniques of graft reduction have led to the development of splitting livers where the left lobe (or left lateral segments) is transplanted into a paediatric recipient and the right lobe is grafted usually into an adult recipient (split-liver)[78-80]. This technique was de veloped on the backbench following removal of the cadaveric donor organ. The pro cedure can take approximately two hours and during this time the donor organ is subject to some re -warming that might be detrimental to its initial function. Recently the technique of in situ splitting of cadaveric donor organs has been developed as an extension of the development of living related liver transplantation. The advantage of this technique is that the splitting of the liver is performed during the warm phase dissection prior to organ perfusion and cooling and the organ is the n not subject to re-warming during a subsequent splitting procedure. The results of this technique appear to result in better initial graft function[81-83].
In countries that do not have legal recognition of brainstem death and therefore have no access to cadaveric organs, solid organ transplantation has been limited to living related organ donation and this has led to the development of living related liver transplantation[84]. The increasing donor organ shortfall with the increasing number of potential recipients; despite the option of organ splitting, has meant that even in countries that do recognise brainstem death living related liver transplantation has had to be undertaken[85-88]. The organ shortfall in the UK for patients with liver disease is less than in other countries and the number of units performing this procedure is small with only 12 being performed in 1999[89,90]. The greatest experience with this technique has been with adult-to-child left lateral lobe because of the obvious size discrepancy and donor to recipient weight ratios[91] but increasing experience of the technique has led to the expansion of the technique to include adult-to-adult donation[92-96]. The increasing demand for liver transplantation in the UK and the reduction in cadaveric donor organs [90] suggest that this technique is likely to become established practice but careful preoperative evaluation of the donor is needed[97-100].
The one-year survival following liver transplantation has improved from approximately 30% in the 1960s and 1970s to more than 80% in the 1990s[14,101,102]. The immediate complications following liver transplantation include primary non-function, haemorrhage and acute renal failure. The incidence of these is significantly influenced by the quality of the donor liver and technical aspects of the transplant operation itself. Over the last 10 years in the UK there has been an increase in the use of marginal organs[46] but this has been offset by improvements in technical aspects of the surgical procedure, per-operative anaesthetic management and post-operative intensive care management. In our own unit the incidence of these complications between 1985 to 1989 and 1995 to 1999 was; primary non-function 1.9% and 1.7%, return to theatre for pack removal or haemorrhage 8.4% and 2.4% and post-operative renal failure 18.6% and 16.4% respectively (unpublished data). Despite the use of an increasingly marginal donor pool the incidence of these complications hastherefore reduced.
Primary non-function may be due to pre-existing but occult problems in the donor, poor retrieval or preservation, or injury caused by reperfusion (post-reperfusion syndrome). The clinical picture mimics acute fulminant hepatic failure and death rapidly follow unless urgent regrafting can be undertaken. Fortunately primary non-function is rare although primary dysfunction occurs in 5% to 10% of cases and is associated with a worse long-term outcome[103,104].
The majority of routine liver transplants require minimal or no transfused blood. In our own series 47% of liver transplants required four units or less of blood per-operatively (unpublished data). Patients with severe portal hypertension and previous major upper abdominal operations can pose a major surgical challenge, meticulous haemostasis, venovenous bypass, warming of blood and blood products and strict control of coagulation parameters will usually be effective.
A significant number of transplant candidates already have impaired renal function and a combination of factors lead to a rise in the serum creatinine after surgery[105-107]. This will usually respond to optimisation of hydration and pharmacological manipulation but a proportion of patients will develop anuria and require renal replacement therapy at least in the short term[108].
Histological evidence of acute rejection can be documented in approximately 80% of liver grafts at the end of the first week but many of these do not require additional immunosuppression if other parameters of graft function are improving[109]. Histological evidence of severe cellular rejection and less severe histological forms associated with significant biochemical abnormalities (approximately 30% of liver grafts) are usually treated with high dose steroids[110,111]. Steroid resistant rejection may respond to other agents including monoclonal (OKT3) and polyclonal antibodies (ATG) or by switching immunosuppression regimes[112,113]. Chronic or irreversible rejection in the liver is a biliary rather than a vascular phenomenon in which the small bile radicals are destroyed[114,115]. This can occur very early on after grafting and if progressive leads to loss of the graft although predicting which patients might require regrafting can be difficult[116,117]. Chronic rejection accounts for approximately 5% of graft loss within the first three to five years following transplantation [118]. Lower rates of chronic rejection and graft salvage in early chronic rejection may occur with newer immunosuppressive regimes[119-121]. Histological examination of the transplanted liver in stable long-term patients often shows evidence of chronic post-transplant hepatitis[122]. The causes of the histological changes are unknown although unrecognised viral infections may be responsible for some cases and the steroid sparing immunosuppression regimes may also be partly responsible.
Serious cytomegalovirus (CMV) infections tend to be primary (transmitted by the donor liver) rather than reactivation infections and should be avoidable if CMV -matched donors are used. Clinical infection usually presents between four and eight weeks with fever and leucopenia but asymptomatic sero-conversion does not require treatment. This will respond well to a combination of reduction in base line immunosuppression and ganciclovir therapy[123]. The traditional serological tests vary between centres, take time and are less sensitive than PCR tests[124]. In patients with symptoms specific to an organ histological analysis should be used in conjunction with PCR tests[125,126]. Significant CMV infection is associated with acute rejection and may result in a worse long-term outcome[127]. The routine use of prophylactic ganciclovir reduces the incidence of clinical CMV infection although a high index of suspicion and prompt treatment will also result in negligible mortality[128-132].
Biliary complications are a significant problem in most units undertaking liver transplantation and these include bile leaks, anastomotic strictures, non-anastomotic strictures of the donor bile duct and sludge formation. The overall incidence in adults is approximately 10% but is higher in children[74,133]. In our own series the overall incidence of biliary complications requiring intervention is 12%, this rises to 27% in those patients undergoing re-transplantation (unpublished data). The ability to image the biliary tree effectively using ultrasound, MRI cholangiography, endoscopic retrograde cholangiography (ERCP) or percutaneous transhepatic cholangiography (PTC) has led to most biliary complications being managed without reoperation[134]. The presence of a major biliary disruption or an associated biliary obstruction is an indication for urgent biliary reconstruction[135]. Biliary obstruction without leakage will usually be evident from simple ultrasound, can be confirmed by ERCP or PTC and can usually be managed without recourse to open surgery[136-138]. Non-anastomotic biliary strictures involving the confluence or intra -hepatic bile ducts are a rare but serious complication that were once attributed to prolonged preservation times[139]. These strictures are complicated but a proportion can be resolved using a PTC approach by a skilled radiologist although a number of cases will require regrafting. In any patient with a biliary complication patency of the hepatic artery should be confirmed, as hepatic artery thrombosis will cause ischaemia and necrosis of the biliary tree[140]. The late biliary complications seen after transplantation are usually obstruction with possible secondary sepsis and cholangitis. The commonest cause is an anastomotic stricture, with or without stone or sludge formation in the proximal dilated biliary tree. An ERCP may enable duct clearance, dilatation of any stricture and stent insertion. Most strictures will recur and therefore formal biliary reconstruction is usually required.
Hepatic arterial thrombosis (HAT) after liver transplantation occurs most frequently in the first postoperative month and leads to graft necrosis, intra-hepatic abscess or biliary necrosis and bile leakage. In all suspected cases patency of the artery should be checked with Doppler ultrasound and confirmed with spiral CT or angiography[141-143]. Per-cutaneous attempts at revascularizati on of stenosed or thrombosed hepatic arteries can be attempted and urgent thrombectomy has been successful in some cases but the majority of cases of early HAT will need regrafting[144-148]. Late arterial thrombosis may be occult and if asymptomatic can probably be ignored. In our own series HAT has occurred in 4.6% of adult grafts and 9.1% of paediatric grafts (unpublished data). Technical problems account for the majority of cases but over transfusion at the time of surgery, producing a high a haematocrit, has been reported as a risk factor[149,150].
Malignancy is well recognised as a potential complication of long term immunosup pression. Longer survival is seen with the liver compared to other solid organ transplants and therefore the time exposed to the risk of malignancy is greater. The most common malignancies seen secondary to prolonged immunosuppression are the lymphoproliferative diseases and lymphoma and skin malignancy[151,152]. Reduction in the level of immunosuppression is often enough to treat lymphoproliferative disease[153]. A proportion of liver transplants are performed for primary hepatic malignancy and paradoxically the donor liver (free from malignancy at the time of transplant) is the commonest site of recurrence. The predilection for circulating malignant cells to return and then grow in the liver is well recognised.
The widespread INTRODUCTION of cylosporine A in the early 1980s was responsible for the improvement in liver graft survival from 35% to 70% survival at one-year[154]. Immunosuppression with cylosporine, azathioprine and steroids remained the main immunosuppressive regimen until the development of tacrolimus in 1989[155]. Tacrolimus was initially used to salvage grafts failing from rejection on cylosporine based regimens[156] but has subsequently been increasingly used as first line immunosuppression by many units. Although structurally different to cylosporine, it also acts by inhibiting calcineurin and subsequent interleukin (IL) 2 production and therefore prevents T cell proli feration[157]. Three prospective randomised trials have compared the efficacy of tacrolimus and cylosporine in liver transplant recipients[158-160]. The incidence of rejection was significantly lower with tacrolimus in all studies but there was no difference in one-year patient and graft survival. Long-term follow up has shown a trend towards en hanced survival in patients treated with tacrolimus[161]. The toxicity profile of tacrolimus is similar to that of cylosporine (nephrotoxicity, neurotoxicity, hypertension and diabetogenic potential) but without the gingival hyperplasia and hirsutism commonly seen with cylosporine[162].
Mycophenolate mofetil (MMF) is another new agent that blocks purine metabolism by inhibiting inosine monophosphate dehydrogenase in T and B lymphocytes[163]. The role of MMF in liver transplant recipients remains to be fully defined but initial reports suggest that when combined with tacrolimus the incidence of acute rejection is reduced[164,165]. MMF has haematological and gastrointestinal side effects but is not nephrotoxic and may be useful in patients with compromised renal function so that the dose of tacrolimus can be reduced[166]. New immunosuppressants continue to be developed and some are currently under evaluation including sirolimus (inhibits action of IL2), basiliximab (chimeric IL2 receptor monoclonal antibody) and daclizumab (humanised IL2 receptor monclonal antibody)[167,168]. Polyclonal antibody therapy that has previously been used to treat steroid resistant rejection has however, been rendered almost obsolete by current immunosuppressant protocols. In our own centre the current immunosuppression regimen is tacrolimus combined with azathioprine and prednisolone, with steroid taper and withdrawal over three months. MMF is used in place of azathioprine to allow low dose tacrolimus regime ns in those patients with renal impairment prior to transplantation and is also used in place of azathioprine in those patients undergoing retransplantation for chronic rejection.
The available immunosuppressive options will continue to increase and with it the permutations of immunosuppressive regimens. This may make it difficult to effectively evaluate individual regimens. Immunosuppression will however, continue to be a balancing act, with over immunosuppression culminating in toxicity, life threatening infections and malignancy and under immunosuppression leading to rejection and graft loss.
In our own series 10% of nearly 2000 liver transplants were regrafts, although the proportion of patients requiring a regraft is decreasing[169]. HAT accounts for 30% of regrafts, primary non-function for 16%, chronic rejection for 31% and recurrent disease for 6%, although the incidence of HAT and primary non-function is decreasing and the incidence of recurrent disease (PSC and HCV) is increasing[169]. Early re-transplantation is technically straight forward and usually performed for HAT or primary non-function. In an era of donor shortage and donor/recipient number mismatch the role of re-transplantation has been questioned but the outcome of re- transplantation is good with survival rates only slightly worse than those achieved for the first graft[170].
One-year survival rates for elective liver transplant in patients with benign disease now exceed 90% in many centres, with predicted 10 year survival rates expected to exceed 70%[102,171]. Patients transplanted for AFHF have a worse one-year survival with higher post-operative death rates usually related to cerebral complications and multi-organ failure. Experienced centres have however, obtained one-year survival rates of approximately 70%[172-174]. The long-term outcome for patients undergoing liver transplant for AFHF is as good as those transplanted for chronic disease. The increasing interest in living related transplantation offers a new opportunity for those patients with AFHF who cannot wait for a cadaveric organ[175]. The outcome in children undergoing liver transplantation is equally good, even in high-risk groups such as children age under 1 year in whom donor organ shortage might prevent grafting at the optimal time[75].
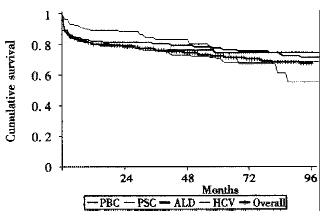
Survival rates for patients grafted for primary liver cancer (HCC) are less good however, patients transplanted for asymptomatic lesions up to 3 cm in diameter have survival rates close to those seen in patients grafted for benign disease[32]. In our own unit overall survival (including fulminant hepatic failure) at one-year is 81% for adults and 86% for children with different long -term survival depending on disease type (Figure 4).
The most serious issue currently facing liver transplant physicians is the short fall in donor organs needed to meet demand. This deficit is greater in the US than in the UK. If the UK could increase its rates of organ donation to levels seen in other European centres and split all livers that meet appropriate criteri a (approximately 25% of UK cadaveric organs) then current organ demand could be met. Patient demand will however, mean that increasingly transplant physicians will be asked to justify why certain categories of patient are not considered suitable for transplantation. The limited supply of cadaveric organs allows these physicians to justify transplantation criteria on the basis of the scarcity of this resource. The continued success of living related liver transplant programmes around the world is likely to lead to increasing pressure to relax the criteria for liver transplantation for those patients able to provide their ‘own’ source of suitable transplant organs. This will require strict control and the application of new National guidelines if the UK is to avoid an expensive and potentially dangerous situation in the application of universal standards of care. A successful UK living related programme would certainly help to ease the deficit in urgent organs for those with AFHF and could address the deficit that currently exists for liver transplantation in chronic liver disease but we believe that this should only occur after the UK has exhausted the potential that is currently untapped in potential cadaveric organs.
The use of genetically modified xenografts could be potential major breakthrough for organ recipients but is not easily applicable to liver failure patients and there remain many biological and ethical obstacles before these organs become a sustainable source[176].
| 1. | Hughes MD, Raskino CL, Pocock SJ, Biagini MR, Burroughs AK. Prediction of short-term survival with an application in primary biliary cirrhosis. Stat Med. 1992;11:1731-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Markowitz JS, Martin P, Conrad AJ, Markmann JF, Seu P, Yersiz H, Goss JA, Schmidt P, Pakrasi A, Artinian L. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology. 1998;28:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 375] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Mutimer D, Pillay D, Shields P, Cane P, Ratcliffe D, Martin B, Buchan S, Boxall L, O'Donnell K, Shaw J. Outcome of lamivudine resistant hepatitis B virus infection in the liver transplant recipient. Gut. 2000;46:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Nery JR, Weppler D, Rodriguez M, Ruiz P, Schiff ER, Tzakis AG. Efficacy of lamivudine in controlling hepatitis B virus recurrence after liver transplantation. Transplantation. 1998;65:1615-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Perrillo R, Rakela J, Dienstag J, Levy G, Martin P, Wright T, Caldwell S, Schiff E, Gish R, Villeneuve JP. Multicenter study of lamivudine therapy for hepatitis B after liver transplantation. Lamivudine Transplant Group. Hepatology. 1999;29:1581-1586. [PubMed] |
| 6. | Deuffic S, Poynard T, Valleron AJ. Correlation between hepatitis C virus prevalence and hepatocellular carcinoma mortality in Europe. J Viral Hepat. 1999;6:411-413. [PubMed] [DOI] [Full Text] |
| 7. | Samuel D, Feray C. Recurrence of hepatitis C virus infection after liver transplantation. J Hepatol. 1999;31 Suppl 1:217-221. [PubMed] |
| 8. | Testa G, Crippin JS, Netto GJ, Goldstein RM, Jennings LW, Brkic BS, Brooks BK, Levy MF, Gonwa TA, Klintmalm GB. Liver transplantation for hepatitis C: recurrence and disease progression in 300 patients. Liver Transpl. 2000;6:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 138] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Lavezzo B, Rizzetto M. Treatment of recurrent hepatitis C virus infection after liver transplantation. J Hepatol. 1999;31 Suppl 1:222-226. [PubMed] |
| 10. | Ben-Ari Z, Mor E, Shaharabani E, Bar-Nathan N, Shapira Z, Tur-Kaspa R. Combination of interferon-alpha and ribavirin therapy for recurrent hepatitis C virus infection after liver transplantation. Transplant Proc. 2000;32:714-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Muramatsu S, Ku Y, Fukumoto T, Iwasaki T, Tominaga M, Kusunoki N, Yoon S, Kuroda Y. Successful rescue of severe recurrent hepatitis C with interferon and ribavirin in a liver transplant patient. Transplantation. 2000;69:1956-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Walsh K, Alexander G. Alcoholic liver disease. Postgrad Med J. 2000;76:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Pereira SP, Howard LM, Muiesan P, Rela M, Heaton N, Williams R. Quality of life after liver transplantation for alcoholic liver disease. Liver Transpl. 2000;6:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Hartley P, Petruckevitch A, Reeves B, Rolles K. The National Liver Transplantation audit: an overview of patients presenting for liver transplantation from 1994 to 1998. On behalf of the Steering Group of the UK Liver Transplantation Audit. Br J Surg. 2001;88:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Subramaniam R, Doig CM, Bowen J, Bruce J. Initial response to portoenterostomy determines long-term outcome in patients with biliary atresia. J Pediatr Surg. 2000;35:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Trey C, Davison CS. The management of fulminat hepatic failure. Progress in Liver Disease. New York: Grune and Stratton 1970; 282-298. |
| 17. | Makin A, Williams R. The current management of paracetamol overdosage. Br J Clin Pract. 1994;48:144-148. [PubMed] |
| 18. | Jones AL, Simpson KJ. Review article: mechanisms and management of hepatotoxicity in ecstasy (MDMA) and amphetamine intoxications. Aliment Pharmacol Ther. 1999;13:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Neuberger JM. Halothane and hepatitis. Incidence, predisposing factors and exposure guidelines. Drug Saf. 1990;5:28-38. [PubMed] |
| 20. | Vasudeva R, Woods B. Isoniazid-related hepatitis. Dig Dis. 1997;15:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Simmonds P, Davidson F, Lycett C, Prescott LE, MacDonald DM, Ellender J, Yap PL, Ludlam CA, Haydon GH, Gillon J. Detection of a novel DNA virus (TTV) in blood donors and blood products. Lancet. 1998;352:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 282] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Fagan EA, Harrison TJ. Exclusion in liver by polymerase chain reaction of hepatitis B and C viruses in acute liver failure attributed to sporadic non-A, non-B hepatitis. J Hepatol. 1994;21:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Smith SL, Ciferni M. Liver transplantation for acute hepatic failure: a review of clinical experience and management. Am J Crit Care. 1993;2:137-144. [PubMed] |
| 24. | Lidofsky SD. Liver transplantation for fulminant hepatic failure. Gastroenterol Clin North Am. 1993;22:257-269. [PubMed] |
| 25. | Kirsh BM, Lam N, Layden TJ, Wiley TE. Diagnosis and management of fulminant hepatic failure. Compr Ther. 1995;21:166-171. [PubMed] |
| 26. | Detre K, Belle S, Beringer K, Daily OP. Liver transplantation for fulminant hepatic failure in the United States: October 1987 through December 1991. Clin Transplant. 1994;8:274-280. [PubMed] |
| 27. | O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [PubMed] |
| 28. | Nolkemper D, Kemper MJ, Burdelski M, Vaismann I, Rogiers X, Broelsch CE, Ganschow R, Müller-Wiefel DE. Long-term results of pre-emptive liver transplantation in primary hyperoxaluria type 1. Pediatr Transplant. 2000;4:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Jansen PL. Diagnosis and management of Crigler-Najjar syndrome. Eur J Pediatr. 1999;158 Suppl 2:S89-S94. [PubMed] |
| 30. | Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-94. Lancet. 1997;350:1142-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 369] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 31. | Ismail T, Angrisani L, Gunson BK, Hübscher SG, Buckels JA, Neuberger JM, Elias E, McMaster P. Primary hepatic malignancy: the role of liver transplantation. Br J Surg. 1990;77:983-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 632] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 33. | Dalgic A, Mirza DF, Gunson BK, D'Silva M, Mayer AD, Buckels JA, McMaster P. Role of total hepatectomy and transplantation in hepatocellular carcinoma. Transplant Proc. 1994;26:3564-3565. [PubMed] |
| 34. | Strong RW. Transplantation for liver and biliary cancer. Semin Surg Oncol. 2000;19:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Strong RW. Transplantation for liver and biliary cancer. Semin Surg Oncol. 2000;19:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Clements D, Hubscher S, West R, Elias E, McMaster P. Epithelioid haemangioendothelioma. A case report. J Hepatol. 1986;2:441-449. [PubMed] |
| 37. | Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 353] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 38. | Donovan JP, Zetterman RK, Burnett DA, Sorrell MF. Preoperative evaluation, preparation, and timing of orthotopic liver transplantation in the adult. Semin Liver Dis. 1989;9:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Rosen HR, Shackleton CR, Martin P. Indications for and timing of liver transplantation. Med Clin North Am. 1996;80:1069-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Jansen PL. New criteria for liver transplantation in adults: the combined Groningen and Rotterdam protocol. Neth J Med. 1998;52:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Fletcher LM, Halliday JW, Powell LW. Interrelationships of alcohol and iron in liver disease with particular reference to the iron-binding proteins, ferritin and transferrin. J Gastroenterol Hepatol. 1999;14:202-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | George DK, Powell LW, Losowsky MS. The haemochromatosis gene: a co-factor for chronic liver diseases问号. J Gastroenterol Hepatol. 1999;14:745-749. [PubMed] |
| 43. | Kuo P. Pulmonary hypertension: considerations in the liver transplant candidate. Transpl Int. 1996;9:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Kirsch JP, Howard TK, Klintmalm GB, Husberg BS, Goldstein RM. Problematic vascular reconstruction in liver transplantation. Part II. Portovenous conduits. Surgery. 1990;107:544-548. [PubMed] |
| 45. | Dalgic A, Mirza DF, Gunson BK, Mutimer DJ, Mayer AD, Buckels JA, McMaster P. Pretransplant investigations of primary liver tumours with minimal access surgery. Transplant Proc. 1994;26:3566-3567. [PubMed] |
| 46. | Mirza DF, Gunson BK, Da Silva RF, Mayer AD, Buckels JA, McMaster P. Policies in Europe on "marginal quality" donor livers. Lancet. 1994;344:1480-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Crowley H, Lewis WD, Gordon F, Jenkins R, Khettry U. Steatosis in donor and transplant liver biopsies. Hum Pathol. 2000;31:1209-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Urena MA, Moreno Gonzalez E, Romero CJ, Ruiz-Delgado FC, Moreno Sanz C. An approach to the rational use of steatotic donor livers in liver transplantation. Hepatogastroenterology. 1999;46:1164-1173. [PubMed] |
| 49. | Yoong KF, Gunson BK, Neil DA, Mirza DF, Mayer AD, Buckels JA, McMaster P. Impact of donor liver microvesicular steatosis on the outcome of liver retransplantation. Transplant Proc. 1999;31:550-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | BMA Medical Ethics Committee. Organ donation in the 21st century: time for a consolidated approach. London: BMA; 2000; . |
| 51. | Gore SM, Cable DJ, Holland AJ. Organ donation from intensive care units in England and Wales: two year confidential audit of deaths in intensive care. BMJ. 1992;304:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Padbury RT, Attard A, Mirza DF, Olliff S, Gunson BK, Mayer AD, Buckels JA, McMaster P. Extended preservation of the liver with UW solution--is it justifiable问号. Transplantation. 1994;57:1490-1493. [PubMed] |
| 53. | Spiess BD, Tuman KJ, McCarthy RJ, DeLaria GA, Schillo R, Ivankovich AD. Thromboelastography as an indicator of post-cardiopulmonary bypass coagulopathies. J Clin Monit. 1987;3:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 179] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | García-Huete L, Domenech P, Sabaté A, Martínez-Brotons F, Jaurrieta E, Figueras J. The prophylactic effect of aprotinin on intraoperative bleeding in liver transplantation: a randomized clinical study. Hepatology. 1997;26:1143-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Porte RJ, Molenaar IQ, Begliomini B, Groenland TH, Januszkiewicz A, Lindgren L, Palareti G, Hermans J, Terpstra OT. Aprotinin and transfusion requirements in orthotopic liver transplantation: a multicentre randomised double-blind study. EMSALT Study Group. Lancet. 2000;355:1303-1309. [PubMed] |
| 56. | Shaw BW, Martin DJ, Marquez JM, Kang YG, Bugbee AC, Iwatsuki S, Griffith BP, Hardesty RL, Bahnson HT, Starzl TE. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 356] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 57. | Belghiti J, Panis Y, Sauvanet A, Gayet B, Fékété F. A new technique of side to side caval anastomosis during orthotopic hepatic transplantation without inferior vena caval occlusion. Surg Gynecol Obstet. 1992;175:270-272. [PubMed] |
| 58. | Hesse UJ, Defreyne L, Pattyn P, Kerremans I, Berrevoet F, de Hemptinne B. Hepato-venous outflow complications following orthotopic liver transplantation with various techniques for hepato-venous reconstruction in adults and children. Transpl Int. 1996;9 Suppl 1:S182-S184. [PubMed] |
| 59. | Lerut J, Gertsch P. Side-to-side cavo-cavostomy: a useful aid in "complicated" piggy-back liver transplantation. Transpl Int. 1993;6:299-301. [PubMed] |
| 60. | Meunier B, Bardaxoglou E, Chareton B, Landen S, Camus C, Roumeas J, Launois B. ["Piggyback" method in hepatic transplantation]. Chirurgie. 1993;119:682-685. [PubMed] |
| 61. | Jovine E, Mazziotti A, Grazi GL, Ercolani G, Masetti M, Morganti M, Pierangeli F, Begliomini B, Mazzetti PG, Rossi R. Piggy-back versus conventional technique in liver transplantation: report of a randomized trial. Transpl Int. 1997;10:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | Lerut JP, Molle G, Donataccio M, De Kock M, Ciccarelli O, Laterre PF, Van Leeuw V, Bourlier P, de Ville de Goyet J, Reding R. Cavocaval liver transplantation without venovenous bypass and without temporary portocaval shunting: the ideal technique for adult liver grafting问号. Transpl Int. 1997;10:171-179. [PubMed] |
| 63. | Verran DJ, Asfar SK, Ghent CN, Grant DR, Wall WJ. Biliary reconstruction without T tubes or stents in liver transplantation: report of 502 consecutive cases. Liver Transpl Surg. 1997;3:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Ben-Ari Z, Neville L, Davidson B, Rolles K, Burroughs AK. Infection rates with and without T-tube splintage of common bile duct anastomosis in liver transplantation. Transpl Int. 1998;11:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 65. | Rolles K, Dawson K, Novell R, Hayter B, Davidson B, Burroughs A. Biliary anastomosis after liver transplantation does not benefit from T tube splintage. Transplantation. 1994;57:402-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Davidson BR, Rai R, Kurzawinski TR, Selves L, Farouk M, Dooley JS, Burroughs AK, Rolles K. Prospective randomized trial of end-to-end versus side-to-side biliary reconstruction after orthotopic liver transplantation. Br J Surg. 1999;86:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Keck H, Langrehr JM, Knoop M, Lohmann R, Bechstein WO, Blumhardt G, Neuhaus P. Reconstruction of bile duct using the side-to-side anastomosis in 389 orthotopic liver transplantations. Transplant Proc. 1995;27:1250-1251. [PubMed] |
| 68. | Vougas V, Rela M, Gane E, Muiesan P, Melendez HV, Williams R, Heaton ND. A prospective randomised trial of bile duct reconstruction at liver transplantation: T tube or no T tube问号. Transpl Int. 1996;9:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Buckels JA. Paediatric liver transplantation: review of current experience. J Inherit Metab Dis. 1991;14:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Tan KC, Malcolm GP, Reece AS, Calne RY. Surgical anatomy of donor extended right trisegmentectomy before orthotopic liver transplantation in children. Br J Surg. 1991;78:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 71. | Emond JC, Whitington PF, Thistlethwaite JR, Alonso EM, Broelsch CE. Reduced-size orthotopic liver transplantation: use in the management of children with chronic liver disease. Hepatology. 1989;10:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Strong R, Ong TH, Pillay P, Wall D, Balderson G, Lynch S. A new method of segmental orthotopic liver transplantation in children. Surgery. 1988;104:104-107. [PubMed] |
| 73. | Srinivasan P, Vilca-Melendez H, Muiesan P, Prachalias A, Heaton ND, Rela M. Liver transplantation with monosegments. Surgery. 1999;126:10-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Chardot C, Candinas D, Mirza D, Gunson B, Davison S, Murphy MS, Kelly D, John P, McMaster P, Mayer D. Biliary complications after paediatric liver transplantation: Birmingham's experience. Transpl Int. 1995;8:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 75. | Beath SV, Brook GD, Kelly DA, Cash AJ, McMaster P, Mayer AD, Buckels JA. Successful liver transplantation in babies under 1 year. BMJ. 1993;307:825-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Stevens LH, Emond JC, Piper JB, Heffron TG, Thistlethwaite JR, Whitington PF, Broelsch CE. Hepatic artery thrombosis in infants. A comparison of whole livers, reduced-size grafts, and grafts from living-related donors. Transplantation. 1992;53:396-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 110] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | de Ville de Goyet J, Hausleithner V, Reding R, Lerut J, Janssen M, Otte JB. Impact of innovative techniques on the waiting list and results in pediatric liver transplantation. Transplantation. 1993;56:1130-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Kazemier G, Hesselink EJ, Lange JF, Terpstra OT. Dividing the liver for the purpose of split grafting or living related grafting: a search for the best cutting plane. Transplant Proc. 1991;23:1545-1546. [PubMed] |
| 79. | Broelsch CE, Emond JC, Whitington PF, Thistlethwaite JR, Baker AL, Lichtor JL. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg. 1990;212:368-75; discussion 375-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 426] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 80. | Mirza DF, Achilleos O, Pirenne J, Buckels JA, McMaster P, Mayer AD. Encouraging results of split-liver transplantation. Br J Surg. 1998;85:494-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Reyes J, Gerber D, Mazariegos GV, Casavilla A, Sindhi R, Bueno J, Madariaga J, Fung JJ. Split-liver transplantation: a comparison of ex vivo and in situ techniques. J Pediatr Surg. 2000;35:283-29; discussion 283-29;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Goss JA, Yersiz H, Shackleton CR, Seu P, Smith CV, Markowitz JS, Farmer DG, Ghobrial RM, Markmann JF, Arnaout WS. In situ splitting of the cadaveric liver for transplantation. Transplantation. 1997;64:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 136] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 83. | Olausson M, Bäckman L, Friman S, Mjörnstedt L, Krantz M, Broelsch CE, Rogiers X. In situ split liver procedures in cadaver and living-related donors. Transplant Proc. 1997;29:3094-3095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Makuuchi M, Kawarazaki H, Iwanaka T, Kamada N, Takayama T, Kumon M. Living related liver transplantation. Surg Today. 1992;22:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Chen CL, Chen YS, Liu PP, Chiang YC, Cheng YF, Huang TL, Eng HL. Living related donor liver transplantation. J Gastroenterol Hepatol. 1997;12:S342-S345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Otte JB, Reding R, de Ville de Goyet J, Sokal E, Lerut J, Janssen M, Rosati R, Hayez JY, Libert F, Paul K. Experience with living related liver transplantation in 63 children. Acta Gastroenterol Belg. 1999;62:355-362. [PubMed] |
| 87. | Chen CL, Chen YS, Chiang YC, Cheng YF, Huang TL, Eng HL. Paediatric liver transplantation: a 10 year experience in Taiwan. J Gastroenterol Hepatol. 1996;11:S1-S3. [PubMed] [DOI] [Full Text] |
| 88. | Rogiers X, Broering DC, Mueller L, Burdelski M. Living-donor liver transplantation in children. Langenbecks Arch Surg. 1999;384:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Rogiers X; UNOS. U.S. Facts About Transplantation. In: UNOS; 1999; Available from: http://www.unos.org/Newsroom/critdata main.htm.. |
| 90. | Rogiers X; UKTSSA. Transplant Update. In: UKTSSA; 2000; Available from: http ://www.uktransplant.org.uk/b2a.asp.. |
| 91. | Tanaka K, Uemoto S, Tokunaga Y, Fujita S, Sano K, Nishizawa T, Sawada H, Shirahase I, Kim HJ, Yamaoka Y. Surgical techniques and innovations in living related liver transplantation. Ann Surg. 1993;217:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 436] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 92. | Hashikura Y, Makuuchi M, Kawasaki S, Matsunami H, Ikegami T, Nakazawa Y, Kiyosawa K, Ichida T. Successful living-related partial liver transplantation to an adult patient. Lancet. 1994;343:1233-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 280] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 93. | Ichida T, Matsunami H, Kawasaki S, Makuuchi M, Harada T, Itoh S, Asakura H. Living related-donor liver transplantation from adult to adult for primary biliary cirrhosis. Ann Intern Med. 1995;122:275-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Kato T, Nery JR, Morcos JJ, Gyamfi AR, Ruiz P, Molina EG, Tzakis AG. Successful living related liver transplantation in an adult with fulminant hepatic failure. Transplantation. 1997;64:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Kawasaki S, Makuuchi M, Matsunami H, Hashikura Y, Ikegami T, Nakazawa Y, Chisuwa H, Terada M, Miyagawa S. Living related liver transplantation in adults. Ann Surg. 1998;227:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 317] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 96. | Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg. 2000;231:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 159] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 97. | Morimoto T, Ichimiya M, Tanaka A, Ikai I, Yamamoto Y, Nakamura Y, Takada Y, Inomata Y, Honda K, Inamoto T. Guidelines for donor selection and an overview of the donor operation in living related liver transplantation. Transpl Int. 1996;9:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 98. | Sterneck MR, Fischer L, Nischwitz U, Burdelski M, Kjer S, Latta A, Malago M, Petersen J, Pothmann W, Rogiers X. Selection of the living liver donor. Transplantation. 1995;60:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Renz JF, Mudge CL, Heyman MB, Tomlanovich S, Kingsford RP, Moore BJ, Snyder JD, Perr HA, Paschal AL, Roberts JP. Donor selection limits use of living-related liver transplantation. Hepatology. 1995;22:1122-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 100. | Baker A, Dhawan A, Devlin J, Mieli-Vergani G, O'Grady J, Williams R, Rela M, Heaton N. Assessment of potential donors for living related liver transplantation. Br J Surg. 1999;86:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Starzl TE, Porter KA, Putnam CW, Schroter GP, Halgrimson CG, Weil R, Hoelscher M, Reid HA. Orthotopic liver transplantation in ninety-three patients. Surg Gynecol Obstet. 1976;142:487-505. [PubMed] |
| 102. | Starzl TE; UKTSSA. One Year Survival By Centre. In: UKTSSA; 1985- 1995; Available from: http;//www.uktransplant.org.uk/pdf/liver/30to31livaud.pdf.. |
| 103. | Pokorny H, Gruenberger T, Soliman T, Rockenschaub S, Längle F, Steininger R. Organ survival after primary dysfunction of liver grafts in clinical orthotopic liver transplantation. Transpl Int. 2000;13 Suppl 1:S154-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Alvares-da-Silva MR, Waechter FL, Francisconi CF, Barros E, Thomé F, Traiber C, Fonseca DL, Zingani JM, Sampaio JA, Pinto RD. Risk factors for postoperative acute renal failure at a new orthotopic liver transplantation program. Transplant Proc. 1999;31:3050-3052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 105. | Bilbao I, Charco R, Balsells J, Lazaro JL, Hidalgo E, Llopart L, Murio E, Margarit C. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin Transplant. 1998;12:123-129. [PubMed] |
| 106. | Bilbao I, Charco R, Balsells J, Lazaro JL, Hidalgo E, Llopart L, Murio E, Margarit C. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin Transplant. 1998;12:123-129. [PubMed] |
| 107. | Van Roey G, Moore K. The hepatorenal syndrome. Pediatr Nephrol. 1996;10:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 108. | Fisher NC, Nightingale PG, Gunson BK, Lipkin GW, Neuberger JM. Chronic renal failure following liver transplantation: a retrospective analysis. Transplantation. 1998;66:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 248] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 109. | Dousset B, Hubscher SG, Padbury RT, Gunson BK, Buckels JA, Mayer AD, Elias E, McMaster P, Neuberger JM. Acute liver allograft rejection--is treatment always necessary问号. Transplantation. 1993;55:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Neuberger J. Incidence, timing, and risk factors for acute and chronic rejection. Liver Transpl Surg. 1999;5:S30-S36. [PubMed] |
| 111. | Demetris A, Adams D, Bellamy C, Blakolmer K, Clouston A, Dhillon AP, Fung J, Gouw A, Gustafsson B, Haga H. Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology. 2000;31:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 362] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 112. | Berard JL, Velez RL, Freeman RB, Tsunoda SM. A review of interleukin-2 receptor antagonists in solid organ transplantation. Pharmacotherapy. 1999;19:1127-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 113. | Brusa P, Casullo R, Dosio F, Cattel L, Beltramini S, Chiappetta R, Tosetti L, Andorno E, Salizzoni M. OKT3 monitoring in the treatment of steroid-resistant acute rejection of hepatotransplant recipients. Eur J Drug Metab Pharmacokinet. 1998;23:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 114. | Hubscher SG, Buckels JA, Elias E, McMaster P, Neuberger J. Vanishing bile-duct syndrome following liver transplantation--is it reversible问号. Transplantation. 1991;51:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 115. | Neil DA, Adams DH, Gunson B, Hubscher SG. Is chronic rejection of liver transplants different from graft arteriosclerosis of kidney and heart transplants问号. Transplant Proc. 1997;29:2539-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 116. | Lowes JR, Hubscher SG, Neuberger JM. Chronic rejection of the liver allograft. Gastroenterol Clin North Am. 1993;22:401-420. [PubMed] |
| 117. | Hübscher S. Diagnosis and grading of liver allograft rejection: a European perspective. Transplant Proc. 1996;28:504-507. [PubMed] |
| 118. | Garcia RF, Garcia CE, McMaster P. Chronic rejection of the liver: the role of immunosuppression. BioDrugs. 2000;14:283-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 119. | Fung JJ, Starzl TE. FK506 in solid organ transplantation. Ther Drug Monit. 1995;17:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 120. | Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology. 2000;47:215-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 121. | Plosker GL, Foster RH. Tacrolimus: a further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs. 2000;59:323-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 197] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 122. | Hubscher SG. Chronic hepatitis in liver allografts. Hepatology. 1990;12:1257-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 123. | Turgeon N, Fishman JA, Basgoz N, Tolkoff-Rubin NE, Doran M, Cosimi AB, Rubin RH. Effect of oral acyclovir or ganciclovir therapy after preemptive intravenous ganciclovir therapy to prevent cytomegalovirus disease in cytomegalovirus seropositive renal and liver transplant recipients receiving antilymphocyte antibody therapy. Transplantation. 1998;66:1780-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 124. | Evans PC, Soin A, Wreghitt TG, Alexander GJ. Qualitative and semiquantitative polymerase chain reaction testing for cytomegalovirus DNA in serum allows prediction of CMV related disease in liver transplant recipients. J Clin Pathol. 1998;51:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Colina F, Jucá NT, Moreno E, Ballestín C, Fariña J, Nevado M, Lumbreras C, Gómez-Sanz R. Histological diagnosis of cytomegalovirus hepatitis in liver allografts. J Clin Pathol. 1995;48:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 126. | Mutimer D. CMV infection of transplant recipients. J Hepatol. 1996;25:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 127. | Rosen HR, Corless CL, Rabkin J, Chou S. Association of cytomegalovirus genotype with graft rejection after liver transplantation. Transplantation. 1998;66:1627-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 128. | Winston DJ. Prevention of cytomegalovirus disease in transplant recipients. Lancet. 1995;346:1380-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 129. | Winston DJ, Wirin D, Shaked A, Busuttil RW. Randomised comparison of ganciclovir and high-dose acyclovir for long-term cytomegalovirus prophylaxis in liver-transplant recipients. Lancet. 1995;346:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 178] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 130. | Gane E, Saliba F, Valdecasas GJ, O'Grady J, Pescovitz MD, Lyman S, Robinson CA. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. The Oral Ganciclovir International Transplantation Study Group [corrected]. Lancet. 1997;350:1729-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 347] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 131. | Umana JP, Mutimer DJ, Shaw JC, McLeish PJ, Buchan A, Martin B, Neuberger JM, Elias E, McMaster P. Cytomegalovirus surveillance following liver transplantation: does it allow presymptomatic diagnosis of cytomegalovirus disease问号. Transplant Proc. 1992;24:2643-2645. [PubMed] |
| 132. | Gavaldà J, de Otero J, Murio E, Vargas V, Rosselló J, Calicó I, Margarit C, Pahissa A. Two grams daily of oral acyclovir reduces the incidence of cytomegalovirus disease in CMV-seropositive liver transplant recipients. Transpl Int. 1997;10:462-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 133. | Bhatnagar V, Dhawan A, Chaer H, Muiesan P, Rela M, Mowat AP, Williams R, Tan KC, Heaton ND. The incidence and management of biliary complications following liver transplantation in children. Transpl Int. 1995;8:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 134. | Keogan MT, McDermott VG, Price SK, Low VH, Baillie J. The role of imaging in the diagnosis and management of biliary complications after liver transplantation. AJR Am J Roentgenol. 1999;173:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 135. | Davidson BR, Rai R, Nandy A, Doctor N, Burroughs A, Rolles K. Results of choledochojejunostomy in the treatment of biliary complications after liver transplantation in the era of nonsurgical therapies. Liver Transpl. 2000;6:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 136. | Rizk RS, McVicar JP, Emond MJ, Rohrmann CA, Kowdley KV, Perkins J, Carithers RL, Kimmey MB. Endoscopic management of biliary strictures in liver transplant recipients: effect on patient and graft survival. Gastrointest Endosc. 1998;47:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 137. | Saab S, Martin P, Soliman GY, Machicado GA, Roth BE, Kunder G, Han SH, Farmer DG, Ghobrial RM, Busuttil RW. Endoscopic management of biliary leaks after T-tube removal in liver transplant recipients: nasobiliary drainage versus biliary stenting. Liver Transpl. 2000;6:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 138. | Johnston TD, Gates R, Reddy KS, Nickl NJ, Ranjan D. Nonoperative management of bile leaks following liver transplantation. Clin Transplant. 2000;14:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 139. | Feller RB, Waugh RC, Selby WS, Dolan PM, Sheil AG, McCaughan GW. Biliary strictures after liver transplantation: clinical picture, correlates and outcomes. J Gastroenterol Hepatol. 1996;11:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 140. | Margarit C, Hidalgo E, Lázaro JL, Murio E, Charco R, Balsells J. Biliary complications secondary to late hepatic artery thrombosis in adult liver transplant patients. Transpl Int. 1998;11 Suppl 1:S251-S254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 141. | Nolten A, Sproat IA. Hepatic artery thrombosis after liver transplantation: temporal accuracy of diagnosis with duplex US and the syndrome of impending thrombosis. Radiology. 1996;198:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 142. | Hellinger A, Roll C, Stracke A, Erhard J, Eigler FW. Impact of colour Doppler sonography on detection of thrombosis of the hepatic artery and the portal vein after liver transplantation. Langenbecks Arch Chir. 1996;381:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 143. | Legmann P, Costes V, Tudoret L, Girardot C, Hazebroucq V, Uzan E, Fery-Lemonnier E, Bonnin A. Hepatic artery thrombosis after liver transplantation: diagnosis with spiral CT. AJR Am J Roentgenol. 1995;164:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 144. | Sheiner PA, Varma CV, Guarrera JV, Cooper J, Garatti M, Emre S, Guy SR, Schwartz ME, Miller CM. Selective revascularization of hepatic artery thromboses after liver transplantation improves patient and graft survival. Transplantation. 1997;64:1295-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 93] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 145. | Pinna AD, Smith CV, Furukawa H, Starzl TE, Fung JJ. Urgent revascularization of liver allografts after early hepatic artery thrombosis. Transplantation. 1996;62:1584-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 110] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 146. | Drazan K, Shaked A, Olthoff KM, Imagawa D, Jurim O, Kiai K, Shackelton C, Busuttil R. Etiology and management of symptomatic adult hepatic artery thrombosis after orthotopic liver transplantation (OLT). Am Surg. 1996;62:237-240. [PubMed] |
| 147. | Orons PD, Zajko AB, Bron KM, Trecha GT, Selby RR, Fung JJ. Hepatic artery angioplasty after liver transplantation: experience in 21 allografts. J Vasc Interv Radiol. 1995;6:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 148. | Figueras J, Busquets J, Dominguez J, Sancho C, Casanovas-Taltavull T, Rafecas A, Fabregat J, Torras J, Jaurrieta E. Intra-arterial thrombolysis in the treatment of acute hepatic artery thrombosis after liver transplantation. Transplantation. 1995;59:1356-1357. [PubMed] |
| 149. | Buckels JA, Tisone G, Gunson BK, McMaster P. Low haematocrit reduces hepatic artery thrombosis after liver transplantation. Transplant Proc. 1989;21:2460-2461. [PubMed] |
| 150. | Tisone G, Gunson BK, Buckels JA, McMaster P. Raised hematocrit--a contributing factor to hepatic artery thrombosis following liver transplantation. Transplantation. 1988;46:162-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 151. | Glez-Chamorro A, Jimenez C, Moreno-Glez E, Glez-Pinto I, Loinaz C, Gomez R, Garcia I, Alonso O, Palma F, Grande C. Management and outcome of liver recipients with post-transplant lymphoproliferative disease. Hepatogastroenterology. 2000;47:211-219. [PubMed] |
| 152. | Hsi ED, Singleton TP, Swinnen L, Dunphy CH, Alkan S. Mucosa-associated lymphoid tissue-type lymphomas occurring in post-transplantation patients. Am J Surg Pathol. 2000;24:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 153. | Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP, Rosenthal JT, Hakala TR, Shaw BW, Hardesty RL. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;1:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 826] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 154. | Iwatsuki S, Starzl TE, Todo S, Gordon RD, Esquivel CO, Tzakis AG, Makowka L, Marsh JW, Koneru B, Stieber A. Experience in 1, 000 liver transplants under cyclosporine-steroid therapy: a survival report. Transplant Proc. 1988;20:498-504. [PubMed] |
| 155. | Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 763] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 156. | Demetris AJ, Fung JJ, Todo S, McCauley J, Jain A, Takaya S, Alessiani M, Abu-Elmagd K, Van Thiel DH, Starzl TE. FK 506 used as rescue therapy for human liver allograft recipients. Transplant Proc. 1991;23:3005-3006. [PubMed] |
| 157. | Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3063] [Cited by in RCA: 3200] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 158. | No authors listed. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. European FK506 Multicentre Liver Study Group. Lancet. 1994;344:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 548] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 159. | No authors listed. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. The U.S. Multicenter FK506 Liver Study Group. N Engl J Med. 1994;331:1110-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 760] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 160. | Fung JJ, Eliasziw M, Todo S, Jain A, Demetris AJ, McMichael JP, Starzl TE, Meier P, Donner A. The Pittsburgh randomized trial of tacrolimus compared to cyclosporine for hepatic transplantation. J Am Coll Surg. 1996;183:117-125. [PubMed] |
| 161. | Wiesner RH. Long-term comparison of tacrolimus versus cyclosporine in liver transplantation. The US FK Study Group. Transplant Proc. 1998;30:1399-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 162. | Van Buren D, Payne J, Geevarghese S, MacDonell R, Chapman W, Wright JK, Helderman JH, Richie R, Pinson CW. Renal function in primary liver transplant recipients receiving neoral (cyclosporine) versus prograf (tacrolimus). Transplant Proc. 1998;30:1401-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 163. | Allison AC, Almquist SJ, Muller CD, Eugui EM. In vitro immunosuppressive effects of mycophenolic acid and an ester pro-drug, RS-61443. Transplant Proc. 1991;23:10-14. [PubMed] |
| 164. | Klupp J, Glanemann M, Bechstein WO, Platz KP, Langrehr JM, Keck H, Settmacher U, Radtke C, Neuhaus R, Neuhaus P. Mycophenolate mofetil in combination with tacrolimus versus Neoral after liver transplantation. Transplant Proc. 1999;31:1113-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 165. | Platz KP, Mueller AR, Neuhaus P. Indications for mycofenolate mofetil therapy after liver transplantation. Transplant Proc. 1997;29:2880-2881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 166. | Hebert MF, Ascher NL, Lake JR, Emond J, Nikolai B, Linna TJ, Roberts JP. Four-year follow-up of mycophenolate mofetil for graft rescue in liver allograft recipients. Transplantation. 1999;67:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 167. | McAlister VC, Gao Z, Peltekian K, Domingues J, Mahalati K, MacDonald AS. Sirolimus-tacrolimus combination immunosuppression. Lancet. 2000;355:376-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 227] [Article Influence: 8.7] [Reference Citation Analysis (2)] |
| 168. | Eckhoff DE, McGuire B, Sellers M, Contreras J, Frenette L, Young C, Hudson S, Bynon JS. The safety and efficacy of a two-dose daclizumab (zenapax) induction therapy in liver transplant recipients. Transplantation. 2000;69:1867-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 169. | Yoong KF, Gunson BK, Buckels JA, McMaster P, Mayer AD. Repeat orthotopic liver transplantation in the 1990s: is it justified问号. Transpl Int. 1998;11 Suppl 1:S221-S223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 170. | Lerut J, Laterre PF, Roggen F, Mauel E, Gheerardyn R, Ciccarelli O, Donataccio M, de Ville de Goyet J, Reding R, Goffette P. Adult hepatic retransplantation. UCL experience. Acta Gastroenterol Belg. 1999;62:261-266. [PubMed] |
| 171. | Lerut J; UNOS. Graft and Patient Survival Rates at One, Three and Five Years. In: UNOS; 1999; Available from: http://www.unos.org/data/anrpt99/ar99_table 06.htm.. |
| 172. | Devlin J, Williams R. Transplantation for fulminant hepatic failure: comparing tacrolimus versus cyclosporine for immunosuppression and the outcome in elective transplants. European FK506 Liver Study Group. Transplantation. 1996;62:1251-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 173. | Bismuth H, Samuel D, Castaing D, Adam R, Saliba F, Johann M, Azoulay D, Ducot B, Chiche L. Orthotopic liver transplantation in fulminant and subfulminant hepatitis. The Paul Brousse experience. Ann Surg. 1995;222:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 206] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 174. | Atillasoy E, Berk PD. Fulminant hepatic failure: pathophysiology, treatment, and survival. Annu Rev Med. 1995;46:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 175. | Uemoto S, Inomata Y, Sakurai T, Egawa H, Fujita S, Kiuchi T, Hayashi M, Yasutomi M, Yamabe H, Tanaka K. Living donor liver transplantation for fulminant hepatic failure. Transplantation. 2000;70:152-157. [PubMed] |
| 176. | Wolf P. Xenotransplantation of the liver. Ann Transplant. 2000;5:53-54. [PubMed] |
Edited by Rampton DS and Ma JY