Published online Jun 15, 2001. doi: 10.3748/wjg.v7.i3.381
Revised: April 3, 2001
Accepted: April 18, 2001
Published online: June 15, 2001
AIM: To establish a relevant animal model of human gastrointestinal cancer, which can be used for repetitive investigations, so as to improve our understanding and management of carcinogenesis and cancer metastasis.
METHODS: Intact tissues of human colorectal and pancreatic cancers were transplanted in nude mice. The biological characteristics of the original and the corresponding transplanted tumors were investigated by HE staining, PAS staining and immunostaining. The metastases in the livers and lungs of nude mice were investigated by immunostaining with biotinylated mab KL-1 and by RT-PCR using CK20 specific primers.
RESULTS: There were totally 9 of 16 surgical specimens growing in nude mice subcutaneously and/or orthotopically (4 of 6 colorectal and 5 of 10 pancreatic cancer). Tumor cell content of the specimens and freezing of tissue specimens are important factors influencing the growth of transplanted tumor. In the group of fresh tumor tissues with greater than 50% tumor cell content, the success rate of the transplantation was 100% (3 cases of pancreatic cancer and 3 cases of colorectal cancer). The orthotopically transplanted tumors resemble the original tumor morphologically and biologically, including TAA expression such as CEA by immunohistochemistry, and CEA level in the serum of mice. Ki-67 labeling index and the expression of TAA especially K-ras, 17-1A and RA-96, are associated with the potential of tumor growth in nude mice. Micrometastases in the lungs and livers of tumor bearing mice can be detected by immunostaining with biotinylated mab KL-1 and CK20-specific RT-PCR.
CONCLUSION: An orthotopic transplantation model for human colon and pancreatic cancer in nude mice has been set up. We have also established sensitive detection methods with CK-immunohistochemistry and CK20-RT-PCR to study xenotransplanted human cancer and its metastatic cancer cells in the liver and lung of nude mice. This study may be helpful in understanding the mechanism of cancer metastasis and in developing new diagnostic methods and therapeutic strategies for metastases including micrometastases.
- Citation: Cui JH, Krueger U, Henne-Bruns D, Kremer B, Kalthoff H. Orthotopic transplantation model of human gastrointestinal cancer and detection of micrometastases. World J Gastroenterol 2001; 7(3): 381-386
- URL: https://www.wjgnet.com/1007-9327/full/v7/i3/381.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i3.381
Despite significant improvement in surgical techniques and apparently curative resection, recurrence and metastasis still occur and is the leading cause of solid malignant tumors[1-7]. Current standard diagnostic techniques are not able to detect early dissemination of cancer cells (micrometastases)[8-10], and conventional classification of tumor stages cannot account for the presence or absence of distant micrometastasis in the patients with small primary tumors. Thus, one of the most critical prognostic determinants for the subsequent clinical course is neglected in many patients[9,11-16]. Specimens from individual patients are often difficult to obtain for detailed analyses, but it will be much easier to investigate materials in animal models, for studying the various aspects of tumorigenesis, especially metastasis because any part of the tissues from the model can be taken for detailed investigation.
Most models based on athymic nude mice have been playing an important role in evaluating many anti-cancer drugs. However, human tumors were transplanted subcutaneously and not at an orthotopically relevant organ site. The major problem of this model is that the transplanted tumors are located in an environment quite different from where most human tumors locate. Most subcutaneously transplanted tumors are surrounded by a pseudocapsule, and have little chance to invade and disseminate to the surrounding tissues and rarely metastasize, even when highly aggressive tumors have served as the source of the xenograft[17]. However, human tumor cells implanted orthotopically in the corresponding organs of nude mice can increase the metastatic capability of human tumor cells in nude mice[17-23]. As a relevant model of human gastrointestinal cancer, orthotopically transplanted tumors in nude mice can improve our knowledge about invasive growth and metastasis. Such tumor model would be available for repeated investigation of experimental diagnosis and treatment of early metastasis. It may help design more effective therapeutic strategies[6,19,22-24].
Tumor specimens for transplantation were taken from primary lesions in pancreas, colon and rectum. Surgical tumor specimens which were directly taken from patients were aseptically removed during surgery and immediately transported and processed. Each specimen was separated into three parts: one part for transplantation of fresh specimen, one part for subsequent transplantation of DMSO-frozen specimen (mostly in 1 d to 3 d), and another part was stored at -80 °C for morphological, immunohistochemistry analyses and for RT-PCR. Eight to twelve week old male or female athymic NMRI nude mice, which were obtained from the University Clinic Eppendorf, Hamburg, Germany, were kept in laminar-flow cabinets.
Each cancerous tissue (fresh or frozen tissue) was divided into small pieces about 2 mm in diameter. Mice were anesthetized with a mixture of 12 g·L-1 ketamin Curamed Phama GmbH) and 1.6 g·L-1 xylazin solution (Parke-Davis GmbH), The colon or pancreas was then carefully exposed and a tumor piece was then attached on the serosal surface of the colon or the wall of the pancreas, with 6-0 absorbable transmural suture. The colon or pancreas was then returned to the peritoneal cavity and the abdominal wall and the skin was closed with 6-0 absorbable suture. Immediately thereafter, another piece of the tissue was transplanted subcutaneously into the left frank of the respective mouse. For the purpose of convenience, the DMSO-frozen tissues were also transplanted into another 2-3 mice. Tumor pieces from each patient were transplanted into 2-3 mice in general.
A modified immunoperoxidase procedure, which was introduced in 1981 by Hsu et al, was used as follows. Tumor sections were air dried, fixed with acetone for 10 min prior to staining, transferred into PBS, blocked for 20 min with diluted normal horse serum, incubated for 30 min with primary antibody diluted in 10 g·L-1 BSA/PBS, washed for 5 min in PBS (if quenching of endogenous peroxidase was required, the sections were incubated for 30 min in methanol with 3 mL·L-1 H2O2), incubated for 30 min with a diluted biotinylated secondary antibody solution (biotinylated horse anti-mouse IgG 1:200 in 15 mL·L-1 horse serum/PBS). If the primary antibody was C-T84.66, biotinylated goat anti-human IgA + IgG + IgM (Jackson Inco. 1:1000) was used, washed for 5 min in PBS, incubated for 30 min with "VECTORSTAIN ELITE ABC Reagent", washed for 5 min in PBS, incubated for about 5 min in DAB solution, rinsed in tap water, counterstained and cleared, airdried and mounted. Positive and negative controls were included as mentioned above.
mAb KL-1 and Biotinilated KL-1 anti-cytokeratin (IgG 1, kappa, 4 mg·L-1, Immunotech, Hamburg, Germany); CIP83 (5 mg·L-1, Kalthoff, Kiel, Germany) and chimeric-T84.66 (1:100, Neumaier, Hamburg, Germany) anti-CEA (IgG1, kappa); and MIB1 (2.5 mg·L-1) and Biotinilated MIB1 (10 mg·L-1) anti-ki-67 (IgG 1, Dianova, Hamburg, Germany) were used both for investigating original and transplanted tumors. mAb CA-199, Ra-96 and 17.1A (all 5 mg·L-1, Kalthoff, kiel, Germany) were used for investigating human original tumor only, Biotinilated KL-1 was also used for detecting micrometastatic cells in the livers and lungs of mice.
Serum from nude mice were measured using a Microparticle Enzyme Immunoassay (MEIA) for the quantitative measurement of CEA with the "IMx" immunoassay analyzer (Abbott Laboratories).
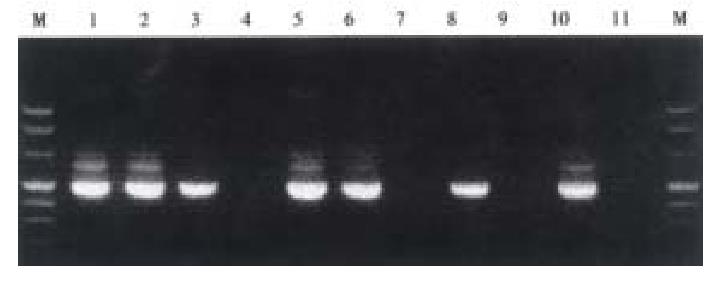
Livers and lungs of nude mice were cut into 10-20 μm slides with cryosection machine, then RNA was extracted using modified single-step RNA isolation method with TRIzol (Gibco BRL, Eggenstein, Germany). The integrity of RNA was checked by gel-electrophoresis. RNA extracted from a pancreatic carcinoma cell line A818.4 was used as positive control, GAPDH (glycerdehyde-3-phosphate dehydrogenase)-RT-PCR was used to monitor cDNA synthesis. The detailed protocol of ck20-specific RT-PCR was described previously[25,26].
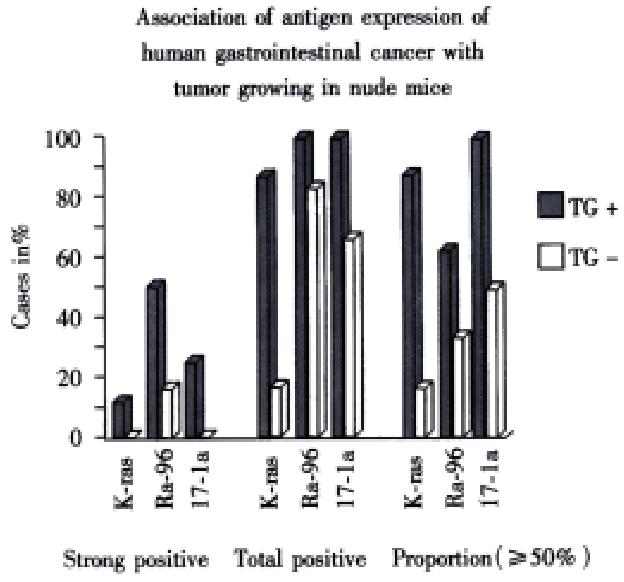
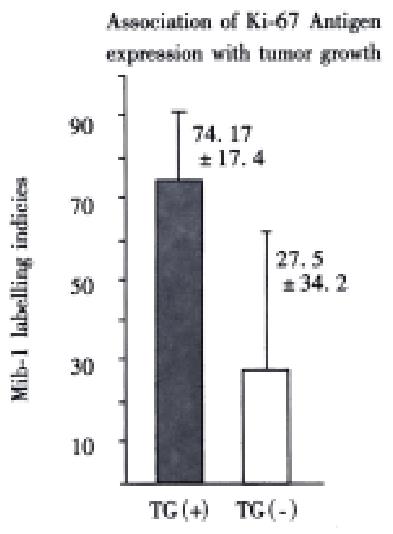
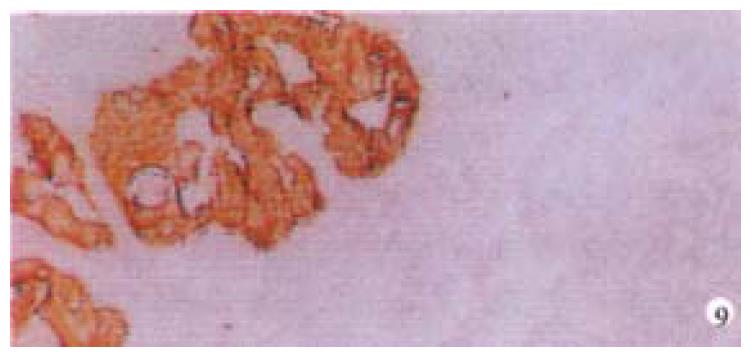
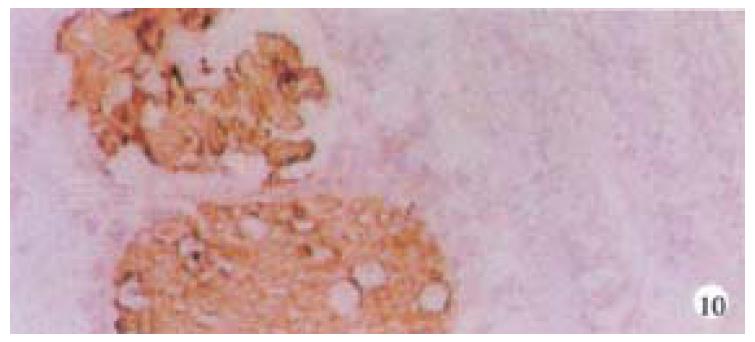
Mice were killed when transplanted tumors reached 1 cm or larger. There were 4 (67%) out of 6 colon cancer, 5 (50%) of 10 pancreatic cancer, totally 9 (56%) of 16 surgical specimens growing in nude mice sc and/or orthotopically. Tumor growth was observed in 0% of frozen tissues and in 63% of fresh tissues with paired samples of original tumors. When retransplanted with xeno-transplanted tumors, 39% of transplants of frozen tissues and 100% of fresh tissues grew in the mice (Table 1). In the group of fresh tumor samples with a tumor cell content ≥ 50%, the success rate was 100% (6/6). While in the group with a cell content of < 50%, the success rate was 40% (2/5), (Table 2). The expression of Ki-67, K-ras, 17.1A and Ra-96 antigens in human colorectal and pancreatic cancer with tumor growth in nude mice was higher than in those without tumor growth (Figure 1, Figure 2). Almost the same morphology and CEA expressions were observed between original human tumors and corresponding xenotransplanted tumors of colon and pancreatic cancer (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8). The serum CEA levels of mice are closely associated with the existence of tumors in nude mice (Table 3). Micrometastases in lungs and livers of tumor-bearing mice: 16 mice transplanted with colorectal, and pancreatic carcinomas from 7 cases of original tumors (5 of the 7 cases had corresponding orthotopic tumors) were investigated for liver and lung micrometastases. In the 16 mice, which were sacrificed around 13-14 weeks after transplantation, macroscopically invisible metastases were found in the lungs of 3 mice by KL-1 immunostaining and CK20-RT-PCR (2 colorectal and 1 pancreatic cancer). Additionally 2 liver metastases from colorectal cancer were detected by CK-20 specific RT-PCR only (Figure 9, Figure 10, Figure 11).
| Type | TG/total (n) | Success rate (%) | ||
| F | D | F | D | |
| Colon | 3/3 (12/12) | 0/3 (6/10)b | 100 (100) | 0 (60) |
| Pancreas | 2/5 (8/8) | 0/5 (1/8) | 40 (100) | 0 (13) |
| Total | 5/8 (20/20) | 0/8 (7/18) | 63 (100) | 0 (39) |
| Tumor | TG/total (n) | Success rate | ||
| ≥ 50% | < 50% | ≥ 50% | < 50% | |
| Colon | 3/3 | 1/1 | 100 | 100 |
| Pancreas | 3/3 | 1/4 | 100 | 25 |
| Total | 6/6 | 2/5 | 100 | 40 |
| With tumor (-x±s, mg·L-1) | Without tumor (-x±s, mg·L-1) | P value | |
| Colon | 19.33 ± 28.77 (n = 17) | 0.1 ± 0.06 (n = 7) | < 0.005 |
| Pancreas | 2.85 ± 1.89 (n = 11) | 0.29 ± 0.2 (n = 7) | < 0.005 |
Developing a relevant animal model of xenotransplantation of human tumors in nude mice could improve our understanding of the biological properties of human gastrointestinal cancer and lead to the development of new effective therapeutic concepts. Human tumor xenografts transplanted SC in athymic nude mice rarely give rise to metastases[24]. However, human tumor cells implanted orthotopically in the corresponding organs of nude mice can increase the metastatic capability of human tumor cells in nude mice[18,24]. Data have shown that desegregated tumor cell suspensions may not always express their fully malignant biological behavior[17,27]. Cell suspensions may have the possibility of seeding tumor cells-"artificial" metastases in the surrounding tissues (i.e. peritoneal cavity) during the process of inoculation. Moreover, inoculation of desegregated tumor cell suspensions does not reflect the situation when metastatic cells spread from intact tissue growing in the human body.
Therefore, we have established an orthotopic transplantation tumor model for human tumors in nude mice with intact tissues of pancreatic, and colorectal cancer. The results showed similar morphology and biological behavior before and after transplantation in this model. Orthotopical transplanted tumors present many of the clinical manifestations of the biological behavior of gastrointestinal cancer in humans, including invasion, metastasis, and antigen expressions of CEA, CK, Ki-67, etc. CEA is a most commonly used tumor marker for gastrointestinal cancers. Recent studies showed that CEAmRNA RT-PCR or CEA immunostaining is frequently used in detection of micrometastases of gastrointestinal tract cancers[1,12,28,29]. CEA levels in serum play an important role in monitoring patients with gastrointestinal cancer[30-34]. We found that serum CEA level is obviously elevated in tumor-bearing mice, suggesting that serum CEA level is a very useful marker in this model. KL-1 reacts with cytokeratins (CK), components of intracytoplasmic network of intermediate filaments (Ifs)[4,8,35], specifically reacts with epithelial tumors. Anti-CK mABs using immunocytochemistry or CK RT-PCR have been widely used for the detection of micrometastases in lymph nodes, venous blood and bone marrow[1,4,12,28,36-42]. It is satisfactory using KL-1 monoclonal antibody to detect micrometastasis in the lungs and livers of the mice in this study. Ki-67, aproliferation-associated antigen[43-46], is expressed in all phases of the cell cycle (G1, S, G2, and M) except for G0[47]. It is thought to be a useful predictor of aggressive tumor behavior and an indicator of patient survival[43]. Mib-1 is raised against recombinant parts of the Ki-67 antigen[48-52]. Our results showed that the original tumors with higher Mib-1 (Ki-67) labeling index had an increased tendency to grow in the nude mice.
Meanwhile, we established the sensitive method to detect disseminated tumor cells (micrometastasis). Sixteen tumor-bearing mice (with 5 orthotopic transplanted tumors) were used for investigating the metastasis of livers and lungs. There was no obvious metastases with naked eye and HE staining but 3 lung micrometastases with KL-1 immunostaining and CK20 RT-PCR, additionally 2 liver metastases with CK20 RT-PCR, suggesting that RT-PCR may be more sensitive than immunostaining. Many factors influence tumor growth in nude mice. On the one hand, tumor tissues are composed of cells with different biological characteristics[7]. On the other hand, different mice may have various reactions to transplantation.
Our data includes the frozen tumor tissue implantation, because the procedure is more flexible and convenient. Unfortunately, freezing of tissue specimens obviously reduced the success rate. However, for tumors with a good cellularity, freezing is a possible procedure. When xenograft tumor tissues were retransplanted (xenografts were taken from mice and frozen, then thawed and transplanted again), the tumor growth rate of pancreatic and colorectal frozen tumor tissues, was nearly 40% and only the colorectal tumors was 60%. This is likely due to the fact that xenograft tumor tissues contain more tumor cells and less damaged cells due to shorter storage time and better preservation. Tumor cell content is an important factor in our study. In the specimens with a tumor cell content greater than 50% (tumor cells vs stroma over 1:1), the success rate of fresh tumor tissues impliantation reached 100% (Table 3).
In this study, we found that TAAs, including C1P83, RA-96, 17.1A, K-ras and Ki-67 expression, were important indicators of tumor growing potential in nude mice.
The authors thank Dr. J. Luettges, Department of Pathology, Kiel University, for investigating the pathological characteristics of the specimens; Dr. N. Zawazawa, for the quantitative measurement of serum of CEA, Institute of Immunology, Kiel University.
| 1. | Maguire D, O'Sullivan GC, Collins JK, Morgan J, Shanahan F. Bone marrow micrometastases and gastrointestinal cancer detection and significance. Am J Gastroenterol. 2000;95:1644-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Nieroda CA, Arnold MW, Barbera-Guillem E, Martin EW. [Lymphadenectomy in colorectal carcinoma]. Chirurg. 1998;69:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Kienle P, Weitz J, Klaes R, Koch M, Benner A, Lehnert T, Herfarth C, von Knebel Doeberitz M. Detection of isolated disseminated tumor cells in bone marrow and blood samples of patients with hepatocellular carcinoma. Arch Surg. 2000;135:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Maehara Y, Hasuda S, Abe T, Oki E, Kakeji Y, Ohno S, Sugimachi K. Tumor angiogenesis and micrometastasis in bone marrow of patients with early gastric cancer. Clin Cancer Res. 1998;4:2129-2134. [PubMed] |
| 5. | Demeure MJ, Doffek KM, Komorowski RA, Wilson SD. Adenocarcinoma of the pancreas: detection of occult metastases in regional lymph nodes by a polymerase chain reaction-based assay. Cancer. 1998;83:1328-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Riesenberg R, Oberneder R, Kriegmair M, Epp M, Bitzer U, Hofstetter A, Braun S, Riethmüller G, Pantel K. Immunocytochemical double staining of cytokeratin and prostate specific antigen in individual prostatic tumour cells. Histochemistry. 1993;99:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Fidler IJ, Radinsky R. Genetic control of cancer metastasis. J Natl Cancer Inst. 1990;82:166-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Thorban S, Roder JD, Siewert JR. Detection of micrometastasis in bone marrow of pancreatic cancer patients. Ann Oncol. 1999;10 Suppl 4:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Yokoyama N, Shirai Y, Hatakeyama K. Immunohistochemical detection of lymph node micrometastases from gallbladder carcinoma using monoclonal anticytokeratin antibody. Cancer. 1999;85:1465-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Czerniecki BJ, Scheff AM, Callans LS, Spitz FR, Bedrosian I, Conant EF, Orel SG, Berlin J, Helsabeck C, Fraker DL. Immunohistochemistry with pancytokeratins improves the sensitivity of sentinel lymph node biopsy in patients with breast carcinoma. Cancer. 1999;85:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Lockett MA, Metcalf JS, Baron PL, O'Brien PH, Elliott BM, Robison JG, Cole DJ. Efficacy of reverse transcriptase-polymerase chain reaction screening for micrometastic disease in axillary lymph nodes of breast cancer patients. Am Surg. 1998;64:539-43; discussion 543-4. [PubMed] |
| 12. | Futamura M, Takagi Y, Koumura H, Kida H, Tanemura H, Shimokawa K, Saji S. Spread of colorectal cancer micrometastases in regional lymph nodes by reverse transcriptase-polymerase chain reactions for carcinoembryonic antigen and cytokeratin 20. J Surg Oncol. 1998;68:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Raj GV, Moreno JG, Gomella LG. Utilization of polymerase chain reaction technology in the detection of solid tumors. Cancer. 1998;82:1419-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Funke I, Schraut W. Meta-analyses of studies on bone marrow micrometastases: an independent prognostic impact remains to be substantiated. J Clin Oncol. 1998;16:557-566. [PubMed] |
| 15. | McDonnell CO, Hill AD, McNamara DA, Walsh TN, Bouchier-Hayes DJ. Tumour micrometastases: the influence of angiogenesis. Eur J Surg Oncol. 2000;26:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Harrison LE, Choe JK, Goldstein M, Meridian A, Kim SH, Clarke K. Prognostic significance of immunohistochemical micrometastases in node negative gastric cancer patients. J Surg Oncol. 2000;73:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Fu X, Hoffman RM. Human RT-4 bladder carcinoma is highly metastatic in nude mice and comparable to ras-H-transformed RT-4 when orthotopically onplanted as histologically intact tissue. Int J Cancer. 1992;51:989-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Gutman M, Fidler IJ. Biology of human colon cancer metastasis. World J Surg. 1995;19:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 19. | Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev 1998-. 1999;17:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 311] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Kobaek-Larsen M, Thorup I, Diederichsen A, Fenger C, Hoitinga MR. Review of colorectal cancer and its metastases in rodent models: comparative aspects with those in humans. Comp Med. 2000;50:16-26. [PubMed] |
| 21. | Capellá G, Farré L, Villanueva A, Reyes G, García C, Tarafa G, Lluís F. Orthotopic models of human pancreatic cancer. Ann N Y Acad Sci. 1999;880:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Funahashi Y, Koyanagi N, Sonoda J, Kitoh K, Yoshimatsu K. Rapid development of hepatic metastasis with high incidence following orthotopic transplantation of murine colon 38 carcinoma as intact tissue in syngeneic C57BL/6 mice. J Surg Oncol. 1999;71:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Pocard M, Middleton S, Northover J, Poupon MF. [Value of human colonic cancer models by surgical cecal implantation in nude mice]. Ann Chir. 1999;53:227-232. [PubMed] |
| 24. | Luo MH, Tang WP, Huang PG, Cai QZ, Li FH, Mo MY. Biological behavior of orthotopic implantation model of human gastric carcinoma in nude mice. Huaren Xiaohua Zazhi. 1998;6:887-890. |
| 25. | Soeth E, Röder C, Juhl H, Krüger U, Kremer B, Kalthoff H. The detection of disseminated tumor cells in bone marrow from colorectal-cancer patients by a cytokeratin-20-specific nested reverse-transcriptase-polymerase-chain reaction is related to the stage of disease. Int J Cancer. 1996;69:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Soeth E, Vogel I, Röder C, Juhl H, Marxsen J, Krüger U, Henne-Bruns D, Kremer B, Kalthoff H. Comparative analysis of bone marrow and venous blood isolates from gastrointestinal cancer patients for the detection of disseminated tumor cells using reverse transcription PCR. Cancer Res. 1997;57:3106-3110. [PubMed] |
| 27. | Kubota T. Metastatic models of human cancer xenografted in the nude mouse: the importance of orthotopic transplantation. J Cell Biochem. 1994;56:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Kijima F, Natsugoe S, Takao S, Aridome K, Baba M, Yoshifumi M, Eizuru Y, Aikou T. Detection and clinical significance of lymph node micrometastasis determined by reverse transcription-polymerase chain reaction in patients with esophageal carcinoma. Oncology. 2000;58:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Noh YH, Im G, Ku JH, Lee YS, Ahn MJ. Detection of tumor cell contamination in peripheral blood by RT-PCR in gastrointestinal cancer patients. J Korean Med Sci. 1999;14:623-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Xu CT, Pan BR, Zhang LZ, Li XX, Wang J. Significance of serum tumor markers CA50 and CEA in gastric cancer. China Natl J New Gastroenterol. 1996;2:16-19. |
| 31. | Lopez JB, Royan GP, Lakhwani MN, Mahadaven M, Timor J. CA 72-4 compared with CEA and CA 19-9 as a marker of some gastrointestinal malignancies. Int J Biol Markers. 1999;14:172-177. [PubMed] |
| 32. | Zhang ZC, Zhang J, Zhang JR. Diagnostic methods and evaluation of metastasis of digestive system neoplasms. Shijie Huaren Xiaohua Zazhi. 1999;7:408-409. |
| 33. | Li M, Wang Y, Yu BM, Zheng MH. Effect of p53 gene mutation and tumor marker on the prognosis of colorectal cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:425-426. |
| 34. | Liu R, Wang YH, Tang Y, Cao GS. Effect of Octreotide on cell-cycle kinetics and serum CEA level in hepatic metastases of colonic adenocarcinoma. China Natl J New Gastroenterol. 1997;3:69-71. |
| 35. | Fishman A, Klein A, Zemer R, Zimlichman S, Bernheim J, Cohen I, Altaras MM. Detection of micrometastasis by cytokeratin-20 (reverse transcription polymerase chain reaction) in lymph nodes of patients with endometrial cancer. Gynecol Oncol. 2000;77:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 36. | Braun S, Pantel K. Micrometastatic bone marrow involvement: detection and prognostic significance. Med Oncol. 1999;16:154-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Cai J, Ikeguchi M, Maeta M, Kaibara N. Micrometastasis in lymph nodes and microinvasion of the muscularis propria in primary lesions of submucosal gastric cancer. Surgery. 2000;127:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Tajima Y, Tomioka T, Ikematsu Y, Ichinose K, Inoue K, Kanematsu T. Immunohistochemical demonstration of cytokeratin is useful for detecting micrometastatic foci from gallbladder carcinoma in regional lymph nodes. Jpn J Clin Oncol. 1999;29:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Güdemann CJ, Weitz J, Kienle P, Lacroix J, Wiesel MJ, Soder M, Benner A, Staehler G, Doeberitz MV. Detection of hematogenous micrometastasis in patients with transitional cell carcinoma. J Urol. 2000;164:532-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Ye FY, Zhang LZ, Wu LL, Liu LJ, Zheng CZ, Xue YF. Expremental study of relation of K19mRNA and lymph node micrometastasis of colorectal cancer. Huaren Xiaohua Zazhi. 1998;6:329-330. |
| 41. | Xia JZ, Yin HR, Zhu ZG, Yan M. Detection of cancer cells in peripheral blood with nested RT-PCR and its significance in patients with gastric carcinomas. World J Gastroenterol. 2000;6:36. |
| 42. | Yang JH, Rao BQ, Wang Y, Tu XH, Zhang LY, Chen SH, Ou-Yang XN, Dai XH. Clinical significance of detecting the circulating cancer cells in peripheral blood from colorectal cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:187-189. |
| 43. | Petrowsky H, Sturm I, Graubitz O, Kooby DA, Staib-Sebler E, Gog C, Köhne CH, Hillebrand T, Daniel PT, Fong Y. Relevance of Ki-67 antigen expression and K-ras mutation in colorectal liver metastases. Eur J Surg Oncol. 2001;27:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Edmonston TB, Cuesta KH, Burkholder S, Barusevicius A, Rose D, Kovatich AJ, Boman B, Fry R, Fishel R, Palazzo JP. Colorectal carcinomas with high microsatellite instability: defining a distinct immunologic and molecular entity with respect to prognostic markers. Hum Pathol. 2000;31:1506-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Takeno S, Noguchi T, Sato T, Uchida Y, Yokoyama S. Multiple gastric carcinomas 21 years after gastrojejunostomy without gastrectomy. Report of a case. Dig Surg. 2000;17:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Kuniyasu H, Yasui W, Shinohara H, Yano S, Ellis LM, Wilson MR, Bucana CD, Rikita T, Tahara E, Fidler IJ. Induction of angiogenesis by hyperplastic colonic mucosa adjacent to colon cancer. Am J Pathol. 2000;157:1523-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Pinder SE, Wencyk P, Sibbering DM, Bell JA, Elston CW, Nicholson R, Robertson JF, Blamey RW, Ellis IO. Assessment of the new proliferation marker MIB1 in breast carcinoma using image analysis: associations with other prognostic factors and survival. Br J Cancer. 1995;71:146-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 136] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 48. | Key G, Becker MH, Baron B, Duchrow M, Schlüter C, Flad HD, Gerdes J. New Ki-67-equivalent murine monoclonal antibodies (MIB 1-3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Invest. 1993;68:629-636. [PubMed] |
| 49. | McGinn CJ, Pestalozzi BC, Drake JC, Glennon MC, Kunugi K, Otterson G, Allegra CJ, Johnston PG, Kinsella TJ. Cell cycle regulation of the G0/G1 transition in 5-fluorouracil-sensitive and -resistant human colon cancer cell lines. Cancer J. 2000;6:234-242. [PubMed] |
| 50. | Helpap B, Köllermann J. Immunohistochemical analysis of the proliferative activity of neuroendocrine tumors from various organs. Are there indications for a neuroendocrine tumor-carcinoma sequence. Virchows Arch. 2001;438:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Dowall JE, Willis P, Prescott R, Lamonby S, Lynch DA. Cell proliferation in type C gastritis affecting the intact stomach. J Clin Pathol. 2000;53:784-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Wang L, Vargas H, French SW. Cellular origin of gastrointestinal stromal tumors: a study of 27 cases. Arch Pathol Lab Med. 2000;124:1471-1475. [PubMed] |
Dr. Jun-Hui Cui, graduated from Zhejiang Medical University in 1984, earned master degree in 1990, studied in the Surgical Department of Kiel University and worked in the Lab of Molecular Oncology of Kiel University from 1994-1997, and achieved MD from Kiel University, Germany, now associate professor of surgery, specialized in colorectal oncology. Adviser of graduated student for master degree, having 20 publications published in key Chinese or English journals.
Edited by Ma JY