INTRODUCTION
It has been well known that there is an association between hyperglycemia and diabetic complication. Patients who develop non-insulin-dependent diabetes mellitus (NIDDM) even at age 65 years may live long enough to develop micro-vascular and neuropathic complication[1]. A cluster of risk factor sincluding hyperglycemia, hyper-insulinaemia, hypertension, dyslipidemia and obesity is called metabolic X-syndrome due to the correlationship of them[2]. Similar to cardiac syndrome X, metabolic syndrome X often induces vascular dysfunction[3-5]. Diet regimen and the control of nutrient entry, with the aim of avoiding glucose and anabolic hormone peaks and reducing the rise of developing long-term complications, are broadly accepted as the basic treatment for diabetes mellitus[6,7].
In the ordinary diet, carbohydrates, which contain far more starch than the other carbohydrates, normally represent the quantitatively greatest part of human diet and the main energies supply even though in diabetes. Glucose represents more than 80 per cent of the final products of carbohydrate digestion. Maltose is a rather important product during starch hydrolysis. Therefore, the digestive process in which various glycohydrolases work successively to hydrolyze starch to the final product glucose and the absorption of glucose in the small intestine could be a target for the control of the nutrient entry. Voglibose, an N-substituted derivative of valiolamine isolated from the fermentation broth of Streptomyces hygroscopicus subsp. limoneus, is a potent and structurally novel inhibitor of the intestinal disaccharidases, which not only can be used for treatment of NIDDM but also for insulin-dependent diabetes mellitus (IDDM)[8-11]. It has a potently inhibitory effect on maltase but with a short inhibitory duration[12,13]. On the other hand, GA[14], a mixture of triterpene glucuronides, which was found in the leaves of the Indian plant, Gymnema sylvestre[15], inhibits glucose absorption of small intestine[16], but it needs a longer time and higher dosage to achieve its maximum effect.
In this experiment, the combinative effect of Voglibose with GA was examined on hydrolysis and absorption of maltose in rat small intestine.
MATERIALS AND METHODS
Animals
Male 8-9-week-old Wistar rats weighing 300 g ± 25 g (Shimizu, Kyoto), were housed in an air-conditioned room at 22 °C ± 2 °C with a nature lighting schedule for 1 to 3 weeks before experiment. They were fed with a standard pellet diet (Oriental Yeast Co., Kyoto) and tap water. Care and treatment of the animals conformed to Tottori University guidelines for the ethical treatment of laboratory animals.
Perfusion of small intestine in vivo
A modified technique of Barry et al[17] was used. Animals fasted overnight with free access to water, were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg bw, Dainabot). The abdominal cavity was opened by a mild line incision. The small intestine 30 cm long from 2 cm caudal ward Treitz’s ligament was used as an in situ loop, which was emptied of its contents with Ringer’s solution. L-shape cannulae were inserted into each end of the selected intestine and connected with a peristaltic pump (SJ-1211H, Atto, Tokyo). The abdominal cavity was closed and the loop was rinsed with Ringer’s solution (145.4 mmol/L NaCl, 5.4 mmol/L KCl, 1.8 mmol/L CaCl2 and 2.4 mmol/L NaHCO3) by uncirculated perfusion for 1 h. Then the intestine was perfused in the recircular mode with 10 mmol/L maltose (Sigma) or maltose plus different dosages of voglibose (Takeda Chemical Industries Ltd., Osaka) and/or GA for 1 h to determine IC50 (concentration of the drug achieving 50% inhibition of maltose absorption or hydrolysis) for each drug. To compare the combinative and individual effects, the animals were randomly separated into four groups in which each loop was perfused for four times (T, R1, R2 and R3). In the first time (T), 10 mmol/L maltose with or without GA (0.5 g/L) and voglibose (2 μmol/L) was perfused. After rinsing for 30 min with Ringer’s solution, perfusion of maltose (10mmol/L) only was repeated 3 times (R1, R2 and R3) to examine the time course of recovery. The perfusates of 4 groups in the first perfusion (T) were as follows: ① control group: 10 mmol/L maltose, ② voglibose group: 2 μmol/L voglibose +10 mmol/L maltose, ③ GA group: 0.5 g/L GA + 10 mmol/L maltose and ④ Combined group: 0.5 g/L GA + 2 μmol/L voglibose +10 mmol/L maltose. All perfusates were dissolved in Ringer’s solution. All solutions were kept at 37 °C and pH was regulated at 7.5-7.8.
Measurement of maltose absorption and hydrolysis
Two samples each containing twenty microlitter of perfusion fluid were taken at an interval of 15 min during the perfusion period to measure the amount of glucose and maltose remained and kept at 0 °C to prevent further hydrolysis in the collected samples. One was used to measure the amount of glucose remaining at time t (Gt) after the beginning of perfusion. Maltose in the other sample was completely hydrolyzed by incubating it with enough alpha-glucosidase (Funakoshi) to determine total glucose remaining at time t (TGt). The amount of glucose was determined by glucose determining kit (Glucose Test B, Wako, Osaka). Thus, the extents of absorption and hydrolysis of maltose at time t were obtained as follows:
Absorption (%) = (TGo - TGt)/TGo × 100
Hydrolysis (%) = (TGo - TGt + Gt)/TGo × 100
where subscript 0 and t represent the perfused time when the sample was taken, and the Go almost equals 0.
GA extraction
Dry-Gymnema sylvestre leaves were obtained from Okinawa, from which GA was extracted with water, ethanol and diethyl carbonate according to slightly modulated Kurihara’s method and freeze-dried to obtain GA powder[16].
Statistical analyses
Statistical analyses were performed with the U test of Mann-Whitney or ANOVA, which was indicated in the result when ANOVA was used. P < 0.05 was considered as significant difference.
RESULTS
Under the present conditions of experiment, about 63% of maltose in the perfusion fluid disappeared during 60 min perfusion as a result of being hydrolyzed to glucose and successive absorption by the intestinal loop. For simplicity we express hereafter this phenomenon as ‘absorption of maltose’, as was defined in Methods.
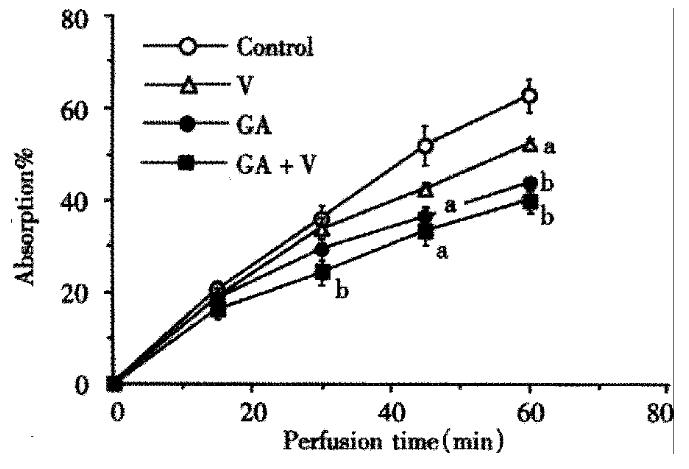
When voglibose was present in the perfusion fluid, the absorption of maltose was inhibited dose dependently with apparent IC50 of about 6.06 × 10-6 mol/L. On the other hand, GA inhibited the maltose absorption with IC50 of 0.85 g/L, whereas the IC50 of voglibose on the hydrolysis of maltose in the loop was 1.8 × 10-6 mol/L. In order to investigate the combined effect of the two inhibitors, we chose rather low doses of the drugs such as concentrations lower than IC50’s. In Figure 1, time courses of the absorption of maltose are shown during 60 min perfusion with or without voglibose (2 μmol/L) and/or GA (0.5 g/L). At such a low dose as 2 μmol/L, voglibose showed slight inhibitory effect on the absorption of maltose and the significant inhibition was observed at 60 min after the beginning of the perfusion. GA (0.5 g/L) exhibited a significant inhibitory effect at 45 min after the beginning of the perfusion. When the two inhibitors co-existed in the perfusion fluid, inhibitory effect was more pronounced. The significant effect was attained at 30 min after the beginning of the perfusion. The inhibition rate of about 37% was achieved at the end of the perfusion and the percentage of maltose absorption was lowest when GA and voglibose were presented in the perfusate (P < 0.05 vs control; ANOVA).
Figure 1 The inhibitory effects of GA (0.
5 g/L), voglibose (V, 2 μmol/L) and the combination (GA + V) on 10 mmol/L maltose absorption. The maltose contained in the fluid at perfusion starting point was taken as 100%. Each bar is expressed as the Mean ± SE. (aP < 0.05, bP < 0.01)
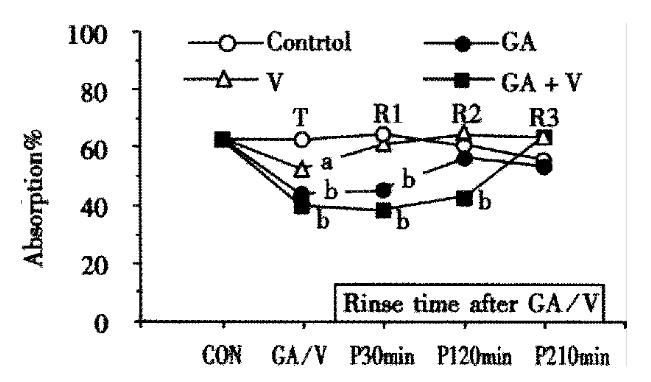
Time courses of the recovery from the inhibited states were compared with each other, where perfusion for 60 min with 10 mmol/L maltose only was repeated three times (R1, R2 and R3) inserting rinsing for 30 min between each perfusion, and the extent of recovery was assessed by the absorption of maltose at the end of each perfusion (Figure 2). As shown in the figure, the effect of voglibose was completely recovered during the first perfusion (R1). The effect of GA was still significant after R1 but completely recovered during R2. On the contrary, the suppressed absorption was still remained significant even after R2 and the complete recovery was attained during R3 in the combination group. The total inhibitory rates of maltose absorption in the 3 times perfusion (T, R1 and R2) were 5.39%, 22.21% and 35.55% in the 2 μmol/L voglibose, 0.5 g/L GA and combination groups respectively.
Figure 2 Alteration of maltose absorption following application with GA and voglibose.
Each point is shown as the maltose absorption during 60 min perfusion of 10 mmol/L maltose following treatment with GA and/or voglibose, except the points of “CON” or “GA/V” which are shown as the absorption during first 1 h perfusion with or without GA and voglibose. Others are the same as in Figure 1.
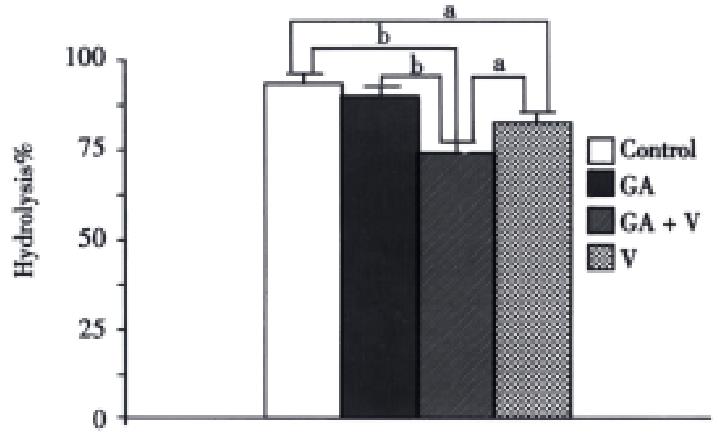
To find the reason of the longer effective duration for the combined group of GA and voglibose, the hydrolytic rate of maltose during R2 in each group was measured (Figure 3). The hydrolysis in the combined group was the lowest among the four groups, although there was no significant difference between the GA and control group.
Figure 3 Hydrolytic rates of maltose in each group during R2 in Figure 2.
The conditions are the same as in Figure 2.
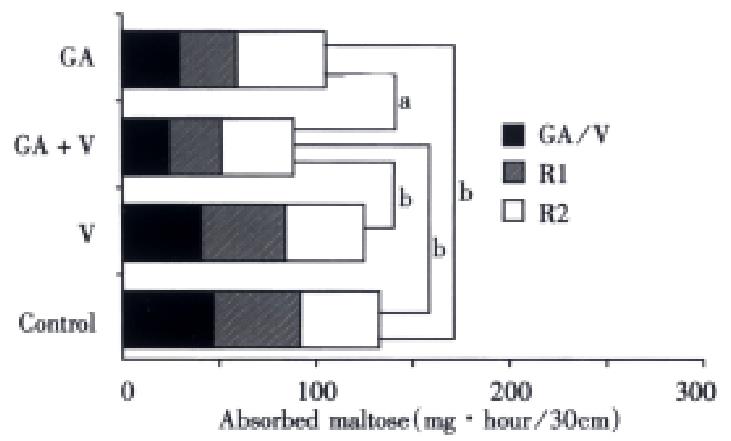
Figure 4 shows the total amount of maltose absorbed during three perfusions (T, R1, and R2) in the rather low doses of voglibose (2 μmol/L) and GA (0.5 g/L). It was easily expected from the result shown in Figure 4, that the smallest amount of maltose was absorbed in case of mixing both inhibitors, reflecting the sustained inhibitory effect on the maltose absorption.
Figure 4 Total maltose absorption in 3 times perfusion during 4 h.
The absolute amounts of maltose absorbed during three perfusions (T, R1 and R2) are compared with each other.
DISCUSSION
In the present study, the combinative effect of voglibose and gymnemic acid (GA) was investigated on the digestion and absorption in rat small intestine using maltose as a substrate. Voglibose is an inhibitor of the alpha-dissaccaridases and expected to suppress the absorption of maltose in small intestine as a result of inhibiting the hydrolysis of maltose. Apparent IC50 determined in the present experiment of perfusion was 6.6 × 10-6 mol/L for the absorption of maltose, which was about three times larger than that for the hydrolysis of maltose (1.8 × 10-6 mol/L). This difference of IC50 could be understood if the rate of hydrolysis of maltose in the intestinal loops was large enough compared to that of absorption of glucose. In fact, when 10mmol/L maltose only was perfused, free glucose appeared progressively in the perfusion fluid. Matsuo et al[18] reported the IC50 of voglibose on maltase originated from rat small intestine to be 6.4 × 10-9 mol/L in vitro. The difference could come from the methods employed in the two experiments.
It has been believed that the anti-diabetic effect of voglibose is due to the inhibition of the hydrolysis of disaccharidases. Recently Hirsh et al[19] reported that voglibose showed the inhibitory activity on the free glucose absorption in vivo with an IC50 near 3 mg/L (1.1 × 10-5 mol/L). The concentration of voglibose used in the present combinative treatment with GA and voglibose was 0.2 × 10-5 mol/L that was less than 1/5 concentration used in Hirshs’ experiment. Therefore, the direct inhibitory effect of voglibose on the glucose absorption, if any, would be negligible in the present experiment.
The depressing effect of GA on the sweet taste sensation in human has been known for a long time[20-26]. The inhibitory effects of GA, a mixture of triterpene glucuronides, from the plant -Gymnema sylvestre, on the glucose absorption in the small intestine and the improvement of glucose tolerance have been noticed since the 1980s[15,16,27,28]. Recently we found that GA could inhibit the absorption of oleic acid and gluocose simultaneously[29]. Although the mechanism of the action of GA has not been fully understood, GA is thought to suppress the active transport of glucose suggesting the involvement of the interaction with Na+ glucose cotransporter and/or ATPase[30-32] on the epithelial cells. The other mechanisms for GA’s actions have been considered as participating in the glucose receptor[33] and insulin release[34].
By combining two inhibitors, faster, more effective and long-lasting inhibition of maltose absorption was achieved than those expected as the additive effect. Namely, under the present conditions, significant suppression of maltose absorption was attained at 30 min after the beginning of the perfusion and interestingly, the inhibitory effect was still significant even at 4 hr after the application, whereas almost complete recovery was attained at same time in the case of applying GA or voglibose alone. Therefore, the more effective reduction of postprandial hyperglycemia and hyperinsulinaemia could be expected with the combination.
In the present stage of our knowledge, the mechanism underlying this synergistic effect is not clear . However, as shown in Figure 3, GA enhanced significantly the inhibitory action of voglibose on maltose hydrolysis even at 4 hr after application, although no effect of GA alone on the hydrolysis of maltose can be observed simultaneously. It is well known that most of the disaccharidases produced by the enterocytes are binding with the membrane (under the unstirred layer) and a small amount of saliva and pancreatic amylase is in the glycocalyx[35,36]. The maltose is hydrolyzed during it passes through the glycocalyx and enterocytes[37]. Recently we have found that GA increased the function of unstirred layer by suppression of intestinal motility[38]. Is it possible that voglibose was kept longer time in/under the unstirred layer by GA.
The most common adverse effect of voglibose is hepatotoxicity and gastrointestinal disturbance[39-43] induced by fermentation of unabsorbed carbohydrate in the bowel and increments of gastrointestinal motility[44]. Unfortunately, the disturbance of digestive system also exists in diabetics[45,46]. Voglibose has only rarely been associated with systemic adverse effects, but in some cases acute ileus, pneumatosis cystoides intestinalis and acute dizziness have been reported[47-49]. These adverse effects tend to increase with higher doses of voglibose. GA may diminish the adverse effects not only by decreasing dosage of voglibose, but also suppressing the intestinal motility[38] and inhibiting the growth of anaerobia[50], because bacterial overgrowth plays a role in the development of gastrointestinal symptoms[51].
In summary, the combined effect of voglibose and GA is first reported here. With the combination, the onset time is shortened and the effective duration was prolonged each other, as a result the total amount of maltose absorption is inhibited significantly. Improvement in postprandial hyperglycemia, hyperinsulinaemia and insulin resistance, treatment of an overweight condition (syndrome[52]) and diminishing of the adverse effects of voglibose in diabetic control can be achieved by this combination.