Published online Apr 15, 2001. doi: 10.3748/wjg.v7.i2.259
Revised: February 22, 2001
Accepted: March 1, 2001
Published online: April 15, 2001
AIM: To examine the role of p38 during acute experimental cerulein pancreatitis.
METHODS: Rats were treated with cerulein with or without a specific JNK inhibitor (CEP1347) and/or a specific p38 inhbitor (SB203580) and pancreatic stress kinase activity was determined. Parameters to assess pancreatitis included trypsin, amylase, lipase, pancreatic weight and histology.
RESULTS: JNK inhibition with CEP1347 ameliorated pancreatitis, reducing pancreatic edema. In contrast, p38 inhibition with SB203580 aggravated pancreatitis with higher trypsin levels and, with induction of acinar necrosis not normally found after cerulein hyperstimulation. Simultaneous treatment with both CEP1347 and SB203580 mutually abolished the effects of either compound on cerulein pancreatitis.
CONCLUSION: Stress kinases modulate pancreatitis differentially. JNK seems to promote pancreatitis development, possibly by supporting inflammatory reactions such as edema formation while its inhibition ameliorates pancreatitis. In contrast, p38 may help reduce organ destruction while inhibition of p38 during induction of cerulein pancreatitis leads to the occurrence of acinar necrosis.
- Citation: Fleischer F, Dabew R, ke BG, Wagner A. Stress kinase inhibition modulates acute experimental pancreatitis. World J Gastroenterol 2001; 7(2): 259-265
- URL: https://www.wjgnet.com/1007-9327/full/v7/i2/259.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i2.259
Acute pancreatitis is a common gastrointestinal disorder but remains enigmatic in its unpredictable clinical course ranging from mild to very severe, and life threatening pancreatitis. Due to our limited understanding of its underlying pathophysiology, the treatment of acute pancreatitis is still confined to general supportive measures with no causal approach. In order to better understand the pathophysiology of pancreatitis, we characterized the molecular mechanisms of the pancreatic stress response.
Members of the mitogen-activated protein kinase (MAPK) cascade are considered to play key roles in signal transduction pathways activated by a wide range of stimuli[1]. The three best characterized members of this growing family of serine/threonine kinases are extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK) and p38. While ERK responds vigorously to growth factors and certain hormones, JNK and p38 are rather activated by stress stimuli and are widely believed to be part of the cellular stress response machinery[1-5]. Activation of these kinases requires phosphorylation of both tyrosine and threonine residues by upstream dual specific kinases. Active MAPK are responsible for the phosphorylation of a variety of effector proteins including several transcription factors[1].
In the pancreas, it has been shown that hyperstimulation with the cholecystokinin (CCK) receptor agonist cerulein, which induces acute pancreatitis in rats, can activate JNK in rat pancreas[6,7]. We and others have recently found that p38 is also expressed in the pancreas and rapidly activated by cerulein[8-10]. Due to the pattern and time course of activation in response to a variety of different secretagogues, JNK has been proposed as an important mediator early during cerulein-induced pancreatitis[6,10]. In addition, we have also shown that treatment with CEP1347, a specific inhibitor of the JNK pathway[11,12], ameliorates cerulein pancreatitis[13].
However, no data on the effects of p38 inhibition on the course of experimental pancreatitis is available so far. There is evidence that p38 may actually promote the protection through influencing HSP27 phosphorylation and actin stabilization [8,14]. Therefore, we have studied the effects of p38 inhibition with SB 203580 on the course of cerulein pancreatitis, and compared the effects of p38 inhibition to those of JNK inhibition as well as inhibition of both kinases simultaneously. Our data show that both kinases influence pancreatitis differentially in that JNK inhibition ameliorates pancreatitis while p38 inhibition aggravates cerulein pancreatitis, resulting in the development of necrosis.
Sodium dodecyl sulfate (SDS), polyacrylamide, molecular weight and isoelectric focusing standards were products of BioRad (Hercules, CA, USA); nitrocellulose membranes were obtained from Schleicher and Schuell (Keene, NH, USA). All other chemicals were from Sigma (St. Louis, MO, USA). CEP-1347 was kindly provided by Cephalon Inc (West-Chester, PA, USA).
Acini were prepared as previously described[15]. Briefly, pancreata from white male Sprague Dawley rats were digested with purified collagenase and dispersed by pipetting through polypropylene pipettes of decreasing orifice, followed by filtration through a 150 µm nytex screen. Acini were purified by centrifugation through 4% bovine serum albumin (wt/vol) and then preincubated for 30 min at 37 °C in N-2-hydroxyethyl-piperazine-N’-2-ethane-sulfonic acid (HEPES)-buffered Ringer solution (HR), pH 7.4, supplemented with 11.1 mM glucose, minimal Eagle’s medium amino acids, 5 g/L bovine serum albumin, and 0.1 g/L soybean trypsin inhibitor. Buffers were gassed with 100% O2. Acini were then kept resting at room temperature for 2 hours with and without 20 µM CEP-1347 and/or 50 µM SB203580. Incubation at 37 °C was then continued and acini stimulated with the indicated concentrations of cerulein. After 30 min stimulation, acini were pelleted and homogenized for assessement of MAPK, JNK and p38 activity in kinase/IP buffer, containing 50 mM glycerophos-phate, 2 mM Na3V04, 1 mM each of NaF, EGTA, EDTA, DTT, PMSF, benzamidine, 10 mg/L each of aprotinine and leupeptin, 0.1% β-mercaptoethanol (v/v), 0.03% Brij 35 (w/w), 5% glycerol in phosphate buffered saline (pH 7.4, 150 mM NaCl, 16 mM Na2HPO4·2H2O and 4 mM NaH2PO4·2H2O). Supernatants were used for measurement of amylase release, expressed as % total amylase content.
Rats were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg). Following insertion of a tail vein catheter, rats were then treated with a hyperstimulatory dose of cerulein (10 µg/kg i.v.). Controls received isotonic saline only. To assess the effects of stress kinase inhibition on cerulein- induced pancreatic stress kinase activation, rats were treated with 30 mg/kg CEP-1347, SB203580 or both, dissolved in Solutol (Boehringer Mannheim), by subcutaneous (s.c.) injection 4 h prior to cerulein injections. Controls received solutol alone. The rats were then sacrificed 30 min after cerulein injection and pancreata homogenized in stress kinase/IP buffer. This time point was chosen since it is known that pancreatic ERK, JNK and p38 are all strongly activated 30 min after cerulein hyperstimulation in vivo[6-10]. Pancreatic wet weight and trypsin activation as well as serum lipase and amylase were also determined.
To assess effects of cerulein and SB203580 on p38 Map kinase in vivo, activation of pancreatic MAPKAP kinase 2 was assessed following immunoprecipitation in an in vitro assay using recombinant HSP27 as substrate as described[13,16].
One-dimensional gel electrophoresis was performed according to Laemmli, as previously described[17]. Five µg of protein were loaded per lane.
For histological evaluation, freshly removed pancreata were formalin (4%) fixed, ethanol dehydrated and, embedded in paraffin. Six micrometer slices were then stained with H&E and subjected to conventional light microscopy.
Measurement of serum amylase and lipase activity was performed using commercially available kits (Boehringer Mannheim) following the manufacturers instructions.
After incubation with or without cerulein, acini were pelleted (10000 ×g, 1min). Pellets were resuspended in 1mL HEPES buffer without BSA and sonicated. After a second centrifugation step (20000 × g, 2 min), the supernatant was then analysed. Trypsin activity was measured fluorometrically using BOC- Glu-Ala-Arg-MCA as the substrate, according to the method of Kawabata[18] To allow quantification and comparison between experiments, a standard curve with known amounts of trypsin was generated by plotting trypsin concentration (0-100 pg) against flouresence intensity (380lEx/460lEm). Trypsin content in individual samples was then normalized to total protein content (BioRad) using BSA as a standard and expressed as pg trypsin per mg protein.
Analysis was done using standard software (Sigma Plot, Systat). Data were compared by Student’s t test, P < 0.05 was considered significant.
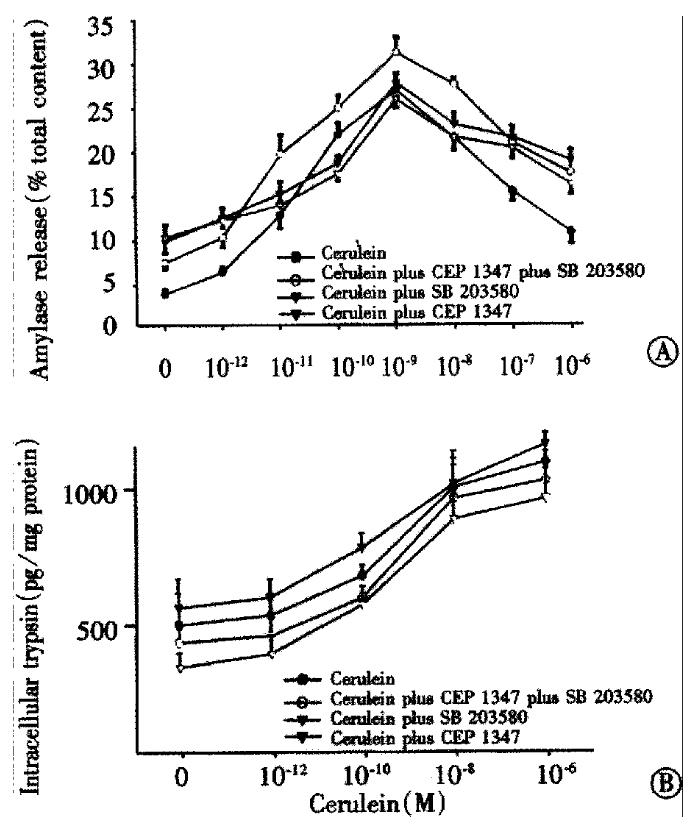
We have previously shown that JNK inhibition does not influence acinar stimulus secretion coupling[13]. We, therefore, investigated effects p38 and/or JNK inhibition on cerulein-induced acinar amylase release (Figure 1A). Cerulein induced a dose dependent secretory response including the typical biphasic inhibition with hyperstimulatory amounts (●). Neither JNK nor p38 inhibition apparently altered the secretory dose response to cerulein. Thus, treatment with either 20 μM CEP1347 (▽), 50 μM SB203580 (▼) or both agents given simultaneously (○) did not alter maximal amylase release not the biphasic dose response. We also measured the effects of stress kinase inhibition on cerulein-induced acinar trypsin activation (Figure 1B). Active trypsin could be found even in unstimulated acini, but treatment with cerulein led to a dose dependent increase of acinar trypsin activity (●). Interestingly, using CEP1347 (▽), we observed a tendency towards reduced trypsin activation while SB203580 (▼) appeared to increase trypsin activation. However, in isolated acini, this effect was not statistically significant.
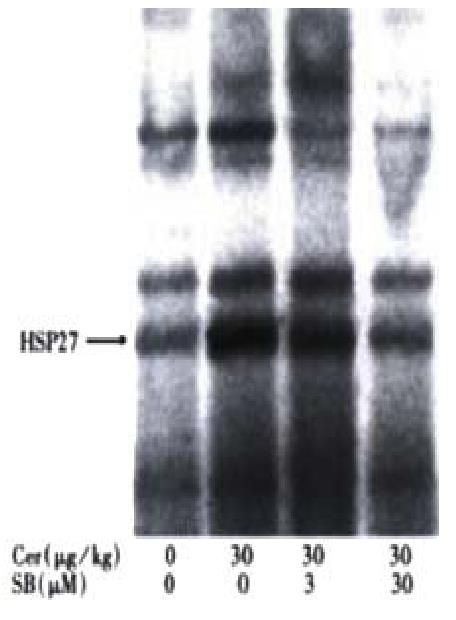
In order to compare the effects of JNK and p38 inhibition in vivo, we tested whether in vivo inhibition of pancreatic p38 activity could be accomplished. In contrast to JNK[9,10], p38 is constitutively active in the pancreas (Figure 2, lane 1). Treatment with 30 mg/kg cerulein resulted in strong p38 activation (lane 2). Treatment with 3 mg/kg SB203580 reduced cerulein-induced p38 activation by half while 30 mg/ kg SB203580 suppressed cerulein-induced p38 activation almost completely (Figure 2, lanes 3 and 4). However, even the highest SB203580 dose could not reduce pancreatic p38 kinase activity below basal levels (Figure 2, lane 4). SB203580 had no apparent effect on cerulein-induced activation of JNK or ERK (not shown).
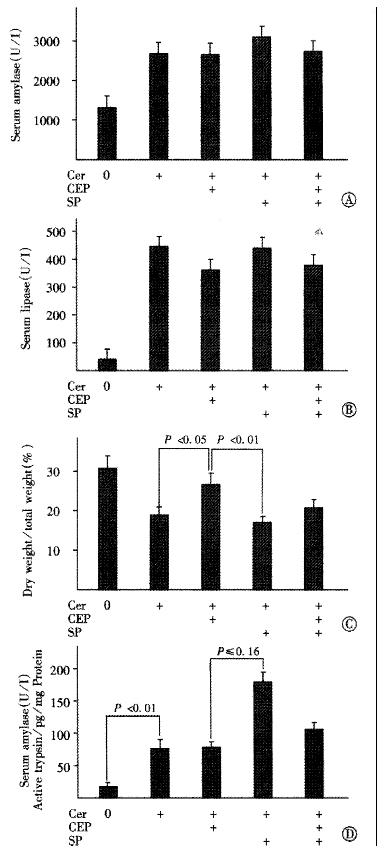
According to our previous results, we used 30mg of either CEP1347[13] and/or SB203580 (Figure 2) given sc 4h prior to cerulein. In accordance to the results on acinar stimulus secretion coupling (Figure 1), both agents failed to alter the increase of serum amylase and lipase levels following pancreatitis induction (Figure 3A and B). As reported previously[13], treatment with CEP1347 to inhibit JNK activation significantly increased the dry to total pancreatic weight ratio after cerulein hyperstimulation, indicating reduced edema formation (Figure 3C). In contrast, SB203580 treatment to inhibit p38 kinase activation had no apparent effect on the cerulein-induced increase of pancreatic water content. However, when given simultaneously, SB203580 treatment abrogated the effects of CEP1347 on the dry to total pancreatic weight ratio. Thus, animals pretreated with both CEP1347 and SB203580, apparently developed similar edema compared to rats treated with cerulein alone.
We also measured the pancreatic trypsin activity in vivo (Figure 3D). Compared to acini, pancreata from untreated rats contained very little active trypsin and trypsin activity was strongly induced following cerulein hyperstimulation. Thus, freshly isolated acini on average contained 500 pg active trypsin/mg protein while whole pancreata from untreated control animals on average contained only 15 pg/mg protein (Figure 1B and Figure 3D). Accordingly, the fold increase of active trypsin after cerulein hyperstimulation was much higher in vivo (5 fold) rising to 72 pg/mg protein, compared with acini in which cerulein hyperstimulation induced only a 2-2.5 fold increase. The higher basal active trypsin content in acini may possibly be explained by the mechanical stress of the acinar preparation.
As in acini, CEP1347 given in vivo did not alter cerulein-induced pancreatic trypsin activation. In contrast, SB203580, interestingly, further increased pancreatic trypsin activation in response to cerulein. This effect was much more pronounced compared to isolated acini, possibly due to lower basal trypsin activity in vivo. Thus, SB203580 pretreated animals had significantly higher pancreatic trypsin levels following cerulein hyperstimulation when compared to animals pretreated with CEP1347 (Figure 3D). Again, when given simultaneously, CEP1347 treatment abrogated the effect of SB203580 on pancreatic trypsin activation.
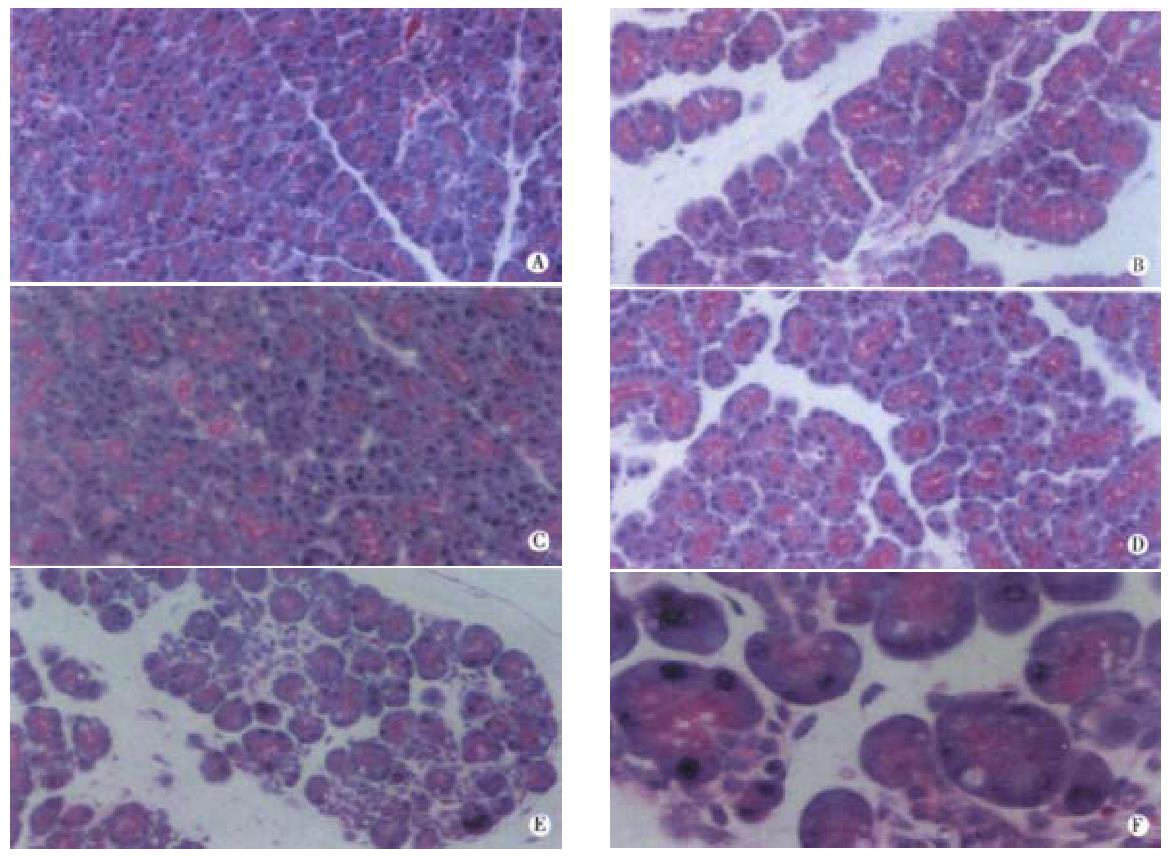
In order to better compare the effects of CEP1347 and SB203580 on cerulein pancreatitis, histological assessment was also performed (Figure 4). Control pancreata showed regular acinar structure with polarized acinar cells grouped around a ductal lumen and lobules were separated by thin septi (Figure 4A). Cerulein induced the typical morphological changes of edematous pancreatitis including widened interstitial spaces and vacuole formation but without necrosis (Figure 4B). In accordance to previous results[13], JNK inhibition through CEP1347 pretreatment strongly reduced edema formation while vacuolisation was still visible (Figure 4C). Interestingly, inhibition of p38 with SB203580 without concomitant JNK inhibition aggravated cerulein pancreatitis with focal areas of necrosis (Figure 4E and F). Effects of either compound alone on cerulein pancreatitis were mutually abolished when both JNK and p38 were inhibited through simultaneous treatment with CEP1347 and SB203580 (Figure 4D) and no major difference in cerulein pancreatitis without any stress kinase inhibition was seen (compare panel B and D).
In our study, we investigated the effects of p38 kinase inhibition on cerulein pancreatitis in comparison to JNK inhibition. We used CEP1347 and SB203580 as tools to specifically inhibit JNK and p38 kinase, respectively. Previously we used CEP1347 for inhibition of pancreatic JNK[13]. CEP 30 mg/kg had no effect on other members of the ERK family. In addition, it has been shown that CEP1347 exhibits between 10-200 fold greater potency towards inhibition of the JNK pathway as compared with other kinases such as protein kinase C, protein kinase A, trk tyrosine kinase, myosin light chain kinase and phosphatidyl inositol 3 kinase[12,13]. We, therefore, believe that CEP1347 can safely be used as a tool to characterize the function of JNK during pancreatic stress. SB203580 has been extensively characterized and widely used as p38 inhibitor and is known to specifically inhibit p38 isoforms α and β but not γ and δ[19]. Nothing is known about pancreatic expression of p38 isoforms, but we (Figure 2) and others[8] have shown that the cerulein-induced p38 activation can be almost completely blocked through SB203580. This indicates that SB203580 is a useful tool to assess the role of p38 kinase in the pancreatic stress reaction early during pancreatitis induction. It is interesting to note that basal p38 kinase activity in the pancreas could not be suppressed by SB203580. This indicates that SB203580 insensitive p38 isoforms may be expressed in the pancreas while only those isoforms inhibitable by SB203580 may be responsive to hyperstimulation stress.
From our data it is clear that neither JNK nor p38 play any role in acinar stimulus secretion coupling, which is in agreement with previous reports by us and others [8,13]. However, our data provides further evidence that stress kinases play an important role early during pancreatitis. Although the exact roles of JNK or p38 for cellular stress responses are still not clear, it has been suggested that these pathways may at least in part be redundant and may substitute each other [20]. Interestingly in our system, p38 and JNK pathways did not appear as redundant systems. JNK inhibition clearly ameliorated cerulein pancreatitis, reducing pancreatic edema formation following cerulein hyperstimulation . This indicates that JNK activation may promote pancreatic injury. Accordingly, JNK activation has been widely reported to induce cellular damage such as apoptotic or necrotic cell death[20].
In contrast, p38 inhibition aggravated cerulein pancreatitis, indicating that p38 activation may support protection of the pancreas against damage through hyperstimulation stress. Recent evidence from other systems indicates that p38 activation can indeed be protective. Thus, p38 is reportedly important for protection observed after ischemic precondition in myocardial cells and it is evidenced that p38 may exert protective effects via its substrate MAPKAPK2 (MAPK activated protein kinase 2)[14,21].
Our investigation does not allow definitive conclusions about the mechanism of SB203580 actions on the course of cerulein pancreatitis. Based on theoretical considerations, some speculations about mechanisms are possible, however. The deletorious effect of p38 inhibition was apparent biochemically with higher pancreatic levels of active trypsin following cerulein hyperstimulation in vivo and, most importantly, histologically with the appearance of necrosis. Premature intracellular trypsin activation has long been thought to play a key role in pancreatitis development[22-24]. Recent evidence to support the importance of active trypsin for the development of pancreatitis came from two sources. First, the discovery of the underlying genetic defect of hereditary pancreatitis showed that, trypsin mutations are sufficient to induce pancreatitis[25,26] second, acinar cells respond to secretory hyperstimulation with rapid conversion of the inactive zymogen precursor trypsinogen to its active form, trypsin[27-29]. Our data could, therefore, be interpreted that p38 kinase inhibition increases pancreatic active trypsin content, allowing necrosis to occur. Our model does not allow us to distinguish whether the active trypsin was mainly present intracellularly or in the pancreatic interstitial space.
Interestingly, it has also been reported that large amounts of trypsinogen are released into the interstitial space during cerulein pancreatitis and that activation of interstitial trypsinogen through concomitant infusion of enterokinase induces necrosis in that model[30]. It is believed that digestive enzymes accumulate in the interstitial space during pancreatitis induction due to missorting of zymogen granules to the basolateral rather than the apical membrane and that missorting is at least in part related to disturbances of the acinar cytoskeletal organization. In the pancreas, it has been demonstrated that secretagogues can activate the p38-MAPKAPK2 pathway[8,10], which has further indicated that secretagogues induce HSP27 (heat shock protein 27) phosphorylation via the p38-MAPKAPK2 pathway[8]. HSP27 phosphorylation via the p38-MAPKAPK2 pathway has further been shown to stabilize the acinar actin cytoskeleton against the effects of secretagogue stimulation[8]. Inaddition, there is evidence that the p38-MAPKAPK2-HSP27 pathway may be important for organ protection in the pancreas.
Although speculative, it appears possible that during cerulein pancreatitis induction, p38 activation may help reduce acinar damage through its action on MAPKAPK2-HSP27 with increased cytoskeletal stability and less accumulation of active trypsin in the interstitial space. In this model, interruption of the p38-MAPKPAK2-HSP27 pathway through SB203580 could then allow necrosis to occur after cerulein hyperstimulation. The exact mechanism of SB203580 action will have to be determined through additional studies.
In conclusion, we have demonstrated that stress kinases play no role in acinar stimulus secretion coupling. We have also shown that inhibition of JNK and p38 kinase oppositely influences the course of cerulein pancreatitis. SB203580 increases pancreatic active trypsin content and also induces necrosis following cerulein hyperstimulation although necrosis is typically not a feature of cerulein pancreatitis. Our current working hypothesis is that p38 kinase activation may stabilize the actin cytoskeleton via the p38-MAPKAPK2-HSP27 pathway. Inhibition of p38 kinase then might increase missorting of secretory granules with higher amounts of trypsinogen/trypsin secretion into the interstitial space and concomitant development of necrosis.
Edited by Ma JY
| 1. | Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143-180. [PubMed] |
| 2. | Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2021] [Cited by in RCA: 2089] [Article Influence: 65.3] [Reference Citation Analysis (6)] |
| 3. | Sánchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis JM, Zon LI. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 815] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 4. | Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2031] [Cited by in RCA: 2054] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 5. | Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1295] [Cited by in RCA: 1331] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 6. | Grady T, Dabrowski A, Williams JA, Logsdon CD. Stress-activated protein kinase activation is the earliest direct correlate to the induction of secretagogue-induced pancreatitis in rats. Biochem Biophys Res Commun. 1996;227:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Dabrowski A, Grady T, Logsdon CD, Williams JA. Jun kinases are rapidly activated by cholecystokinin in rat pancreas both in vitro and in vivo. J Biol Chem. 1996;271:5686-5690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Schäfer C, Ross SE, Bragado MJ, Groblewski GE, Ernst SA, Williams JA. A role for the p38 mitogen-activated protein kinase/Hsp 27 pathway in cholecystokinin-induced changes in the actin cytoskeleton in rat pancreatic acini. J Biol Chem. 1998;273:24173-24180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Metzler W, Höfken T, Weber H, Printz H, Göke B, Wagner AC. Hyperthermia, inducing pancreatic heat-shock proteins, fails to prevent cerulein-induced stress kinase activation. Pancreas. 1999;19:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Wagner AC, Metzler W, Höfken T, Weber H, Göke B. p38 map kinase is expressed in the pancreas and is immediately activated following cerulein hyperstimulation. Digestion. 1999;60:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Glicksman MA, Chiu AY, Dionne CA, Harty M, Kaneko M, Murakata C, Oppenheim RW, Prevette D, Sengelaub DR, Vaught JL. CEP-1347/KT7515 prevents motor neuronal programmed cell death and injury-induced dedifferentiation in vivo. J Neurobiol. 1998;35:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Maroney AC, Glicksman MA, Basma AN, Walton KM, Knight E, Murphy CA, Bartlett BA, Finn JP, Angeles T, Matsuda Y. Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J Neurosci. 1998;18:104-111. [PubMed] |
| 13. | Wagner AC, Mazzucchelli L, Miller M, Camoratto AM, Göke B. CEP-1347 inhibits caerulein-induced rat pancreatic JNK activation and ameliorates caerulein pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2000;278:G165-G172. [PubMed] |
| 14. | Weinbrenner C, Liu GS, Cohen MV, Downey JM. Phosphorylation of tyrosine 182 of p38 mitogen-activated protein kinase correlates with the protection of preconditioning in the rabbit heart. J Mol Cell Cardiol. 1997;29:2383-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 179] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Wagner AC, Wishart MJ, Yule DI, Williams JA. Effects of okadaic acid indicate a role for dephosphorylation in pancreatic stimulus-secretion coupling. Am J Physiol. 1992;263:C1172-C1180. [PubMed] |
| 16. | Groblewski GE, Grady T, Mehta N, Lambert H, Logsdon CD, Landry J, Williams JA. Cholecystokinin stimulates heat shock protein 27 phosphorylation in rat pancreas both in vivo and in vitro. Gastroenterology. 1997;112:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Wagner AC, Weber H, Jonas L, Nizze H, Strowski M, Fiedler F, Printz H, Steffen H, Göke B. Hyperthermia induces heat shock protein expression and protection against cerulein-induced pancreatitis in rats. Gastroenterology. 1996;111:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Kawabata S, Miura T, Morita T, Kato H, Fujikawa K, Iwanaga S, Takada K, Kimura T, Sakakibara S. Highly sensitive peptide-4-methylcoumaryl-7-amide substrates for blood-clotting proteases and trypsin. Eur J Biochem. 1988;172:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Lee JC, Kassis S, Kumar S, Badger A, Adams JL. p38 mitogen-activated protein kinase inhibitors--mechanisms and therapeutic potentials. Pharmacol Ther. 1999;82:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 281] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313-24316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 865] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 21. | Nakano A, Baines CP, Kim SO, Pelech SL, Downey JM, Cohen MV, Critz SD. Ischemic preconditioning activates MAPKAPK2 in the isolated rabbit heart: evidence for involvement of p38 MAPK. Circ Res. 2000;86:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Steer ML. Frank Brooks memorial Lecture: The early intraacinar cell events which occur during acute pancreatitis. Pancreas. 1998;17:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Steer ML, Meldolesi J. The cell biology of experimental pancreatitis. N Engl J Med. 1987;316:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 149] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Whitcomb DC, Preston RA, Aston CE, Sossenheimer MJ, Barua PS, Zhang Y, Wong-Chong A, White GJ, Wood PG, Gates LK. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996;110:1975-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 192] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Amann ST, Toskes PP. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 1039] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 27. | Saluja AK, Donovan EA, Yamanaka K, Yamaguchi Y, Hofbauer B, Steer ML. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology. 1997;113:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Saluja AK, Bhagat L, Lee HS, Bhatia M, Frossard JL, Steer ML. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol. 1999;276:G835-G842. [PubMed] |
| 29. | Hofbauer B, Saluja AK, Lerch MM, Bhagat L, Bhatia M, Lee HS, Frossard JL, Adler G, Steer ML. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol. 1998;275:G352-G362. [PubMed] |
| 30. | Hartwig W, Jimenez RE, Werner J, Lewandrowski KB, Warshaw AL, Fernández-del Castillo C. Interstitial trypsinogen release and its relevance to the transformation of mild into necrotizing pancreatitis in rats. Gastroenterology. 1999;117:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |