INTRODUCTION
Cholesterol gallstone formation involves multiple steps including hepatic secretion of lithogenic bile[1,2] and nucleation and growth of cholesterol crystals within the gallbladder[3]. Besides cholesterol supersaturation[4] and gallbladder hypomotility[2,5], kinetic protein factors that promote or inhibit cholesterol crystallization have been suggested to play key roles in cholesterol gallstone pathogenesis[6,7]. During the past decade, model bile studies indicated that several promoter or inhibitor proteins influence cholesterol crystallization in vitro[8-14]. Immunoglobulins were also identified as nucleating proteins in the gallbladder bile of patients with cholesterol gallstones[15,16]. However, the significance of individual protein factors for the pathogenesis of the human disease remains controversial[17,18].
By preparing crystal adsorbed proteins via sucrose density gradient centrifugation and gel electrophoresis, we identified a subgroup of human biliary proteins that bind to cholesterol crystals, modify crystal morphology and inhibit cholesterol crystallization [19]. We demonstrated that secretory immunoglobulin A (IgA) is a major constituent of this group of cholesterol crystal-binding proteins[20]. To assess the importance of biliary IgA for cholesterol crystal agglomeration into gallstones in vivo, we now visualized and characterized the binding of biliary IgA to the cholesterol crystal surface.
MATERIALS AND METHODS
Chemicals and analytical procedures
Cholesterol was obtained from Eastman Kodak Co. (Rochester, NY, USA) and its purity was verified by HPLC to be > 99%[21]. Sodium taurocholate was obtained from Sigma Chemical Co. (St. Louis, MO, USA) . Concanavalin A and Helix pomatia - lectin-sepharose were purchased from Pharmacia Fine Chemicals (Uppsala, Sweden). All other chemicals were reagents or HPLC grade quality (Sigma Chemical Co.; Merck AG, Darmstadt, Germany).
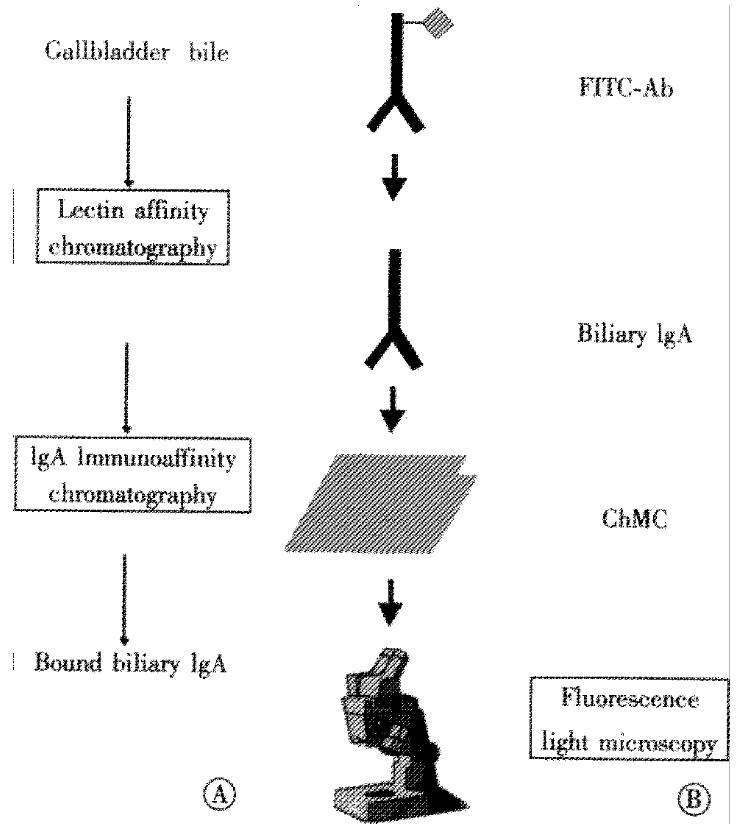
Isolation of biliary IgA by immunoaffinity chromatography (Figure
1A)
Figure 1 (A) Biliary IgA was isolated from human gallbladder bile by lectin affinity in sequence with immunoaffinity chromatography.
(B) Cholesterol crystal binding of biliary IgA to cholesterol monohydrate crystals (ChMC) was visualized microscopically with fluoresceine isothiocyanate-conjugated anti-human IgA antibodies (FITC-Ab).
Gallbladder bile from patients with cholesterol gallstones was obtained by needle aspiration during cholecystectomy. The protocol was approved by the ethical committee of RWTH Aachen. To remove insoluble constituents, bile samples were ultracentrifuged at 100000 ×g for 1 h. Biliary protein fractions were isolated by affinity chromatography using -Concanavalin A and Helix pomatia- lectin, as described[19,20].
Biliary IgA was isolated from the bound protein fraction by immunoaffinity chromatography[20]. Briefly, affinity columns were prepared by coupling rabbit polyclonal antibodies against the human immunoglobulin á-chain (Sigma Chemical Co.) to Aminolink coupling gel (Immunopure- Immoblization Kit, Pierce Chemical Co., Rockford, IL, USA). After washing the column with 1 M NaCl and equilibration with Tris-buffered saline (TBS; 20 mM Tris/HCl, pH 7.4, 200 mM NaCl, 3 mM NaN3), biliary proteins (2-3 mg) were applied to the affinity column overnight. The column was washed extensively with TBS containing 10 mM sodium taurocholate to remove any unbound material and residual lipids. Afterwards, the bound IgA was eluted from the column with 10 mL 0.1 M glycine/HCl (pH 2.5) into a tube containing 500 μL 1 M Tris/HCl (pH 9.5), and dialyzed against 25 mM ammonium bicarbonate. All steps were carried out at 4 °C.
Immunofluorescence studies (Figure
1B)
Cholesterol monohydrate crystals were prepared by recrystallization from 95% (v/v) ethanol according to Igimi and Carey[22]. Five grams cholesterol was dissolved in 400 mL ethanol at 60 °C and cooled slowly to room temperature. To obtain predominantly small, intact crystals, the solution was stirred at 30 rpm. The cholesterol crystals were harvested by filtration through a 0.45 μm micropore filter (Millipore Corp., Bedford, MA) and checked under light microscopy.
Cholesterol monohydrate crystals were resuspended in phosphate buffered saline (PBS; 9 .6 mM phosphate, pH 7.2, 2.7 mM KCl, 137 mM NaCl, 3 mM NaN3) containing 1% (w/v) bovine serum albumin (BSA) to reduce non-specific crystal binding. Samples were incubated at 37 °C for 1 h and shaken at 100 rpm. After washing the crystals three times with PBS containing 0.1% (w/v) BSA (PBS/BSA), IgA was added in PBS/BSA to a final concentration of 200 μg/L. The sample was incubated for 2 h at 37 °C with rapid agitation (100 rpm). After another four washes with PBS/BSA, fluoresceine isothiocyanate (FITC)-conjugated anti-human IgA (Sigma Chemical Co.) was added in PBS/BSA (dilution 1 : 10). The sample was incubated for 1 h at 37 °C. After two final rinses, the suspension was taken up in 1mL PBS/BSA. Samples without IgA or with human IgG (Sigma Chemical Co.) served as controls.
Small specimen (25 μL) were examined under polarizing and fluorescence light microscopy (Leica DM RB, fluorescence filter I3). Microphotographs (Minolta 9000) were processed with Corel PhotopaintTM (Version 7.0; Corel Corporation, Dublin, IL).
RESULTS
Utilizing the information on the identity of cholesterol crystal-binding proteins[20], we isolated biliary IgA by lectin affinity chromatography and immunoaffinity chromatography. The isolation procedure yielded electrophoretically homogeneous preparations of biliary IgA, as judged by silver-stained sodium dodecyl-sulfate polyacrylamide gel electrophoresis and immunoblotting ( not shown), thus confirming the results of our previous study[20].
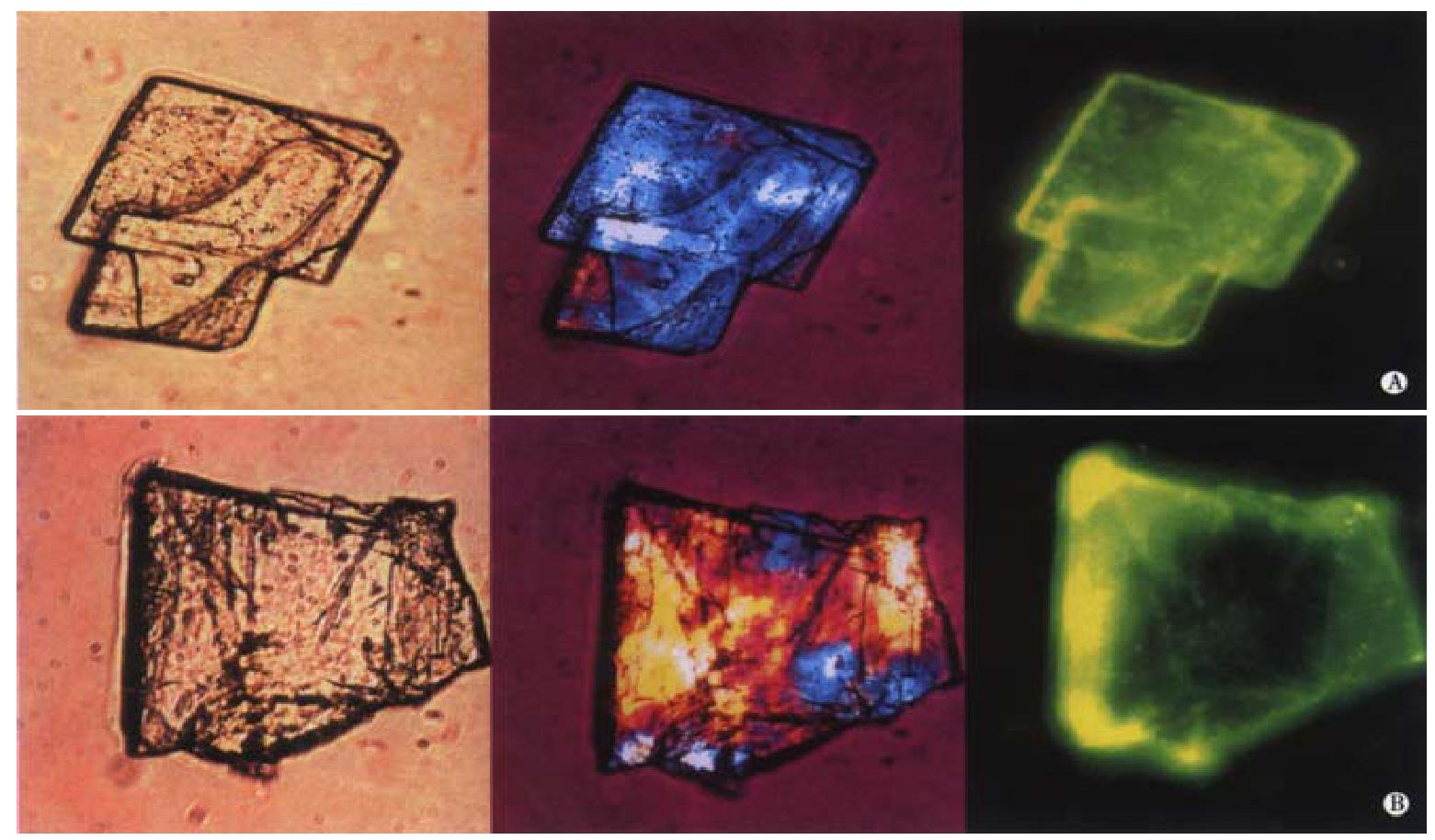
The immunofluorescence studies clearly demonstrate significant binding of biliary IgA to cholesterol monohydrate crystals. After incubation of the cholesterol crystal suspension with biliary IgA, followed by the secondary antibody conjugated to FITC, fluorescence is observed dispersed on the surface of the crystals. The left, middle and right panels of Figure 2A and Figure 2B display two representative cholesterol crystals by light, polarizing and fluorescence light microscopy, respectively. The basic unit is the plate-like cholesterol monohydrate crystal, which shows an euhedral triclinic form with straight edges and a smooth surface (Figure 2A). In addition, the crystals tend to aggregate and to form more irregular structures (Figure 2B). The two right microphotographs of Figure 2A and Figure 2B show how the specific yellow FITC fluorescence is distributed on the crystal surface: Peak fluorescence signals occur at crystal edges as well as screwed and stepwise dislocations, consistent with crystal binding sites with a high affinity to IgA.
Figure 2 Binding of biliary IgA to cholesterol monohydrate crystals.
Crystals were treated with biliary IgA, followed by FITC-Ab (see Figure 1). Left, middle and right panels of show light, polarizing and fluorescence light microphotographs, respectively.
Figure 3 displays control samples with IgG (A) and without IgA (B). The crystals exhibit no or only little spotted fluorescence, with blank crystals set off against the green background activity.
Figure 3 Cholesterol monohydrate crystals with IgG and FITC-Ab incubation (see Figure 1).
Cholesterol monohydrate crystals without IgA incubation (FITC-Ab only). Left and right panels show polarizing and fluorescence light microphotographs, respectively.
Separate experiments were carried out to reduce residual non-specific binding by pre-incubation with BSA (data not shown), and 0.1% (w/v) BSA was used throughout the experiments. This is consistent with recent immunofluorescence studies demonstrating that albumin binds to randomly located little spots on the crystal surface[23], although albumin is not a crystal-binding protein sui generis and does not modulate cholesterol crystallization in model bile[24].
DISCUSSION
Gallbladder bile contains several proteins that exhibit promoting or inhibiting effects on cholesterol crystallization in vitro. Besides physical-chemical determinants[4] and hypomotility of the gallbladder, these kinetic protein factors are assumed to modulate cholesterol gallstone formation[7,25]. Because systematic studies in reconstituted supersaturated human bile (cholesterol saturation index > 1.6) did not affect cholesterol crystallization sequences or crystal detection times[17], biliary proteins might predominantly affect subsequent cholesterol crystal agglomeration and growth into mature stones[26].
Bile formation is a means of delivering immunoglobulin into the biliary system and the intestine. The major biliary immunoglobulin is IgA, which enhances the immune protection of mucus membranes and epithelial cells by binding injurious agents or toxins[27]. In humans, polymeric IgA is the predominant class of biliary immunoglob ulins[28,29]. It is secreted by the liver and the gallbladder[30] as complex (secretory IgA), consisting of IgA monomers and the secretory component that is necessary for the endocytotic path way of IgA across the biliary epithelial cells. Our previous studies demonstrated that secretory IgA inhibits cholesterol crystallization in supersaturated model bile in a dose-dependent manner; maximum crystal growth rate and final crystal concentration were significantly reduced even below physiological concentrations[20]. Furthermore, the presence of IgA and other crystal-binding proteins changed crystal morphology, favoring compact and regular microcrystals. However, direct visualization of IgA binding to the crystal surface was not demonstrated.
The interaction between biliary IgA and cholesterol monohydrate crystals could not be visualized by immunoelectron microscopy using anti-human IgA labeled with colloidal gold (mean particle size 4 nm), because the crystal structures were unstable under the conditions tested. We therefore employed immunofluorescence, which was successfully applied in parallel studies that investigated the protein contents of cholesterol gallstones[31] and biliary sludge[23]. Our fluorescence light microscopy studies confirmed cholesterol crystal binding properties of biliary IgA. These findings are in line with localization of IgA in biliary sludge where it was clearly associated with aggregations of cholesterol crystals, which resemble the concentric cholesterol layers at the periphery of stones[23]. These findings support the hypothesis that crystal-binding IgA might be an important modulator of cholesterol crystal agglomeration into stones and stone growth in vivo.
The interaction between IgA and cholesterol crystals could not only depend on the distinct immunoglobulin class but might predominantly rely on the variable ends of the light chains. This hypothesis is supported by the synthesis of specific antibodies against cholesterol upon injection of cholesterol-rich liposomes in rabbits and mice[32,33]. These antibodies recognize non-oxidized crystalline cholesterol[32], and analyses by solid-phase ELISA revealed that human sera contain varying levels of naturally occurring autoantibodies to cholesterol[34]. Similarly, humans might synthesize and secrete specific IgA antibodies to cholesterol into bile. This would explain why biliary IgA inhibits cholesterol crystal growth, in contrast to IgA from colostrum[20] and other immunoglobulins, which promote cholesterol crystallization in various experimental settings[15,35]. Using a modified ELISA technique for rapid, precise measurement of protein binding to cholesterol crystals[33], we have recently shown that binding of purified biliary IgA is more than 10-fold increased compared to colostrum IgA (Südfeld S, Lammert F, Busch N, Matern S; unpublished observations).
We speculate that crystal growth inhibitors like biliary IgA attach to the most rapidly growing sites like spiral and edge dislocations on the thermodynamically stable monohydrate crystals, thus changing crystal morphology, retarding crystal growth and decreasing final crystal concentration[19]. This concept applies to many crystal growth inhibitors in biomineralization. Concerning formation of other stones in humans, Nakagawa et al reported a glycoprotein that prevents formation of urinary calcium oxalate stones in healthy persons[36]. From human pancreatic calculi, a small acidic glycoprotein could be isolated that inhibits calcium carbonate precipitation[37]. Other glycoproteins regulate the precipitation of calcium salts in bone and dentin[38,39]. Additional examples of crystal growth inhibiting glycoproteins are found in sera of polar fish and insects, whose ‘antifreeze’ proteins prevent freezing by adsorbing to ice crystal surfaces[40,41]. These examples illustrate that the concept of crystal growth inhibition by crystal binding probably applies to all inhibitor proteins in biomineralization.