Published online Apr 15, 2001. doi: 10.3748/wjg.v7.i2.152
Revised: March 19, 2001
Accepted: March 21, 2001
Published online: April 15, 2001
- Citation: Dahl SV, Kircheis G, Haussinger D. Hepatic encephalopathy as a complication of liver disease. World J Gastroenterol 2001; 7(2): 152-156
- URL: https://www.wjgnet.com/1007-9327/full/v7/i2/152.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i2.152
Hepatic encephalopathy (HE) is a frequent complication of chronic liver disease. It is defined as a characteristic functional and reversible alteration of the mental state, due to impaired liver function and/or increased portosystemic shunting.
In the brain of HE patients, neurons appear morphologically normal, but astrocytes show signs of Alzheimer type II degeneration, i.e. nuclear enlargement, peripheral margination of chromatin and prominent nucleoli. Ammonia is generally considered to play a central role in the pathophysiology of HE[1]. In HE, selective alterations of blood-brain barrier permeability, changes in cerebral energy metabolism, an increased GABA-ergic tone, changes in neurotransmitter systems and alterations of gene expression. e.g. of monoamine oxidase, peripheral-type benzodiazepine receptor (PTBR) and neuronal NO synthase are found (reviewed in[1-10]. The reversibility of HE symptoms and the reason for its precipitation by a variety of different factors has not been sufficiently explained yet (Table 1). Central insights into the etiology of HE have arisen from recent in vitro work with astrocytes. Astrocytes are important constituents of the blood-brain barrier, and uptake of substances from the blood into the brain is achieved by transastrocytic transport. Astrocytes communicate directly with neurons[11], regulate neurotransmitter processing and ionic milieu and provide substrates for neurons[12,13]. In brain, astrocytes are the only cells containing glutamine synthetase[14] and represent the major site of cerebral ammonia detoxification. Upon exposure to ammonia, cultured astrocytes develop Alzheimer type II changes. These findings prompted the idea that HE is a disorder of glial cells with a consecutive neuronal dysfunction[7,15,16].
| ·Gastrointestinal or tissue bleeding |
| ·Protein overload |
| ·Sepsis |
| ·Infection |
| ·Catabolism |
| ·Azotemia |
| ·Acidosis |
| ·Sedatives |
| ·Diuretics |
| ·Portocaval shunting (TIPS or surgical) |
| ·Constipation |
Although the symptoms of HE in acute or chronic liver failure are different, there are good reasons to assume that the pathophysiology of both conditions is similar, but may involve different kinetics. In acute liver failure, astrocytes swell and brain edema develops[17]. HE in chronic liver disease is not accompanied by clinical signs of cerebral edema, but evidence for increased cell hydration has been given, as described later. A disturbance of astrocyte hydration is apparently a major pathophysiologic event in both forms.
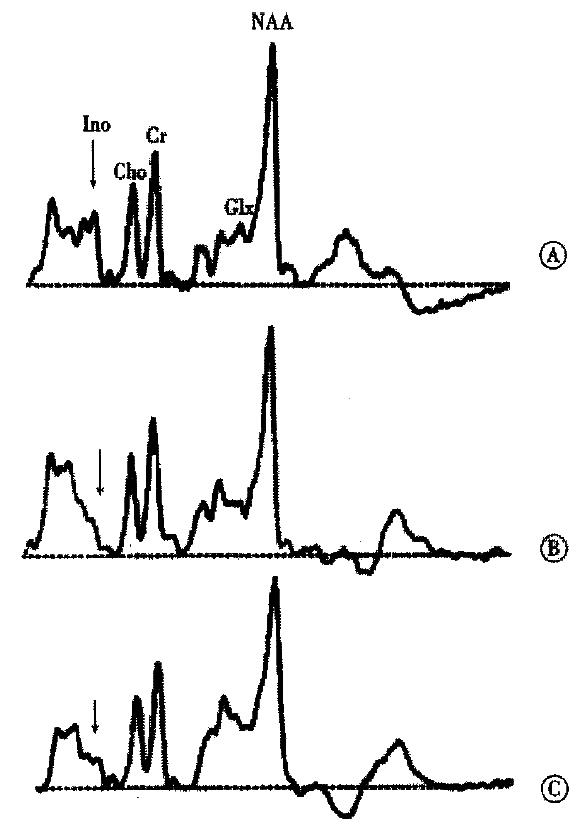
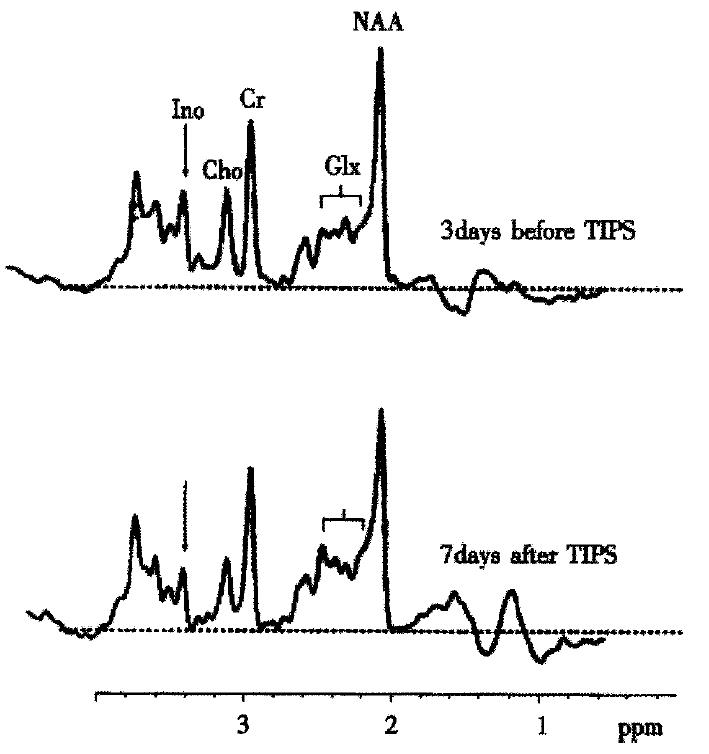
MR-spectroscopy (H-MRS) studies in human brain initiated the idea that a disturbance of astrocyte cell volume homeostasis could be decisive for development of chronic HE[18-20]. 1H-MRS can be used to study metabolic abnormalities in the human brain in vivo and allows a myo-inositol signal to be picked up, which represents an osmosensitive myo-inositol pool[18] of predominantly glial origin[21]. Myo-inositol is an organic osmolyte in astrocytes[22-24]. Such organic osmolytes play a decisive role in cell volume regulation: upon shrinkage, they accumulate inside the cells, in response to cell swelling they can be released from the cells via osmoregulated membrane channels[25,26]. Consistently, in vivo1H-MRS studies on the brain from cirrhotic patients with HE show a depletion of myo-inositol which is accompanied by an increase in the glutamine/ glutamate signal[18,19,27-30], as shown in Figure 1. On the other hand, in cirrhotic patients, the implantation of transjugular intrahepatic-stent-shunt (TIPS) may lead to an aggravation of 1H-MRS changes (Figure 2)[18] and a normalization of MRS findings after transplantation has been described[30]. In vitro studies from the rat have shown similar alterations after portocaval shunting[28]. There is a good correlation between the extent of these 1H-MRS changes and the clinical severity of HE[18,19,29,30]. An increased glutamine/glutamate signal, together with adecrease of inositol signal, is observed in patients after TIPS implantation[18], as depicted in Figure 2. A high sensitivity and specificity of the myo-inositol signal for the diagnosis of HE cirrhotics has been reported[29,30], but these changes have also been reported in asymptomatic stages of hepatic encephalopathy[18,19,29,30].
The MRS findings in HE make an impaired cell volume homeostasis in brain likely and suggest that cellular non-cytotoxic edema[31] is present in hepatic encephalopathy. This edema is the result of an osmotically active intracellular accumulation of glutamine in response to hyperammonemia and a consecutive depletion of releasable myo-inositol and probably other osmolytes. Astrocytes swell in presence of ammonia[7,32]. Ammonia induces brain edema and intracranial hypertension in the portocaval shunted rat in vivo in a largely methionine sulfoximine-sensitive way[33]. PET studies with 15N-ammonia on human brain from encephalopathic patients showed an increased cerebral metabolic rate for ammonia, suggestive of enhanced cerebral uptake of ammonia in HE and a stimulation of glutamine synthesis[34]. Astrocyte swelling also occurs in vitro under the influence of hyponatremia[35,36], some neurotransmitters[35,37], TNF-α[38] and benzodiazepines[7,37]. Apart from myo-inositol, recent data suggest that other organic osmolytes, such as taurine[21,22] and α-glycerophosphorylcholine[22,39], are depleted in order to counteract astrocyte swelling in HE. Small increases in astrocyte water content, as may occur in HE, could already have important functional consequences despite the absence of clinically overt increased intracranial pressure. Cell hydration is an independent signal which regulates cell function and gene expression, reviewed in[40-43] and a variety of different osmosignalling pathways linking cell hydration and cell function have been identified [44]. Extensive work from this laboratory has described the impact of cell hydration on cell function, cytoskeleton and gene expression[45], mainly in liver. In brain, cell hydration is also considered a key trigger of cell function. Swelling of astrocytes in culture activatesviaphosphatidylinositol-3-kinaseextracellular regulated protein kinases (Erks)[36], i.e. members of the MAP kinase family with multiple functions, elevates intracellular calcium concentrations[46] and upregulates the peripheral type benzodiazepine receptor (PBR, reviewed in[47]) at the level of agonist binding[48] and mRNA (D. Häussinger and R. Fischer, unpublished results). Further, astrocyte swelling increases the pH in endocytotic vesicles[49] in an Erk-dependent osmosignalling pathway (R. Fischer and D. Häussinger). Several key findings in HE can thus partially be explained by an increase of astrocyte hydration. The endosomal alkalinization following astrocyte swelling could affect receptor densities and neurotransmitter processing and swelling-induced changes of the activity of plasma membrane transporters may underlie the selective changes in “blood-brain barrier” permeability of HE. Cell swelling stimulates glycogen synthesis and inhibits glycogenolysis[45,50] and the increased deposition of glycogen in astrocytes in animal models of chronic HE[7] may reside on cell swelling. Astrocyte swelling leads to an increased expression of PBR and augments the synthesis of neurosteroids, which are potent modulators of neuronal GABAA activity[7]. Thus the interaction between astrocyte swelling, PBR expression and increased neurosteroid synthesis may explain the increased GABAA-ergic tone in HE[9,51].
An increase in astrocyte hydration, i.e. a low-grade cerebral edema, is a major pathogenetic event in the development of HE and induces a profound alteration of astrocyte function [20] . Altered astrocyte function may eventually lead to a disturbance of glioneuronal communication and present as the clinical syndrome of HE. Bleeding, infection, sedatives or electrolyte imbalance may precipitate HE in the cirrhotic patient (Table 1). Apart from ammonia, an increase of astrocyte hydration is also induced by hyponatremia, benzodiazepines and cytokines. Multiple factors could thus obviously result in a common pathogenetic endpath, i.e. glial swelling with its functional consequences. The osmolyte systems for counteraction of cell swelling are intact in non-cirrhotics and the precipitating conditions are well tolerated. In cirrhosis, however, organic osmolyte depletion is observed in order to compensate for glial glutamine accumulation and further challenges of cell volume can hardly be counteracted. The 1H-MRS findings in nonencephalopathic cirrhotics could represent an early stage of a largely compensated disturbance of astrocyte volume homeostasis, where only few consequences yet for astrocyte hydration and function are observed. In response to HE-precipitating factors, a dysequilibrium of astrocyte volume results and hydration-dependent alterations of glial function will become clinically apparent. This unstable situation may explain the rapid kinetics of HE episodes and why severe brain edema with fatal outcome can occasionally develop in endstage cirrhotics[52].
Usually, HE is due to extensive porto-venous collateral shunting together with a decrease in hepatic function, resulting in increased cerebral ammonia load and diminished ammonia detoxification. Fulminant hepatic failure (FHF) means acute liver failure accompanied by hepatic encephalopathy. Sometimes, HE may be the result of metastatic liver disease[53], portal vein thrombosis[54], congestive heart failure[55] or constrictive pericarditis[56]. Even in the absence of overt liver disease, portosystemic shunting can induce HE[57], reviewed in[58]. Pre-TIPS encephalopathy is an important predictor of death during follow-up after placement of TIPS[59].
The symptoms of encephalopathy in all of these circumstances are characteristic, but unspecific. They range from subtle neuropsychologic derangements to coma. The diagnosis is made be the recognition of an appropriate hepatic disorder and the presence of encephalopathy in the absence of any other likely non-hepatic causes.
Foetor hepaticus and an increased blood ammonia concentration may contribute to the diagnosis.
For study purposes, an exact quantification of HE is required and defined by the West Haven Criteria [60,61]. The PSE index comprises the mental state, asterixis, number connection test results, electroencephalography and arterial blood ammonia concentrations. Subclinical hepatic encephalopathy (SHE) can only be diagnosed by subtle neuropsychological testing[62]. Preliminary results show that hepatic retinopathy, as detected by neurophysiological testing, very sensitively reflects the degree of HE (G. Kircheis and D. H-ussinger, unpublished observation) and responds to HE therapy. At the bedside, HE grade I is characterized by desorientation, whereas grade II HE shows spontaneous or inducible asterixis. In HE grade III, the patient is somnolent, grossly desoriented and precomatose, whereas grade IV represents coma.
Treatment of HE focusses on the pathogenetic events present in the individual patient. The most important therapeutic approach is to identify the precipating factors (Table 1) and to treat them vigorously. The required measures for precipitating factors are: therapy of GI bleeding together with bowel cleaning by lactulose, antibiotic treatment of concurrent infection, protein restriction, parenteral nutrition by an i.v. line and discontinuation of any diuretic therapy or sedatives. In patients with deterioration of HE after TIPS implantation, a reduction stent may sometimes be necessary[63,64]. Apart from treatment of precipitating factors, additional therapeutic measures interfere with ammonia generation/disposal. In placebo-controlled studies, conflicting data exist on the efficacy of most therapeutic substances. Administration of lactulose is considered as gold standard in the treatment of HE, even for subclinical encephalopathy[65], but the beneficial effect of lactulose has not been precisely shown versus placebo, reviewed in[66]. In a recent randomized crossover trial, plasma ammonia and nitrogen balance were significantly better on vegetable protein diet as compared to an isonitrogenousanimal protein diet[67]. Neomycin is equally effective as lactulose, but a placebo-controlled trial on neomycin showed little effectivity[68]. Intravenous ornithine aspartate has proven its effectivity[69,70]and the benefit of orally administered ornithine aspartate is currently evaluated. Most studies on oral branched-chain amino acids (BCAA) showed clinical improvement of latent or low-grade HE and of protein tolerance[71], but studies on i.v. BCAA have not led yet to definite results . Benzodiazepine receptor antagonists have been reported to be of value in HE[72,73] but, to date with only modest success in some patients. In experimental cirrhosis, zinc supplementation reduces blood ammonia and increases liver ornithine transcarbamlyase activity[74] and a positive effect of zinc supplementation has also been shown in clinically overt HE[75].
| 1. | Butterworth RF, Giguère JF, Michaud J, Lavoie J, Layrargues GP. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol. 1987;6:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 289] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Basile AS, Jones EA. Ammonia and GABA-ergic neurotransmission: interrelated factors in the pathogenesis of hepatic encephalopathy. Hepatology. 1997;25:1303-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Ferenci P, Püspök A, Steindl P. Current concepts in the pathophysiology of hepatic encephalopathy. Eur J Clin Invest. 1992;22:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Lockwood AH. Hepatic encephalopathy. Boston: Butterworth-Heinemann. 1992;. |
| 5. | Jalan R, Seery JP, Taylor-Robinson SD. Review article: pathogenesis and treatment of chronic hepatic encephalopathy. Aliment Pharmacol Ther. 1996;10:681-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Norenberg MD, Neary JT, Bender AS, Dombro RS. Hepatic encephalopathy: a disorder in glial-neuronal communication. Prog Brain Res. 1992;94:261-269. [PubMed] |
| 7. | Norenberg MD, Itzhak Y, Bender AS, Baker L, Aguilaa2Mansilla N, Zhou BG, Issacks R. Ammonia and astrocyte function. In: H-ussinger D, Jungermann K, eds. Liver and nervous system. Lancaster:. Kluwer Press. 1998;276-293. |
| 8. | Butterworth RF. Complications of cirrhosis III. Hepatic encephalopathy. J Hepatol. 2000;32:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Bender AS, Norenberg MD. Effect of ammonia on GABA uptake and release in cultured astrocytes. Neurochem Int. 2000;36:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Butterworth RF. Alterations of neurotransmitter-related gene expression in human and experimental portal-systemic encephalopathy. Metab Brain Dis. 1998;13:337-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 11. | Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 770] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 12. | Murphy S ed. Astrocytes-pharmacology and function. San Diego: Academic Press. 1993;. |
| 13. | Kimelberg H, Ransom BR, ed . Neuroglia. New York: Oxford Uni-versity Press. 1995;. |
| 14. | Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 905] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 15. | Norenberg MD, Bender AS. Astrocyte swelling in liver failure: role of glutamine and benzodiazepines. Acta Neurochir Suppl (Wien). 1994;60:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Norenberg MD. Astrocytic-ammonia interactions in hepatic encephalopathy. Semin Liver Dis. 1996;16:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 109] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Blei AT, Larsen FS. Pathophysiology of cerebral edema in fulminant hepatic failure. J Hepatol. 1999;31:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 18. | Häussinger D, Laubenberger J, vom Dahl S, Ernst T, Bayer S, Langer M, Gerok W, Hennig J. Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology. 1994;107:1475-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 281] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Laubenberger J, Häussinger D, Bayer S, Gufler H, Hennig J, Langer M. Proton magnetic resonance spectroscopy of the brain in symptomatic and asymptomatic patients with liver cirrhosis. Gastroenterology. 1997;112:1610-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 131] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Häussinger D, Kircheis G, Fischer R, Schliess F, vom Dahl S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema. J Hepatol. 2000;32:1035-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 306] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Zwingmann C, Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR spectroscopy studies on NH4Cl-induced metabolic alterations and detoxification processes in primary astrocytes and glioma cells. Dev Neurosci. 1998;20:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Cordoba J, Gottstein J, Blei AT. Glutamine, myo-inositol, and organic brain osmolytes after portocaval anastomosis in the rat: implications for ammonia-induced brain edema. Hepatology. 1996;24:919-923. [PubMed] [DOI] [Full Text] |
| 23. | Isaacks RE, Bender AS, Kim CY, Shi YF, Norenberg MD. Effect of osmolality and anion channel inhibitors on myo-inositol efflux in cultured astrocytes. J Neurosci Res. 1999;57:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Paredes A, McManus M, Kwon HM, Strange K. Osmoregulation of Na(+)-inositol cotransporter activity and mRNA levels in brain glial cells. Am J Physiol. 1992;263:C1282-C1288. [PubMed] |
| 25. | Burg MB. Molecular basis of osmotic regulation. Am J Physiol. 1995;268:F983-F996. [PubMed] |
| 26. | Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247-306. [PubMed] |
| 27. | Kreis R, Farrow N, Ross BD. Diagnosis of hepatic encephalopathy by proton magnetic resonance spectroscopy. Lancet. 1990;336:635-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Moats RA, Lien YH, Filippi D, Ross BD. Decrease in cerebral inositols in rats and humans. Biochem J. 1993;295:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Ross BD, Jacobson S, Villamil F, Korula J, Kreis R, Ernst T, Shonk T, Moats RA. Subclinical hepatic encephalopathy: proton MR spectroscopic abnormalities. Radiology. 1994;193:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 132] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Ross BD, Danielsen ER, Blüml S. Proton magnetic resonance spectroscopy: the new gold standard for diagnosis of clinical and subclinical hepatic encephalopathy. Dig Dis. 1996;14 Suppl 1:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Kimelberg HK. Current concepts of brain edema. Review of laboratory investigations. J Neurosurg. 1995;83:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 302] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Norenberg MD, Baker L, Norenberg LO, Blicharska J, Bruce-Gregorios JH, Neary JT. Ammonia-induced astrocyte swelling in primary culture. Neurochem Res. 1991;16:833-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Blei AT, Olafsson S, Therrien G, Butterworth RF. Ammonia-induced brain edema and intracranial hypertension in rats after portacaval anastomosis. Hepatology. 1994;19:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 148] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Lockwood AH, Yap EW, Wong WH. Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cereb Blood Flow Metab. 1991;11:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 231] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Kimelberg HK, O'Connor ER, Kettenmann H. Effects of cell swelling on glial function. In: Lang F, H ussinger D, eds. Interac-tions in cell volume and function. Heidelberg: Springer Verlag. 1993;158-186. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Schliess F, Sinning R, Fischer R, Schmalenbach C, Häussinger D. Calcium-dependent activation of Erk-1 and Erk-2 after hypo-osmotic astrocyte swelling. Biochem J. 1996;320:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Bender AS, Norenberg MD. Effect of benzodiazepines and neurosteroids on ammonia-induced swelling in cultured astrocytes. J Neurosci Res. 1998;54:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 38. | Bender AS, Rivera IV, Norenberg MD. Tumor necrosis factor a induces astrocyte swelling. Trans Am Neurochem. 1992;23:113. |
| 39. | Bluml S, Zuckerman E, Tan J, Ross BD. Proton-decoupled 31P magnetic resonance spectroscopy reveals osmotic and metabolic disturbances in human hepatic encephalopathy. J Neurochem. 1998;71:1564-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Lang F, Stehle T, Häussinger D. Water, K+, H+, lactate and glucose fluxes during cell volume regulation in perfused rat liver. Pflugers Arch. 1989;413:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 41. | Häussinger D. Regulation and functional significance of liver cell volume. Prog Liver Dis. 1996;14:29-53. [PubMed] |
| 42. | Häussinger D, Schliess F, Warskulat U, vom Dahl S. Liver cell hydration. Cell Biol Toxicol. 1997;13:275-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Häussinger D, Wettstein M, Warkulat U, vom Dahl S, Noé B, Schliess F. Cell volume signalling, osmolytes and liver function. Digestion. 1997;58 Suppl 1:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Häussinger D, Schliess F. Osmotic induction of signaling cascades: role in regulation of cell function. Biochem Biophys Res Commun. 1999;255:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Häussinger D. The role of cellular hydration in the regulation of cell function. Biochem J. 1996;313:697-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 390] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 46. | Fischer R, Schliess F, Häussinger D. Characterization of the hypo-osmolarity-induced Ca2+ response in cultured rat astrocytes. Glia. 1997;20:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Butterworth RF. The astrocytic ("peripheral-type") benzodiazepine receptor: role in the pathogenesis of portal-systemic encephalopathy. Neurochem Int. 2000;36:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Itzhak Y, Bender AS, Norenberg MD. Effect of hypoosmotic stress on peripheral-type benzodiazepine receptors in cultured astrocytes. Brain Res. 1994;644:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Busch GL, Wiesinger H, Gulbins E, Wagner HJ, Hamprecht B, Lang F. Effect of astroglial cell swelling on pH of acidic intracellular compartments. Biochim Biophys Acta. 1996;1285:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Baquet A, Hue L, Meijer AJ, van Woerkom GM, Plomp PJ. Swelling of rat hepatocytes stimulates glycogen synthesis. J Biol Chem. 1990;265:955-959. [PubMed] |
| 51. | Jones EA, Ferenci P. Hepatic encephalopathy: GABA ergic neu-rotransmission and the benzodiazepines. In: Conn H, Bircher JO, eds. Hepatic encephalopathy: syndromes and therapies. Lansing, Michigan: Medi-Ed Press. 1995;75-100. |
| 52. | Donovan JP, Schafer DF, Shaw BW, Sorrell MF. Cerebral oedema and increased intracranial pressure in chronic liver disease. Lancet. 1998;351:719-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Sawabe M, Kato Y, Ohashi I, Kitagawa T. Diffuse intrasinusoidal metastasis of gastric carcinoma to the liver leading to fulminant hepatic failure. A case report. Cancer. 1990;65:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Arora A, Sharma MP, Buch P, Mathur M. Paroxysmal nocturnal hemoglobinuria with hepatic vein thrombosis presenting as hepatic encephalopathy. Indian J Gastroenterol. 1990;9:91-92. [PubMed] |
| 55. | Nouel O, Henrion J, Bernuau J, Degott C, Rueff B, Benhamou JP. Fulminant hepatic failure due to transient circulatory failure in patients with chronic heart disease. Dig Dis Sci. 1980;25:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 39] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Arora A, Seth S, Acharya SK, Sharma MP. Hepatic coma as a presenting feature of constrictive pericarditis. Am J Gastroenterol. 1993;88:430-432. [PubMed] |
| 57. | Hassall E, Benson L, Hart M, Krieger DE. Hepatic encephalopathy after portacaval shunt in a noncirrhotic child. J Pediatr. 1984;105:439-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Watanabe A. Portal-systemic encephalopathy in non-cirrhotic patients: classification of clinical types, diagnosis and treatment. J Gastroenterol Hepatol. 2000;15:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 59. | Chalasani N, Clark WS, Martin LG, Kamean J, Khan MA, Patel NH, Boyer TD. Determinants of mortality in patients with advanced cirrhosis after transjugular intrahepatic portosystemic shunting. Gastroenterology. 2000;118:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Conn HO. The hepatic encephalopathies. In: Conn HO, ed. He-patic encephalopathy: syndromes and therapies. Bloomington. Illinois: Medi Ed Press. 1994;1-12. |
| 61. | Conn HO. Quantifying the severity of hepatic encephalopathy. In: Conn HO, ed. Hepatic encephalopathy: syndromes and therapies. Bloomington. Illinois: Medi Ed Press. 1994;13-26. |
| 62. | Conn HO. Subclinical hepatic encephalopathy. In: Conn HO, ed. Hepatic encephalopathy: syndromes and therapies. Bloomington. Illinois: Medi Ed Press. 1994;27-42. |
| 63. | Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, Berger E, Blum U, Gabelmann A, Hauenstein K. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 481] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 64. | Rössle M, Piotraschke J. Transjugular intrahepatic portosystemic shunt and hepatic encephalopathy. Dig Dis. 1996;14 Suppl 1:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Dhiman RK, Sawhney MS, Chawla YK, Das G, Ram S, Dilawari JB. Efficacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci. 2000;45:1549-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Orlandi F, Brunelli E, Benedetti A, Macarri G. Clinical trials of nonabsorbable disaccharide therapy in hepatic encephalopathy. In: Conn HO, Bircher J, eds. Hepatic encephalopathy: syndromes and therapies. Bloomington. Illinois: Medi Ed Press. 1994;243-264. |
| 67. | Bianchi GP, Marchesini G, Fabbri A, Rondelli A, Bugianesi E, Zoli M, Pisi E. Vegetable versus animal protein diet in cirrhotic patients with chronic encephalopathy. A randomized cross-over comparison. J Intern Med. 1993;233:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Strauss E, Tramote R, Silva EP, Caly WR, Honain NZ, Maffei RA, de Sá MF. Double-blind randomized clinical trial comparing neomycin and placebo in the treatment of exogenous hepatic encephalopathy. Hepatogastroenterology. 1992;39:542-545. [PubMed] |
| 69. | Kircheis G, Nilius R, Held C, Berndt H, Buchner M, Görtelmeyer R, Hendricks R, Krüger B, Kuklinski B, Meister H. Therapeutic efficacy of L-ornithine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo-controlled, double-blind study. Hepatology. 1997;25:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 201] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 70. | Rees CJ, Oppong K, Al Mardini H, Hudson M, Record CO. Effect of L-ornithine-L-aspartate on patients with and without TIPS undergoing glutamine challenge: a double blind, placebo controlled trial. Gut. 2000;47:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Plauth M, Egberts EH, Hamster W, Török M, Müller PH, Brand O, Fürst P, Dölle W. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. A double-blind placebo-controlled crossover study. J Hepatol. 1993;17:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Pomier-Layrargues G, Giguère JF, Lavoie J, Perney P, Gagnon S, D'Amour M, Wells J, Butterworth RF. Flumazenil in cirrhotic patients in hepatic coma: a randomized double-blind placebo-controlled crossover trial. Hepatology. 1994;19:32-37. [PubMed] |
| 73. | Ferenci P, Grimm G, Meryn S, Gangl A. Successful long-term treatment of portal-systemic encephalopathy by the benzodiazepine antagonist flumazenil. Gastroenterology. 1989;96:240-243. [PubMed] |
| 74. | Riggio O, Merli M, Capocaccia L, Caschera M, Zullo A, Pinto G, Gaudio E, Franchitto A, Spagnoli R, D'Aquilino E. Zinc supplementation reduces blood ammonia and increases liver ornithine transcarbamylase activity in experimental cirrhosis. Hepatology. 1992;16:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Reding P, Duchateau J, Bataille C. Oral zinc supplementation improves hepatic encephalopathy. Results of a randomised controlled trial. Lancet. 1984;2:493-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 114] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Edited by Ma JY