INTRODUCTION
There are about 30 million patients suffering from chronic hepatitis B in China. Patients with active liver disease carry a high risk of developing cirrhosis and hepatocellular carcinoma. At present, the most effective medicine is IFN-α, but it is too expensive for most patients. Even in the patients treated by IFN-α, the sera negative-convertion rate of HBeAg and HBVDNA is about 40%[1-3]. Previous works in our department by Cai X et al[4] showed that the sera negative-convertion rates of HBeAg in patients with chronic active hepatitis B treated with oxymatrine was 61%. To investigate the inhibiting mechanism of oxymatrine (oxy) on HBV replication, transgenic mice were employed as animal model to observe the changes of HBsAg and HBcAg expression in liver tissues.
MATERIALS AND METHODS
Materials
Matrine injection is the product of Ningxia Pharmaceutical Company Ltd., containing 98% oxymatrine. The restriction enzymes were purchased from PROMEGA. Taq enzyme purchased from SANGON. Immunohistochemical kits were purchased from DAKO. Primers of PCR were designed by ourselves and synthesized by GIBCO. 32P labeled kit was the product of PROMEGA. Other reagents were purchased from WASON, HUAMEI and so on.
Methods
Preparation of animals HBV transgenic mice (official designation: ICR-TgN HBV adr1.2 SMMU) were produced by micro-injection of a 4.2 kb fragment containing the complete HBV genome (adr subtype)[5]. Structural analysis of the transgene revealed that at least one complete uninterrupted HBV genome was present. HBsAg and HBeAg were not detectable in the sera of the mice, but can be detected in livers by immunohistochemical assay (ABC), which was used to determine the expression level of HBV.
PCR and Southern-blot analysis Total tail genome DNA was analyzed by PCR using HBV-specific primers 5’CCCAATGGAACACTCACC [sense], 5’AGGAACCACTGAACAAATGGC [antisense]), generating a 380 bp fragment. Twenty mililiter of PCR products were analyzed by electrophoresis on a 1% agarose gel in the presence of 0.5 mg of ethidium bromide per mililiter. DNA bands were visualized by UV fluorescence. Southern-blot analysis was performed on total genomic DNA by agarose gel electrophoresis of 30 mg restricted genomic DNA. Samples added on nylon filters were hybridized with HBV specific 32P labeled DNA probes.
Histological analysis and electron microscopy were carried out as routine methods. The expression of HBsAg and HBcAg in liver tissues were assessed by immunohistochemical analysis according to Guidotti et al[6].
RESULTS
Effect of oxy at different doses on the expression of HBsAg and HBcAg Twenty-four age and sex-matched mice were divided into four groups. Each group was injected intraperitoneally with oxy at the dosage of 100, 200 and 300 mg/kg or with saline separately once a day for 30 d. Livers were harvested 2 hours after the last injection for immunohistochemical assay. Both HBsAg and HBcAg were positive in livers of all the six mice in the control group (injected with saline). In 100 mg/kg group, HBsAg and HBcAg were positive in two mice, while HBsAg and HBcAg were negative in the other four mice. In the 200 mg/kg group, both HBsAg and HBcAg were negative in all the six mice, none of the six mice had detectable HBV antigen in the livers. In the 300 mg/kg group, HBsAg and HBcAg were positive in two mice, and negative in the other four mice. No pathological changes were found in the transgenic mice. Based on the results, we considered that 200 mg/kg is the ideal dosage of oxy for further study.
The effect of oxy at different time on the expression of HBsAg and HBcAg
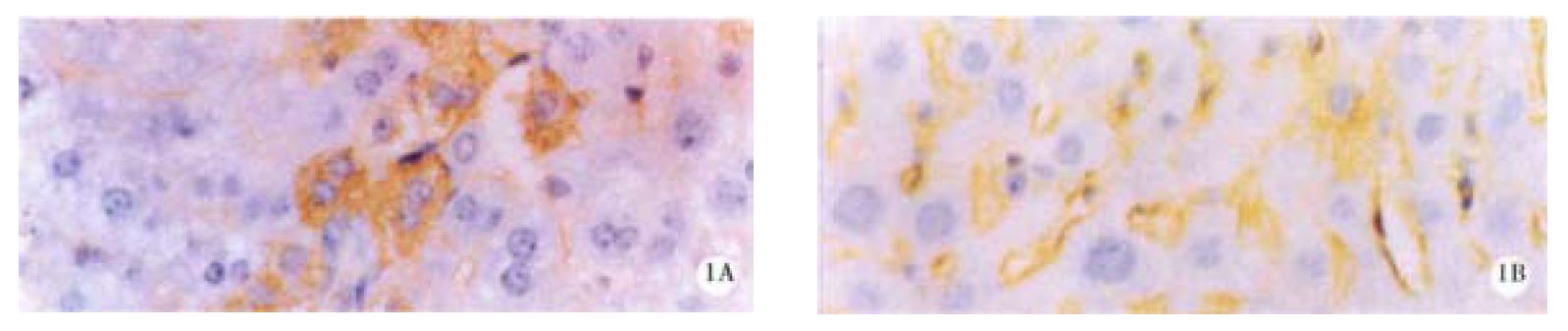
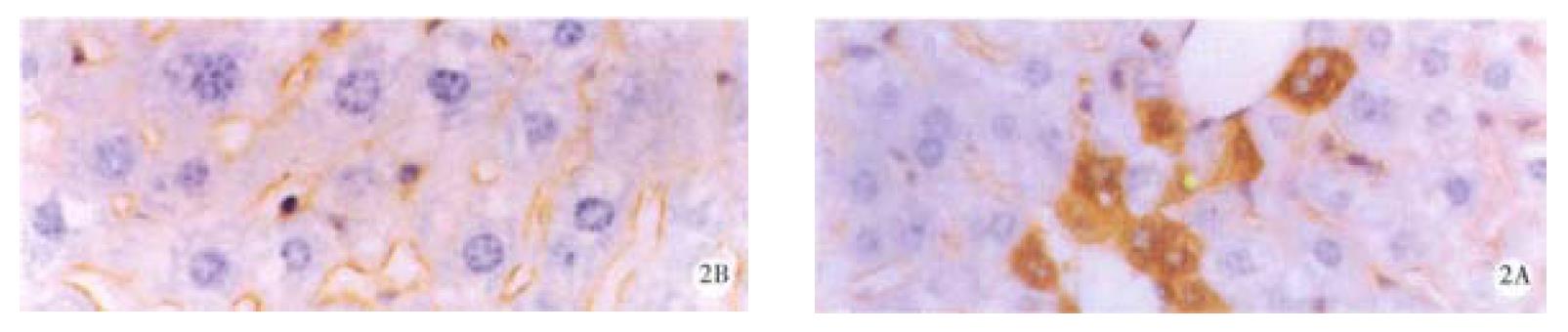
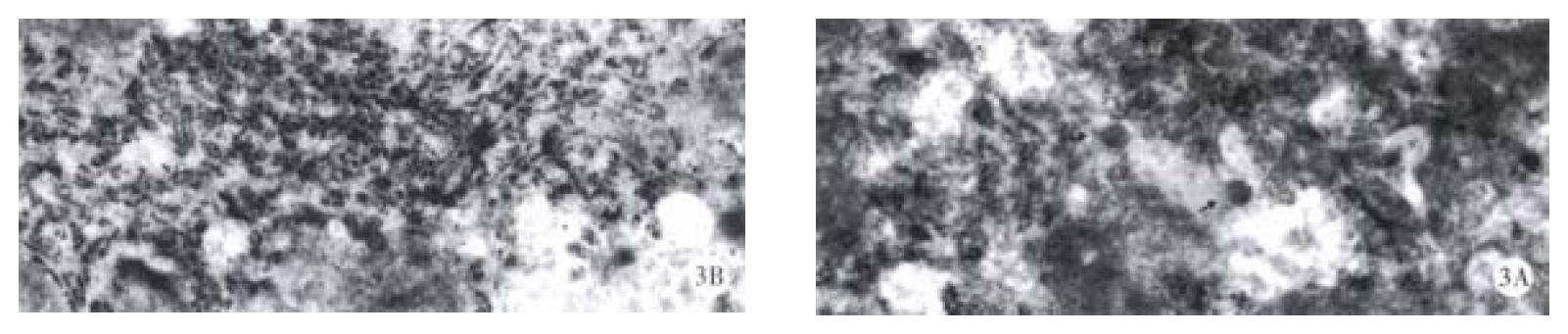
Mice were divided into four groups according to the oxy treatment time, 10 d (group 1), 20 d (group 2), 30 d and 60 d (group 3 and 4). In each group, 4 mice were randomly entered. Each mouse underwent liver biopsy two weeks before the treatment of oxy (200 mg/kg). The liver samples before and after oxy treatment were collected, and immunohistochemical analysis was performed to determine the expression level of HBsAg and HBcAg. All the samples contained HBsAg and HBcAg positive cells, and the positive and negative cells were counted in 5 randomly selected high field vision, and χ2 test was made to compare the HBV expression level before and after oxy treatment. In group 1, the number of HBsAg and HBcAg positive cells was significantly lower than before treatment of oxy in all the four mice livers. In group 2, the similar results were observed (Figure 1 and Figure 2), and Dane-like particles could be found in the livers before oxy treatment under electron microscope and such particles could not be found after the treatment of oxy for 20 d (Figure 3). In group 3, the expression of HBV was decreased only in two of four mice. No difference was observed on the expression of HBsAg and HBcAg between the two liver samples harvested before and after the treatment of oxy in group 4.
Figure 1 HBsAg in liver of the mice before (A) and after (B) treatment with 200 mg/kg oxy for 20 d.
Figure 2 HBcAg in liver of the mice before (A) and after (B) treatment with 200 mg/kg oxy for 20 d.
Figure 3 Dane’s like particles can be seen in the liver of untreated mice (A) and disappeared after treatment with 200 mg/kg oxy for 20 d (B).
DISCUSSION
As a traditional Chinese medicine, Sophra Flavescens Ait has been used for the treatment of many diseases for thousands of years. Its extract, oxy, has long been extensively used in China. It is reported that oxy has a lot of pharmacological functions which can be divided into four classes: ① Anti-bacterial and anti-parasitic actions. It has been reported that oxy can cure acute dysentery, Trichomonas vaginalis and Giardia lamblia infection[7-9], but the mechanism is still unclear. ② Regulating immune reaction. Oxy can stimulate immune response at a low concentration while inhibiting immune response at a high concentration[10]. Recently, more researchers have paid attentions to the immune inhibitory effect of oxy. It has been reported that oxy has many functions such as anti-inflammation,anti-hypersensitivereactions,inhibiting histamine releasing[11-14]. The mechanism may be related to the changes of cAMP in the cell[15] and inhibiting production of cytokine[16]. ③ Inducing production of cytochrome P450. Oxy can increase the content of P450 in the rat liver significantly after treatment of oxy at the dosage of 200 mg/kg for 4d[17]. ④ Anti-virus actions. Liu JX reported that oxy could inhibit coxsackie virus B3 in vivo and in vitro [18,19]. Cai X found that the sera negative convertion rates of HBVDNA and HBeAg were 61.9% and 61.0% in chronic active hepatitis treated by oxy, while such rates in the therapy of IFN-α were 57.9% and 55.3%[4]. In our study, the content of HBV antigen in livers of transgenic mice decreased significantly after treatment of oxy for 10 and 20 d. Dane-like particles disappeared in the liver of transgenic mice after oxy treatment for 20 d. However, HBV expression level returned to normal after treatment by oxy for 60 d. We concluded that oxy can be used as an effective drug in managing HBV infection. There are two features of oxy on HBV: ① HBsAg and HBcAg was down regulated at the same time, ② longer time and larger dose did not yield better effect.
Our results strongly suggested that oxy can significantly inhibit the expression of HBV antigen in transgenic mice and the replication of HBV as well[20]. But how oxy give play to its effect can not be concluded from our experiments. However, based on the previous researches, it seems that oxy may act by two ways: ① oxy acts as an immune reaction regulator: Since HBV transgenic mice were first found by Chisari in 1985[21], in vivo study of HBV has become convenient and objective. Different lineage of HBV transgenic mice has also been found in our country[5,22]. A serial studies by Chisari et al[23-28] have shown that certain soluble products of the immune response, especially IFN-γ, TNF-α, IFN-α, IL-2 and IL-12 could suppress the steady-state content of HBV messenger RNA in the hepatocytes of transgenic mice. Furthermore, these effects were found to be mediated by a post-transcriptional mechanism that selectively accelerates the degradation of cytoplasmic HBV mRNA[27]. The same events were set in motion when HBsAg-specific CTL secreted IFN-γ and induced TNF-α after antigen recognition[23,29]. The interhepatic nucleocapsid particles and replicative intermediates were also eliminated during unrelated virus infection[30,31] or during hepatocellular regeneration after partial hepatectomy[32]. Oxy is a strong immune regulator, Wang HX has reported that oxy can inhibit the competence of LAK cell killing P815 cell by about 70%-80%[33], and Shang HS reported that oxy has the same effect of marcophage on P815 cell[34], which proved that oxy may be an agonist of IL-2. Thus, IL-2 could not be the mediator of oxy inhibiting HBV. Whether other cytokines may be the mediator remains unclear. ② Oxy acts as an inducer of cytochrome P450. HBV antigen is exogenous proteins in mice hepatocytes. mRNA of HBV in hepatocytes may be degraded by cytochrome P450. Therefore, oxy can induce the production and enhance the activity of cytochrome P450[17], hence accelerating the degradation of HBV mRNA and inhibiting HBV replication. Further study is needed.
Oxy is a broad-spectrum anti virus drug, at least to HBV and coxsackie B virus 3 so far. This may give us new hope for the treatment of chronic hepatitis HBV infection including other viral infection such as HCV and HIV infection.
Cirrhosis is a servere consequence of chronic HBV infection and preventing the development of cirrhosis is very difficult[35]. Gan LW et al[36] reported that oxy can inhibit the liver fibrosis induced by CCl4 in rats. Oxy can not only down-regulate HBV expression but also inhibit the liver fibrosis. Based on the two points, we concluded that oxy can be used as an effective drug in managing HBV infection. However, the exact mechanism of oxy inhibiting expression of HBV and liver fibrosis has not yet been fully understood. Further studies both basicaly and clinically are needed.