Published online Feb 15, 2001. doi: 10.3748/wjg.v7.i1.22
Revised: October 22, 2000
Accepted: October 29, 2000
Published online: February 15, 2001
AIM: To test the hypothesis to block VEGF expression of SMMC-7721 hepatomacells may inhibit tumor growth using the rat hepatoma model.
METHODS: Amplifiy the 200 VEGF cDNA fragment and insert it into human U6 gene cassette in the reverse orientation transcribing small antisense RNA which could specifically interact with VEGF165, and VEGF121 mRNA. Construct the retroviral vector containing this antisense VEGF U6 cassette and package the replication-deficient recombinant retrovirus. SMMC-7721 cells were transduced with these virus and positive clones were selected with G418. PCR and Southern blot analysis were performed to determine if U6 cassette integrated into the genomic DNA of positive clone. Transfected tumor cells were evaluated for RNA expression by ribonuclease protection assays. The VEGF protein in the supernatant of parental tumor cells and genetically modified tumor cells was determined with ELISA. In vitro and in vivo growth properties of antisense VEGF cell clone in nude mice were analyzed.
RESULTS: Restriction enzyme digestion and PCR sequencing verified that the antisense VEGF RNA retroviral vector was successfully constructed. After G418 selection, resistant SMMC-7721 cell clone was picked up. PCR and Southern blot analysis suggested that U6 cassette was integrated into the cell genomic DNA. Stable SMMC-7721 cell clone transduced with U6 antisense RNA cassette could express 200 bp small antisense VEGF RNA and secrete reduced levels of VEGF in culture condition. Production of VEGF by antisense transgenea2expressing cells was 65 ± 10 ng/L per 106 cells, 420 ± 45 ng/L per 106 cells in sense group and 485 ± 30 ng/ L per 106 cells in the negative control group, (P < 0.5). The antisense-VEGF cell clone appeared phenotypically indistinguishable from SMMC-7721 cells and SMMC-7721 cells transfected sense VEGF. The growth rate of the antisense-VEGF cell clone was the same as the control cells. When S.C. was implanted into nude mice, growth of antisense-VEGF cell lines was greatly inhibited compared with control cells.
CONCLUSION: Expression of antisense VEGF RNA in SMMC-7721 cells could decrease the tumorigenicity, and antisense-VEGF gene therapy may be an adjuvant treatment for hepatoma.
- Citation: Tang YC, Li Y, Qian GX. Reduction of tumorigenicity of SMMC-27721 hepatoma cells by vascular endothelial growth factor antisense gene therapy. World J Gastroenterol 2001; 7(1): 22-27
- URL: https://www.wjgnet.com/1007-9327/full/v7/i1/22.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i1.22
Neovascularization is critical for supporting the rapid growth of solid tumors[1]. Tumor angiogenesis appears to be achieved by the expression of angiogenic agents within solid tumors that stimulate host vascular endothelial cell mitogenesis and possibly chemotaxis. One such protein, vascular endothelial growth factor (VEGF) or vascular permeability factor[2-5], is a selective endothelial cell mitogen and angiogenic agent .Many tumor cell lines secrete VEGF in vitro, suggesting that this diffusible molecule is a mediator of tumor angiogenesis. The clinical results showed high levels of VEGF expression in primary hepatoma, elevated levels of flt-1, the receptors of VEGF in hepatoma blood vessels, and the relationship between VEGF levels and hepatoma invasion and transfer[6]. These data indicated that VEGF and its receptors play important roles in the development of hepatoma vasculature and progressive growth of hepatoma.
In this study, we used the SMMC-7721 hepatoma cell line which has a high expression of VEGF, as established model for human hepatoma. The strategy of exogenous expression of antisense VEGF transcribed by POL III promoter in the SMMC-7721 cell line was applied to assess the feasibility of disrupting the VEGF/VEGF receptor pathway of angiogenesis and decreasing their tumorigenicity in vivo.
Bam HI, T4 DNA ligase was purchased from Promega Company. RNase A and RNase T1 were the products of MBI Fermentas, G418 was purchased from Sigma Company and SMMC-7721 cell line from Chinese Academy of Cell Biology.
Construction of vectors VEGF antisense vector to generate the VEGF anti-sense vector, a DNA fragment containing 250 bp of human VEGF cDNA, was ligated in reverse orientation in the sal I, xho I sites of the U6 cassette, and subcloned into the Bam HI site of pLXSN vector. Expression of the antisense molecule in pLXSN was driven by POL III promoter of U6 cassette. The pLXSN vector also contained the G418 resistance gene driven by the simian virus (SV40) promoter. To generate infectious virions, PA317 packaging cells were transfected with pLXSN-U6-as-VEGF and selected in the culture medium containing 500 mg/L G418. Virus-containing supernatants were harvested and used to infect SMMC-7721 cells.
Genetic modification of SMMC-7721 cells The SMMC-7721 cells were incubated with the viral stock containing 8 mg/L polybrene. On the following day, the cells were split and selected in 500 mg/L G418. Cultures were added every 3-4 d with the fresh G418 supplemented media for 14 d. Resistant colonies were expanded, and subcloned and the clone which produced the reduced levels of VEGF was selected for further research.
Ribonuclease protection assay A 200 bp VEGF PCR product was cloned into T7, T3 vector pBlueScript-SK, the plasmid was linearized by EcoR V, treated with proteinase K and purified. The α-32P UTP sense VEGF RNA was generated by addition of T7 polymerase. Ribonuclease protection assays were made as follows, 20 µg of total cellular RNA was hybridized with RNA probes overnight at 45 °C. The remaining single-stranded probe RNA and unhybridized RNA were digested with a mixture of RNase A and RNase T1, added yeast RNA, extracted by phenol, precipitated by ethanol, seperated on 7 M urea/polyacrylamide gels, and then exposed to X-ray film.
PCR, southern blot analysis PCR was performed on genomic DNA isolated from human SMMC-7721 cells and individual clones of transfected cells using a sense primer corresponding to the U6 promoter (5’-TATACTAAGTCGACTCCTATGTGCTGG-3’) and an antisense primer corresponding to the VEGF cDNA (5’-TAGAGAGGGCAGAATCATCACG-AAGTGG-3’). Using the NeoR primer, the sense primer is 5’-CAAGATGGAATTGCACGCAGG-3’, the reversal primer is 5’-CCCGCTCAGAAAGAACTCGTC-3’. The PCR was performed using the following protocol: 95 °C 1 min, 60 °C 1 min, 72 °C 1 min 30 s; in the last cycle, extend 10 min at 72 °C. Southern blot, 20 µg genomic DNA was digested overnight, electrophoresed on 1% agarose gels, transferred onto Hybond N nylon membrane, and hybridized with the DIG labeled NeoR probe at 68 °C for 6 h, the membrane was washed in 2 × SSC for 5 min × 2, and 0.1 × SSC for 15 min. The fragments were visualized by chemiluminescent, and exposed to X-ray film.
Quantitation of VEGF The supernatant of parental or transfected SMMC-7721 cells were measured by ELISA. To generate the conditional medium, the cells were seeded onto 3.0 × 105/well plates. The media was changed next day to MEM/0.5% bovine serum albumin/1% dialyzed fetal calf serum for another 24 h. The media was then replaced by the fresh MEM and cells were allowed to grow for another 48 h. The CM was generated by centrifugation at 14000 rpm at 4 °C for 15 min, then for ELISA analysis according to the manufacturer’s instructions.
In vitro growth rate SMMC-7721 hepatoma cells and cells transfected with antisense, and sense-VEGF were cultured at 1 × 104 and grown under standard culture conditions. Cell count was made every 24 h for a total of 144 h. The total number of cells from duplicate experiments was determined as a function of time.
Determination of in vivo tumor growth Subcutaneous inoculation and tumor growth measurements were carried out, 1 × 106 cells of the parental SMMC-7721 cells or antisense, sense expressing clones were injected into the flank of normal BALB/C nude mice. Tumors were measured in two dimensions every 5 d for 25 d. Tumor volume was calculated using the formula v = l × w2/2, where v = volume (mm3), l = long diameter, and w = short diameter.
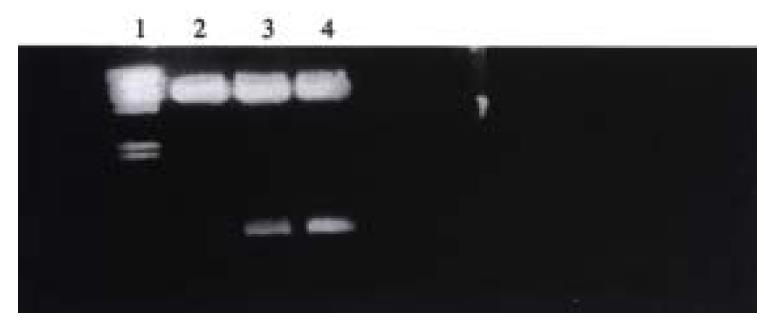
All of the major transcriptional promoter elements for U6 RNA polymerase III are upstream of the transcription start, which has a potential advantage of the less exogenous RNA coding sequence. Another advantage of the U6 promoter is that U6 gene is heavily expressed in human cells. The U6 cassette contained the first 5’ initial 27 nucleotides and 3’ stem 19 nucleotides for transcript terminator and the U6+27 transcript was predicted to be most stable because of the γ-phosphomethyl-GTP cap. The fragment of VEGF was cloned into U6 cassette through sense or antisense direction, and verified by DNA sequence. U6 cassette containing sense or antisense VEGF was cleaved by Hind III, Pst I digestion and cloned into pBlue-SK vector, and subcloned into Bam HI restriction site of retroviral expression vector. The positive plasmid was verified by Bam HI digestion (Figure 1).
Following transfection by recombinant antisense VEGF or sense VEGF retrovirus, the SMMC-7721 cells were selected by antibiotic G418, the individual clones were isolated and expanded. And the selected clone expressed low VEGF for further analysis and was referred to as anti-1. This clone was evaluated for gene expression in ribonuclease protection assays. In this assay, hybridization of RNA with the complementary RNA probe protects the probe from the subsequent digestion with RNase A and RNase T1. From Figure 2, it can be seen that 200 bp antisense VEGF RNA was only expressed in SMMC-7721 transfected by pLXSN U6 antisense VEGF.
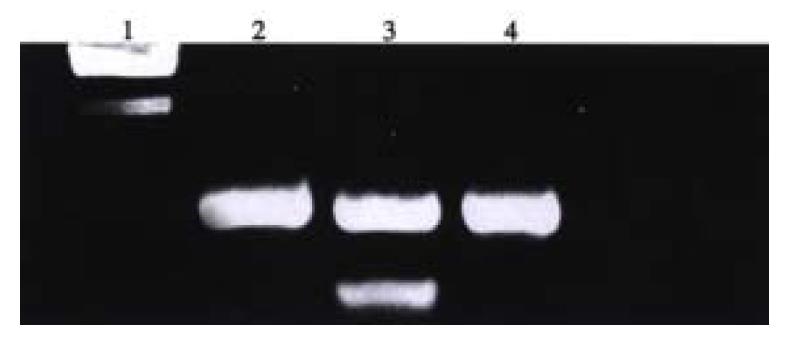
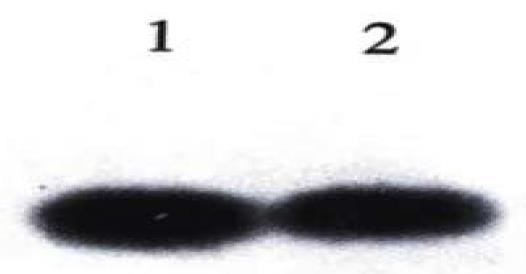
PCR analysis of DNA isolated from the antisense-VEGF, sense VEGF clones showed foreign gene integration into the genomic DNA, and the results of PCR using the specific primer, showed that the antisense VEGF U6 gene cassette had inserted the genomic DNA of SMMC-7721 cells. Southern blot analysis was performed on genomic DNA of these antisense VEGF, senseVEGF clones to verify again that there was foreign integrated cDNA (Figure 3, Figure 4).
In order to determine if the expression of the antisense VEGF transgene reduced production of secreted protein, supernatant from control-transduced (SMMC-7721 sense VEGF) and antisense VEGF transduced cells were assayed for VEGF by ELISA. Production of VEGF by antisense transgene-expressing cells was 65 + 10 ng/L per 106 cells, as compared with 420 + 45 ng/L per 106 cells in sense group and 485 + 30 ng/L per 106 cells in negative control group, P < 0.05.
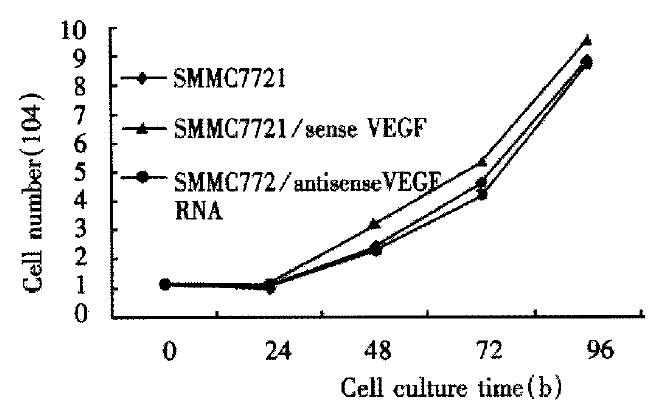
The antisense-VEGF cell lines appeared phenotypically indistinguishable from normal SMC-7721 cells and SMMC-7721 transfected sense VEGF cells. And growth rates of antisense-VEGF cell lines were the same as the control cells (Figure 5).
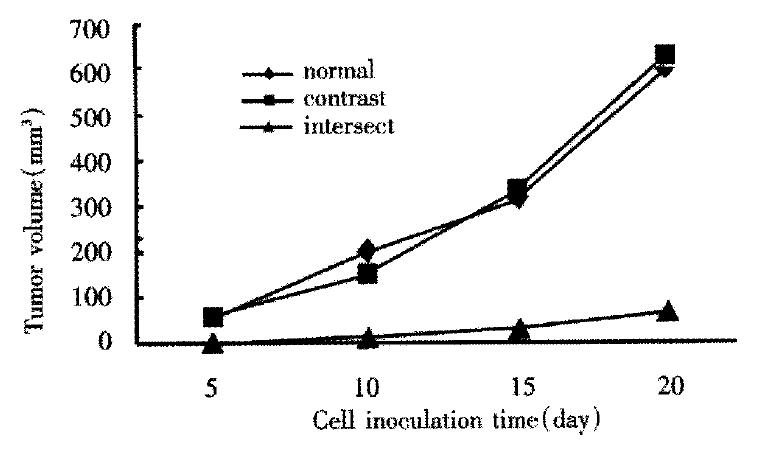
Control SMMC-7721 cells and antisense-VEGF SMMC-7721 cells were s.c. injected into nude mice, tumor volumes were measured every 5 d. Tumor growth was detectable and measurable for control SMMC-7721 cells 5 d post-implantation, while the antisense VEGF cell lines gave rise to tumors. Examination of mice at 25 d post-implantation revealed that the negative control SMMC-7721 group produced tumors of 630.92 ± 85 mm3, sense-VEGF SMMC-7721 group produced tumors of 601.07 ± 52 mm3, while the antisense VEGF SMMC-7721 group produced tumors of 76.33 ± 20 mm3. This experiment demonstrates that the reduced tumorigenicity of antisense-VEGF SMMC-7721 cells in nude mice may be attributed to the reduced expression of VEGF (Figure 6).
Angiogenesis, the formation of new blood vessels , is essential for both tumor growth and metastasis[7-10]. Tumor angiogensis is a process controlled by certain chemicals produced in cancer cells. These chemicals stimulate endothelail cells to form new blood vessels. Candidates as major physiological stimulators include VEGF[11], bFGF. VEGF, and its receptors play critical roles in tumor-associated angiogenesis and represent good targets for therapeutic intervention[12-15]. VEGF was initially termed vascular permeability factor, its first function was discovered by Dvorak and colleagues[16]. There are several VEGF isoforms, in which VEGF121 and VEGF165 are readily secreted. Unlike bFGF, VEGF is a very specific mitogen for vascular endothelial cells. It also functions as a potent pro-survival factor for endothelial cells in nearby formed vessels and this may be one of its most important functions[17,18].
It is reported that VEGF is an angiogenic factor most closely associated with the neovascularization in solid tumors. VEGF is expressed by vast majority of cancers at elevated levels and blocks its activity by specific neutralizing antibodies to VEGF[19,20]. VEGF-toxin conjugates[21], aptamers[21] and small molecule VEGF receptor antagonists[22] could inhibit the growth of cancer in animal models. In human hepatocellular carcinoma, abundant tumor vascularity was observed. And vascular endothelial growth factor gene and protein expression was analyzed by means of Northern hybridization and immunohistochemistry, increased expression of VEGF has been reported in hepatocellular carcinoma cells (HCC)[23-26]. So VEGF gene expression is significantly associated with angiogenesis of HCC. Tang Zhao You et al studied the angiogenesis induced by liver cancer with different metastatic potentials using corneal micropocket model in nude mice. It was suggested that highly metastatic liver cancer was more angiogenic than low metastatic cancer and liver tissue[26]. In HCC with metastasis, mRNA of VEGF is closely related to the growth of HCC as well as its metastasis[27].
In China, the hepatocarcinogenesis is closely related with the hepatitis virus[28], the results of the researches showed that, after viral infection,there is abnormal expression of oncogene such as ras, bcl-2, especially P53[29-33], and there is also a possible link between oncogenes and tumor angiogenesis. Expression of mutant ras can lead to a marked induction of a potent paracrine stimulator of angiogenesis. In addition, hypoxia stimulates expression of VEGF and tumor angiogenesis[34-40]. The results of therapeutic experiments showed that the chimeric protein consisting of DT390-VEGF165 or DT390-VEGF exon7 can efficiently kill the HepG2 and gastric carcinoma cells and may kill vascular endothelial cells in the cancer[41,42] and antiangiogenesis inhibitor TNP-470 plus lipiodol greatly decreased the hepatoma growth in animal models which depend on the reduction of microvessel density[43].
Blocking the interaction between the VEGF and its receptor can inhibit the growth of tumor through the antiangiogenesis effect[44-47]. From our previous experiment, we found VEGF expression in hepatoma cell line SMMC-7721 cells. We there fore sought to determine if inhibition of secretion of VEGF in SMMC-7721 tumor cells would inhibit the growth of this tumor in animal model.
In order to improve the expression of the antisense VEGF RNA in the target cell, we constructed the retrovirus vector containing the human U6 promoter cassette that had the POL III promoter to transcribe the small therapeutic RNA in the nuclei of cells. Compared with other transcriptional promoters such as POL II, tRNA, there are two advantages of U6 promoter: ① high expression in human cells, and ② the therapeutic RNA contains less unnecessary RNA encoding the intragenic ptomoter. In the same time, we amplified a common VEGF cDNA and inserted reversely into U6 cassette[48-52]. U6 promoter transcribed a small antisense VEGF RNA fragment that could specifically interacted with VEGF165 and VEGF121 mRNA. Our previous results, verified that U6 cassette could effectively express antisense VEGF RNA molecules and decreased the expression of mRNA VEGF165, and VEGF121. Then U6 cassette that expressed antisense VEGF RNA was inserted into the retroviral construct. After packaging the recombinant retrovirus, this cassette was introduced into the SMMC-7721 cells. Ribonuclease protection analysis using the RNA probe specific for antisense VEGF demonstrated that there was antisense VEGF RNA expression in the SMMC-7721 cells genetically modified by antisense U6 cassette. The antisense clone selected for further study showed radical decrease in VEGF protein in supernatant compared with the sense and negative SMMC-7721 cell group. Inhibition of VEGF expression in SMMC-7721 cells resulted in severely impaired growth of this tumor in vivo. This may be related with the reduced levels of VEGF produced by the antisense-VEGF-transfected SMMC-7721 cell clone, and this resulted in a decrease of number of tumor blood vessels. Our findings demonstrate that the inhibition of VEGF is sufficient to control the tumor growth in vivo. The antisense VEGF srategy offers a way for gene therapy as an adjuvant treatment for hepatoma.
| 1. | Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3206] [Cited by in RCA: 3243] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 2. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6569] [Article Influence: 252.7] [Reference Citation Analysis (0)] |
| 3. | Lewin M, Bredow S, Sergeyev N, Marecos E, Bogdanov A, Weissleder R. In vivo assessment of vascular endothelial growth factor-induced angiogenesis. Int J Cancer. 1999;83:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Millauer B, Longhi MP, Plate KH, Shawver LK, Risau W, Ullrich A, Strawn LM. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996;56:1615-1620. [PubMed] |
| 5. | Asano M, Yukita A, Matsumoto T, Kondo S, Suzuki H. Inhibition of tumor growth and metastasis by an immunoneutralizing monoclonal antibody to human vascular endothelial growth factor/vascular permeability factor121. Cancer Res. 1995;55:5296-5301. [PubMed] |
| 6. | Li J, Wang WL, Liu B. Angiogenesis and apoptosis in human hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:1057-1060. |
| 7. | Jia L, Chen TX, Sun JW, Na ZM, Zhang HH. Relationship be-tween microvessel density and proliferating cell nuclear antigen and prognosis in colorectal cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:74-76. |
| 8. | Gao GL, Yang Y, Yang S, Ren CW. Relationship between prolif-eration of vascular andothelial cells and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:282-284. |
| 9. | Yoshiji H, Kuriyama S, Ways DK, Yoshii J, Miyamoto Y, Kawata M, Ikenaka Y, Tsujinoue H, Nakatani T, Shibuya M. Protein kinase C lies on the signaling pathway for vascular endothelial growth factor-mediated tumor development and angiogenesis. Cancer Res. 1999;59:4413-4418. [PubMed] |
| 10. | Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9-19. [PubMed] |
| 11. | Danielsen T, Rofstad EK. The constitutive level of vascular endothelial growth factor (VEGF) is more important than hypoxia-induced VEGF up-regulation in the angiogenesis of human melanoma xenografts. Br J Cancer. 2000;82:1528-1534. [PubMed] |
| 12. | Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 863] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 13. | Cheng SY, Huang HJ, Nagane M, Ji XD, Wang D, Shih CC, Arap W, Huang CM, Cavenee WK. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc Natl Acad Sci USA. 1996;93:8502-8507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 233] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2567] [Cited by in RCA: 2543] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 15. | Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523-1530. [PubMed] |
| 16. | Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029-1039. [PubMed] |
| 17. | Wong EY, Morgan L, Smales C, Lang P, Gubby SE, Staddon JM. Vascular endothelial growth factor stimulates dephosphorylation of the catenins p120 and p100 in endothelial cells. Biochem J. 2000;346 Pt 1:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9-22. [PubMed] |
| 19. | Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593-4599. [PubMed] |
| 20. | Zhu Z, Rockwell P, Lu D, Kotanides H, Pytowski B, Hicklin DJ, Bohlen P, Witte L. Inhibition of vascular endothelial growth factor-induced receptor activation with anti-kinase insert domain-containing receptor single-chain antibodies from a phage display library. Cancer Res. 1998;58:3209-3214. [PubMed] |
| 21. | Ramakrishnan S, Olson TA, Bautch VL, Mohanraj D. Vascular endothelial growth factor-toxin conjugate specifically inhibits KDR/flk-1-positive endothelial cell proliferation in vitro and angiogenesis in vivo. Cancer Res. 1996;56:1324-1330. [PubMed] |
| 22. | Seo MS, Kwak N, Ozaki H, Yamada H, Okamoto N, Yamada E, Fabbro D, Hofmann F, Wood JM, Campochiaro PA. Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am J Pathol. 1999;154:1743-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Suzuki H, Seto K, Shinoda Y, Mori M, Ishimura Y, Suematsu M, Ishii H. Paracrine upregulation of VEGF receptor mRNA in endothelial cells by hypoxia-exposed hep G2 cells. Am J Physiol. 1999;276:G92-G97. [PubMed] |
| 24. | El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 242] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Jin-no K, Tanimizu M, Hyodo I, Nishikawa Y, Hosokawa Y, Doi T, Endo H, Yamashita T, Okada Y. Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroenterol. 1998;33:376-382. |
| 26. | Sun HC, Li XM, Xue Q, Chen J, Gao DM, Tang ZY. Study of angiogenesis induced by metastatic and non-metastatic liver cancer by corneal micropocket model in nude mice. World J Gastroenterol. 1999;5:116-118. [PubMed] |
| 27. | Yamaguchi R, Yano H, Iemura A, Ogasawara S, Haramaki M, Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 274] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Gu GW, Zhou HG. New concept in etiology of liver cancer. Huaren Xiaohua Zazhi. 1998;6:185-187. |
| 29. | Feng DY, Chen RX, Peng Y, Zheng H, Yan YH. Effect of HCV NS(3) protein on p53 protein expression in hep atocarcinogenesis. World J Gastroenterol. 1999;5:45-46. [PubMed] |
| 30. | Peng XM, Peng WW, Chen Q, Yao JL. Apoptosis, Bcl-2 and P53 protein expressions in tissues from hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:834-836. |
| 31. | Zheng SX, Liu LJ, Shao YS, Zheng QP, Ruan YB, Wu ZB. Rela-tionship between ras p53 gene RNA and protein expression and HCC metastasis. Huaren Xiaohua Zazhi. 1998;6:104-105. |
| 32. | Yuan FP, Huang PS, Wang Y, Gong HS. Relationship between EBV infection in Fujian HCC and HBV and P53 protein expression. Shijie. Huaren Xiaohua Zazhi. 1999;7:491-493. |
| 33. | Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1008] [Cited by in RCA: 948] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 34. | Yamagishi S, Yonekura H, Yamamoto Y, Fujimori H, Sakurai S, Tanaka N, Yamamoto H. Vascular endothelial growth factor acts as a pericyte mitogen under hypoxic conditions. Lab Invest. 1999;79:501-509. [PubMed] |
| 35. | Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1877] [Cited by in RCA: 1969] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 36. | Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322-3331. [PubMed] |
| 37. | Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1997;94:8104-8109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 798] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 38. | Raleigh JA, Calkins-Adams DP, Rinker LH, Ballenger CA, Weissler MC, Fowler WC, Novotny DB, Varia MA. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res. 1998;58:3765-3768. [PubMed] |
| 39. | Kim KW, Bae SK, Lee OH, Bae MH, Lee MJ, Park BC. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res. 1998;58:348-351. [PubMed] |
| 40. | Biroccio A, Candiloro A, Mottolese M, Sapora O, Albini A, Zupi G, Del Bufalo D. Bcl-2 overexpression and hypoxia synergistically act to modulate vascular endothelial growth factor expression and in vivo angiogenesis in a breast carcinoma line. FASEB J. 2000;14:652-660. [PubMed] |
| 41. | Pan X, Ke CW, Pan W, He X, Cao GW, Qi ZT. Killing effect of DT/VEGF system on gastric carcinoma cell. Shijie Huaren Xiaohua Zazhi. 2000;8:393-396. |
| 42. | Pan X, Pan W, Ni CR, Ke CW, Cao GW, Qi ZT. Killing effect of tetracy cline-controlled expression of DT/VEGF system on liver cell cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:867-873. |
| 43. | Cao W, Wang ZM, Liang ZH, Zhang HX, Wang YQ, Huan Y, Li WX, Pan BR. Effects of angiogenesis inhibitor TNP-470 with lipiodol in arterial embolization of liver cancer in rabbit model. Shijie Huaren Xiaohua Zazhi. 2000;8:629-632. |
| 44. | Brekken RA, Overholser JP, Stastny VA, Waltenberger J, Minna JD, Thorpe PE. Selective inhibition of vascular endothelial growth factor (VEGF) receptor 2 (KDR/Flk-1) activity by a monoclonal anti-VEGF antibody blocks tumor growth in mice. Cancer Res. 2000;60:5117-5124. [PubMed] |
| 45. | Drevs J, Hofmann I, Hugenschmidt H, Wittig C, Madjar H, Müller M, Wood J, Martiny-Baron G, Unger C, Marmé D. Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Res. 2000;60:4819-4824. [PubMed] |
| 46. | Takayama K, Ueno H, Nakanishi Y, Sakamoto T, Inoue K, Shimizu K, Oohashi H, Hara N. Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res. 2000;60:2169-2177. [PubMed] |
| 47. | Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15-R24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 852] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 48. | Ohkawa J, Taira K. Control of the functional activity of an antisense RNA by a tetracycline-responsive derivative of the human U6 snRNA promoter. Hum Gene Ther. 2000;11:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Lobo SM, Ifill S, Hernandez N. Cis-acting elements required for RNA polymerase and (r) transcription in the human U2 and U6 snRNA promoters. Nucleic Acids Res. 1990;18:2891-2899. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Chalker DL, Sandmeyer SB. Sites of RNA polymerase III transcription initiation and Ty3 integration at the U6 gene are positioned by the TATA box. Proc Natl Acad Sci USA. 1993;90:4927-4931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Goomer RS, Kunkel GR. The transcriptional start site for a human U6 small nuclear RNA gene is dictated by a compound promoter element consisting of the PSE and the TATA box. Nucleic Acids Res. 1992;20:4903-4912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Good PD, Krikos AJ, Li SX, Bertrand E, Lee NS, Giver L, Ellington A, Zaia JA, Rossi JJ, Engelke DR. Expression of small, therapeutic RNAs in human cell nuclei. Gene Ther. 1997;4:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 166] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
Edited by Ma JY