Published online Jun 15, 2000. doi: 10.3748/wjg.v6.i3.384
Revised: January 13, 2000
Accepted: January 22, 2000
Published online: June 15, 2000
AIM: To propose a hypothesis defining the absorption, distribution, metabolism and elimination of traditional Chinese recipe (TCR)component in blood of healthy subjects and patients, and estimate its correctness.
METHODS: The pharmacokinetics (PK) of same dose of drug was studied in the animal model of traditional Chinese syndrome (S) and healthy animals. The classification, terminology, concept and significance of the hypothesis we re set forth with evidence provided in the present study. The hypotheses consist ed of traditional Chinese syndrome PK (S-PK) and traditional Chinese recipe PK (R-PK). Firstly, the observed tetramethylpyrazine (TMP) PK in healthy, chronica lly reserpinized rats (rat model of spleen deficiency syndrome, RMSDS) and RMSDS treated with Sijunzi decoction (SJZD) for confirmation were used to verify S-P K; secondly, the ferulic acid (FA) PK in healthy and high molecular weight dextran (HMWD)-induced rabbit model with blood stasis syndrome (RDBSS) was also used to verify S-PK; and lastly, TMP PK parameters in serum of healthy rats after orally taken -Ligusticum wallichii (LW), LW and Salvia miltiorrhiza (LWandS M) decoctions were compared to verify R-PK.
RESULTS: The apparent first-order absorption [Ka, (13.61 ± 2.56) h-1], area under the blood drug concentration-time curve [AUC, (24.88 ± 9.76) μg·h-1·mL-1], maximum drug concentration [Cmax, (4.82 ± 1.23) μg·mL-1] of serum TMP in RMSDS were increased markedly (P < 0.05) compared with those [Ka = (5.41 ± 1.91) h-1, AUC = (5.20 ± 2.57) μg·h-1·mL-1, Cmax = (2.33 ± 1.77) μg·mL-1] of healthy rats (HR). The apparent first-order rate constant for α and β distribution phase [α = (0.38 ± 0.09) h-1, β = (0.06 ± 0.03) h-1], the apparent first-order intercom partmental transfer rate constants [K10 = (0.24 ± 0.07) h-1, K12 = (0.11 ± 0.02) h-1, K21 = (0.11 ± 0.02) h-1] of serum TMP in RMSDS were decreased significantly (P < 0.01) compared with those [K10 = (0.88 ± 0.20) h-1, K12 = (1.45 ± 0.47) h-1, K21 = (0.72 ± 0.22) h-1] of HR. However, no apparent differences occurred between HR and RMSDS treated with SJZD. The serum FA concentration and its AUC [(5.6690 ± 2.3541) μg·h-1·mL-1] in RMBSS were also higher than those [AUC = (2.7566 ± 0.8232) μg·h-1·mL-1] of healthy rabbits (P < 0.05). The Ka (11.51 ± 2.82) h-1, AUC (0.84 ± 0.17) μg·h-1·mL-1 of LW and SM-derived TMP in serum were much lower (P < 0.05) than those [Ka = (19.58 ± 4.14) h-1, AUC = (1.27 ± 0.26) μg·h-1·mL-1] of LW-derived TMP in serum after oral decoctions.
CONCLUSION: The SDS and blood stasis syndrome state could affect significantly the pharmacokinetic parameters of drugs and the abnormal SDS pharmacokinetic parameters could be normalized by SJZD. The combination of Chinese medicine in TCR could reciprocally affect the pharmacokinetic parameters of other components absorbed into the systemic circulation. These results support the S and R-PK hypothesis.
- Citation: Huang X, Ren P, Wen AD, Wang LL, Zhang L, Gao F. Pharmacokinetics of traditional Chinese syndrome and recipe: a hypothesis and its verification (I). World J Gastroenterol 2000; 6(3): 384-391
- URL: https://www.wjgnet.com/1007-9327/full/v6/i3/384.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i3.384
Three progresses in the related subjects arouse us to propose hypotheses defining the traditional Chinese syndrome (S) and recipe PK (S-PK and R-PK) [1-6]. Firstly, in the field of traditional Chinese medicine (TCM), their characteristic PK has not developed as a consequence of what are th e chemical components absorbed into the circulation after administering traditional Chinese recipe (TCR) is not clear yet. Then, the progresses in successful detection of TCR derived component in serum from 1985 to 1989 supported the concept of above hypothesis[7,8]. Finally, the theoretical and practical achievements of chronopharmacokinetics are not only an example but also enhanced our intention of presenting the hypothesis[9-11].
The investigation of TCR-derived compound in vivo affords a sound basis for advancing the hypothesis of S and R-PK[2-8,12,13] and the inspiration from the theory and practice of chronopharmacokinetics greatly encourages us[9-11]. The knowledge dealing with the rhythmic changes of pharmacokineti c phenomenon in living organisms is called chronopharmacokinetics[14], in which, PK of administering drug at different time is not constant and is related to its therapeutic and toxic effects[14]. Similarly, are there possible differences of serum drug concentration and its pharmacokinetic parameters between different TCS and different Chinese medicines of TCR? In other words, does TCS state and the drugs combination in TCR affect significantly the blood drug concentration and their pharmacokinetic parameters after oral administration? We have postulated the above positive differences in the previously published hypotheses[1-6].
The four applications according to above concepts have been supported by National Natural Science Foundation of China since 1991. Under the support of grants, we choose Ligusticum wallichii (LW) prescriptions and its chemical component s as the examples to verify the above hypotheses.
Tetramethylpyrazine phosphate (TMPP) intravenous infusion in 2 mL ampule (25 g/L, lot No. 90101) and sodium ferulate (SF) were purchased from Guangdong Limin Pharmaceutical Factory (Shaoguang, China); reserpine injection solution in 1 mL (1 mg, lot No. 901008) was purchased from Red-Flag Pharmaceutical Factory of Shanghai Medical University (Shanghai, China).
LW was purchased from Dujiangyan City Pharmaceutical Company (Sichuan, China) which was identified by Professor Hu ZH (the Department of Botany, Northwest University, Xi'an, China). Salvia miltiorrhiza (SM), Panax ginseng C.A.Mey, Atractylodes macrocephala Koidz, Poria cocos (Schw.) Wolf, Glycyrrhiza uralensis Fisch were purchased from Xi'an Pharmaceutical Company (Xi'an, China). All organic reagents were bought from Xi'an Chemical Reagent Factory. All chemicals were of analytical reagent grade unless otherwise stated. Methaqualonum (internal standard) was presented by Institute of Materia Medica, Beijing Medical University (Beijing, China). Coumarin (internal standard) was purchased from Sigma. High molecular weight dextran (HMWD) (Mr 500000) was bought from Tianjin Air Force hospital (Tianjin, China).
The following main instruments were used in the experiment: Shimadzu LC-6A HPLC system; SPD-6A ultraviolet detector; shimadzu QP-1000 gas chromatography-mass spectrometer. 7650 infrared spectrometer. RH-90 NMR meter.
The S-PK hypothesis indicates that the pharmacokinetic differences between the different TCS have statistical significance and R-PK hypothesis means that one of TCM could influence markedly the pharmacokinetic parameters of other TCM-derive d components in blood when administered together in same TCR. The above two pharmacokinetic characteristics are related to the therapeutic, toxic responses and theory of TCM.
The preparation of SJZD (TCR): it consists of Panax ginseng C.A.Mey, Atra ctylodes macrocephala Koidz, Poria cocos (Schw.) Wolf and Glycyrrhiza uralensis Fisch (2:2:2:1). The 7000 g of SJZD drugs were divided into 7 parts. Each part of 1000 g was macerated with 7000 mL of distilled water (drug:water = 1:7, v/v) at room temperature for 1 h and then boiled for 40 min. The residues were boiled by same volume of water for 40 min once again. Firstly, the two boiled water extracts were mixed, then filtered through several layers of cotton gauze to remove the coarse particles and concentrated by evaporation, repeated preparation of the other parts of SJZD in the same way. The mixture of the seven parts of extraction gives the final concentration of 3 g·mL-1.
Serum TMP and FA PK in healthy and modeled animals were studied to verify the hypotheses. Healthy animals and animal models were divided into five groups and the latter included rat model of spleen deficiency syndrome (RMSDS) treated with SJ ZD; and rabbit model with blood stasis syndrome (RMBSS). Group 1 (HR-1, n = 72): healthy male Wistar rats weighing 240 g ± 20 g afforded from the Experimental Animal Center, the Fourth Military Medical University were injected ip with normal saline (0.1 mL·kg-1·d-1, 14 d). Group 2 (RMSDS, n = 72): healthy Wistar rats were injected ip with reserpine (0.5 mg·kg-1·d-1, 14 d). Group 3 (RMSDS treated by SJZD, n = 72): healthy Wistar rats were prepared the same as Group 2 and treated with intragastric administration of SJZD (30 g·kg-1·d-1, 14 d). The above three groups were given po 10 mg·kg-1 of TMPP 24 h after the final injection of normal saline or reserpine. Group 4 (HR-2, n = 6): healthy male New Zealand white rabbits afforded from the Animal Center of our university were injected intravenously with normal saline (15 mL·kg-1). Group 5 (RMBS, n = 6): rabbits were prepared by intravenous injection of 100 g/L HMWD in normal saline (15 mL·kg-1). FA was injected intravenously of 5 mg·kg-1 30 min after the end of injection of normal saline or HMWD. Rats and rabbits were fasted 12 h before administration.
The 0.5 mL of serum samples of rats were obtained by decapitation after a single oral TMPP at 0.083 h, 0.5 h, 1.0 h, 3 h, 5 h, 8 h, 12 h and 24 h, respectively. At each time point, 6 samples were obtained. The rabbit blood sample of 0.5 mL was directly withdrawn and collected from the ear vein at 2 min, 5 min, 10 min, 20 min, 30 min, 45 min, 60 min, 90 min, 120 min, 150 min and 180 min after intravenous FA. The blood samples were centrifuged (3000 r·min-1, 5 min), and 0.2 mL of the resulting serum was used.
Determination of serum TMP concentration[15-17]: the chromatographic system (Shimadzu LC-6A, Japan) consisted of two pumps (LC-6A), a sample injector (Rheodyne, model 7125), a SPD-6AV detector (Shimadzu) and a C-R3A data processor (Shimadzu). A Shim-Pach CLC-ODS column (particles 5 μm, 150 mm ± 4.6 mm ID) was used for quantitative analysis; AUFs was 0.04, the flow rate was 1 mL/min, t he paper speed was 3 mm/min, column temperature was 38 °C, the detection wavelength was 280 nm, the mobile phase consisted of methanol and water (72:28 v/v). The serum concentration data of HR-1 and RMSDS were analyzed by t he 3P87 (Chinese Pharmacological Association) software.
Determination of serum FA concentration[18]: the detector was set at 320 nm (AUFs: 0.01); the mobile phase was acetonitrile 0.1 mL·min-1 phosphoric acid (pH2.5) (3:7, v/v); the other chromatographic condition s were the same as those in determining serum TMP concentration.
Serum sample contained TMP: internal standard of Methaqualonum (428 ng) was added to 5 mL of ground conical centrifuge tube containing 100 μL of methanol. The mixture was oscillated on vortex mixer and evaporated to dryness at 48 °C on water bath under the stream of nitrogen. Successively added 0.2 mL of blank rat serum, the different amount of TMPP (0.044 μg·mL-1, 0.087 μg·mL-1, 0.168 μg·mL-1, 0.336 μg·mL-1, 0.671 μg·mL-1 and 1.342 μg·mL-1), 0.2 mL 0.05 mol/L of NaOH solution and 2 mL of trichloromethane to the above centrifugate. After the mixture was vortex mixed again for 15 s, it was centrifuged at 3000 rmin-1 for 10 min. The organic layer was transferred into another 5 mL ground conical centrifuge tube contained 100 μL of 1 mol/L hydrochloric acid-methanol solution (50 mL/L) and evaporated again to dryness on water bath at 48 °C under a stream of nitrogen. The residue was dissolved with 100 μL of methanol solution and then 20 μL was determined by HPLC each time[15].
Serum sample contained FA: to 0.2 mL of rabbit serum added 0.4 mL of acetonitrile contained 1.5 μg of coumarin internal standard. The mixture was vortex mixed and then the deproteinated precipitate was separated by centrifugation (3000 r·min-1, 5 min). The supernatant was evaporated at 60 °C water bath under a stream of nitrogen. The residue was dissolved in 60 μL acetonitrile and 20 μL of solution was directly injected into HPL C system for determination. To each 0.2 mL of rabbit blank sera 20 to 800 μg·L-1 of FA were added respectively. Their serum samples were analyzed separately by the corresponding method described above[18].
The preparation of LW decoction, LWandSM (LW:SM = 3:1) decoction: the dried roots of them were pounded to about 2 mm ± 4 mm ± 4 mm pieces. The other procedure was the same as those for preparing SJZD. The final concentration of L W decoction and LWandSM decoction were 3 g·mL-1 and 4 g·mL-1, respectively. TMP content of LW and LWandSM decoctions were determined before administration[17].
Only healthy rats were used to verify R-PK. Twelve Wistar rats were divided into two groups. LW decoction (30 g·kg-1) was given intragastricly to one group (n = 6) and LWandSM decoction (40 g·kg-1) was given to another group (n = 6). The blood samples of 0.2 mL each were obtained by cutting the animal tails at 0.083 h, 0.25 h, 0.50 h, 0.75 h, 1.00 h, 1.50 h, 2.00 h, 3.00 h and 5.00 h after oral TCR.
Analytical method: all quantitative but qualitative detections of TMP were the same as those described above. Chromato graphic conditions: Shim-Pach CLC-ODS column (particles 5 μm, 150 mm ± 4.6 mm ID) was also used for quantitative analysis; the half-preparative column (particles 10 μm, 250 mm ± 10 mm ID) was used for the separation and purification of LW decoction components in serum; Nebulizer and vaporizer temperatures were 250 °C. The drift voltage was 70eV.
The identification of LW-derived component in serum[17]: the related component was firstly separated, purified, enriched and then identified by 3-dimentional HPLC, mass spectrum, and NMR. Calibration curve and statistical analysis were the same as the correspondings described above.
The results are expressed as -x±s. Comparison of serum drug concentration and pharmacokinetic parameters between controls and studied model of LW and LWandSM decoctions were made by t test for paired samples. Differences were considered significant when P < 0.05.
One of the chemical components in serum after oral administration of LW extract to rats was identified as TMP by three-dimensional HPLC, UV, IR, MS and NMR (data not shown)[17].
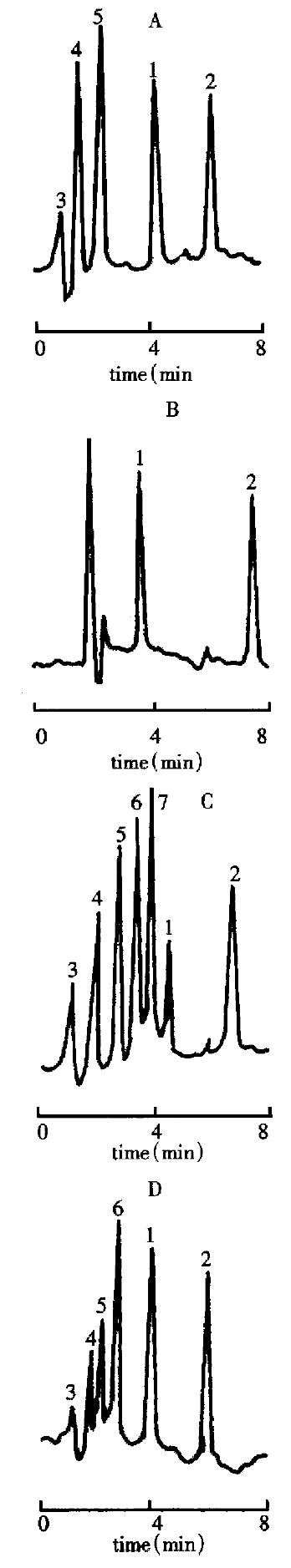
The chromatographic retention time of TMP, methaqualone (internal standard), FA and coumarin (internal standard) were 3.992 min, 6.223 min, 3.82 min and 7.68 min, respectively (Figure 1, A-B)[17,18]. They were separated well under their own chromatographic conditions. The ratios between the peak areas of the TMP and methaqualon (internal standard) in serum of rat, and of the FA and coumar in (internal standard) in serum of rabbit were calculated to make the calibration curves and the good linearity over the range (220-6710) μg·mL-1 (TMP, r = 0.9990, n = 6) and (20-800) μg·mL-1 (FA, r = 0.9986, n = 6) were obtained. Their equation of the curves were Y = 0.1889 + 0.0440X (TMP) and Y = 0.5826X + 0.1718 (FA). If the concentration of FA was above 800 μg·mL-1, the s ample was diluted. The detection limit of TMP and FA in sera were 68 mg·L-1 and 15 mg·L-1 respectively with a signal-to-noise ratio at 3.
The recoveries of TMP and FA contents from sera of rat and rabbit were determine d. Their results are shown in Table 1.
| Drug | Added (mg/L) | Found + SD (mg/L) | Recovery + RSD (%) |
| TMP | 0.2200 | 0.2126 ± 0.0081 | 96.64 ± 3.18 |
| TMP | 0.8388 | 0.8294 ± 0.0181 | 98.99 ± 2.18 |
| TMP | 6.7100 | 0.6657 ± 0.1973 | 99.34 ± 2.96 |
| FA | 0.0300 | 0.0280 ± 0.0009 | 95.00 ± 1.60 |
| FA | 0.4500 | 0.43804 ± 0.0043 | 97.40 ± 1.00 |
| FA | 0.7000 | 0.6883 ± 0.0053 | 98.30 ± 0.80 |
The precision in sera with three levels (0.22 μg·mL-1, 0.8388 μg·mL-1 and 6.7100 μg·mL-1) of TMP and (0.030 μg·mL-1, 0.450 μg·mL-1 and 0.700 μg·mL-1) of FA was detected (Table 2).
| Drug | Amount added (mg/L) | Interday cv (%) | Intraday cv (%) |
| TMP | 0.2200 | 2.45 | 5.57 |
| TMP | 0.8388 | 1.25 | 5.67 |
| TMP | 6.7100 | 1.44 | 4.72 |
| FA | 0.030 | 5.5 | 6.1 |
| FA | 0.450 | 3.2 | 4.3 |
| FA | 0.700 | 2.1 | 3.8 |
RMSDS: Four days after ip injection of reserpine, RMSDS showed decrease in activity, severe muscular hypotonia, palpebral ptosis and diarrhea, which were similar to those reported in our previously paper[15] and accorded with the published diagnostic standard of spleen deficiency syndrome by Chinese Association of Integrated Traditional and Western Medicine[19]. In RMSDS, the body weights of rats were reduced by 40% (242 g ± 22 g vs 145 g ± 20 g), and in RMSDS treated with SJZD, they had not shown any significant changes (results not shown).
The above analytical method of TMP was used to study TMP PK following oral TMPP. The pharmacokinetic profiles of oral TMPP in HR-1, RMSDS and SJZD treated RMSD S were the 2-compartment models according to the calculation of serum TMPP concentration-time data (Figure 2), which was in agreement with the results obtained from human and rats[20,21]. The serum concentrations of TMP in RMSDS w ere higher than those in HR-1 (P < 0.01, Figure 2). The pharmacokinetic parameters of TMPP in RMSDS (Table 3) with the exception of V/F(C) an d T peak were significantly different from those in HR-1(P < 0.01); ka, T1/2 ka, AUC, absorption of TMPP; α, β, T1/2β, T1/2α, CLs, K12, and K10 demonstrated that RMSDS decreased the rate of the distribution, transportation and clearance of TMPP (P < 0.01). The state of the syndrome in RMSDS affected obviously the absorption, distribution, metabolism and excretion of TMPP.
| Parameter | Healthy rat | RMSDS | SJZD |
| α (h-1) | 2.83 ± 0.70 | 0.38 ± 0.09b | 2.33 ± 0.65 |
| β (h-1) | 0.22 ± 0.02 | 0.06 ± 0.30b | 0.24 ± 0.05 |
| Ka (h-1) | 5.41 ± 1.91 | 13.61 ± 2.56 | 5.28 ± 1.39 |
| K10 (h-1) | 0.88 ± 0.20 | 0.24 ± 0.07b | 0.82 ± 0.11 |
| K12 (h-1) | 1.45 ± 0.47 | 0.11 ± 0.02b | 1.39 ± 0.68 |
| K21 (h-1) | 0.72 ± 0.22 | 0.11 ± 0.02b | 0.77 ± 0.18 |
| t1/2a (h) | 0.14 ± 0.09 | 0.05 ± 0.04b | 0.16 ± 0.10 |
| t1/2α (h) | 0.27 ± 0.11 | 1.92 ± 0.44b | 0.31 ± 0.09 |
| t1/2β (h) | 3.19 ± 0.39 | 13.35 ± 5.92b | 3.21 ± 0.28 |
| AUC (μg·h·m L-1) | 5.20 ± 2.57 | 24.88 ± 9.76b | 5.11 ± 6.81 |
| Tp (h) | 0.30 ± 0.05 | 0.34 ± 0.03 | 0.33 ± 0.02 |
| Cmax (μg/mL) | 2.33 ± 1.17 | 4.82 ± 1.23b | 2.18 ± 1.14 |
| VFC (μg/mL) | 2.81 ± 1.30 | 2.03 ± 0.58 | 2.19 ± 0.98 |
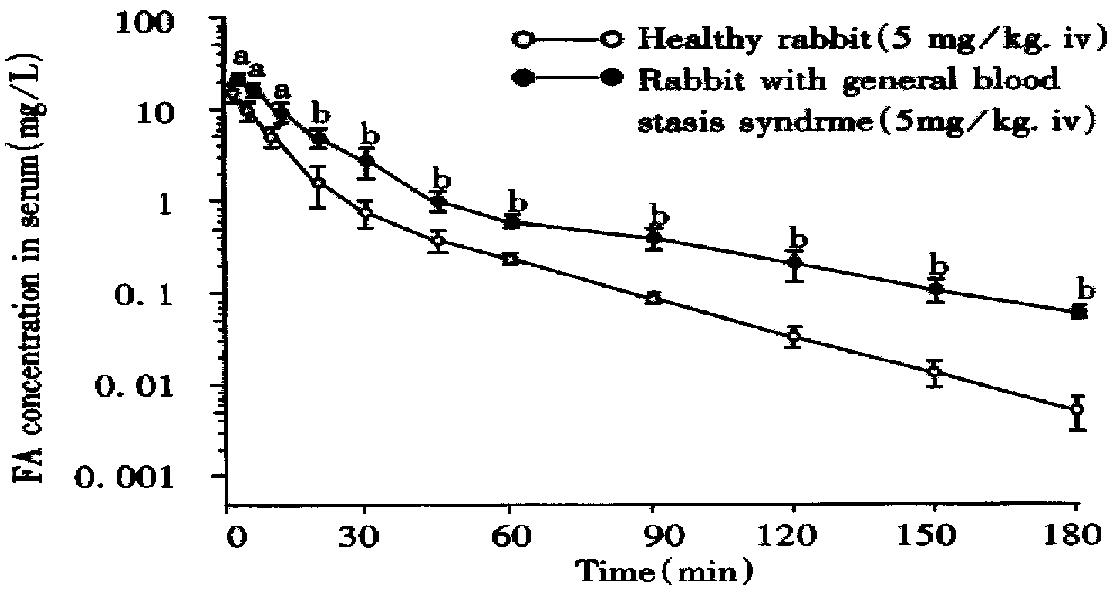
RMBSS: the above method of detecting FA used to study PK in healthy rabbit and FA serum concentration in RMBSS compartment model was fitted and the n pharmacokinetic parameters were calculated with a MCPKP program on a COMPAQ 80-386 computer. Compartmental analysis yielded a two-compartment open model. A rapid distribution phase followed by a slower elimination phase was observed. The mean pharmacokinetic parameters are given in Table 4 and serum concentration-time data in Figure 3.
The results of detecting TMP concentrations in LW and LWandSM decoctions were 574.50 g·kg-1·L-1 and 463.02 g·kg-1·L-1, respectively.
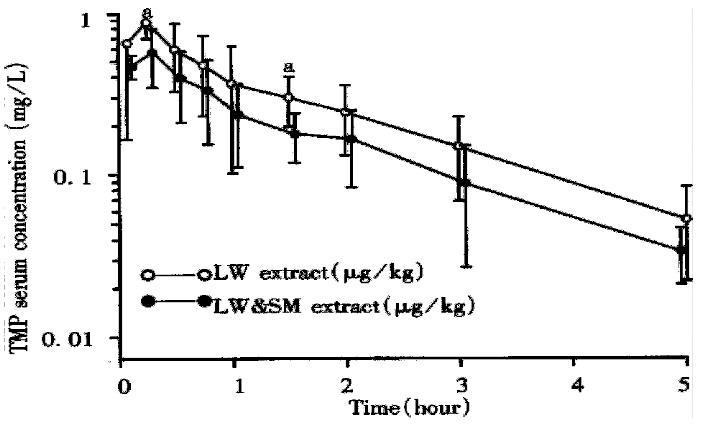
The chromatograms of TMP and methaqualonum, the latter was added into serum, and then extracted, were the same as shown in Figure 1A, 1B. The serum TMP chromatogram after administration of LW or LWandSM decoctions was different from that of TMP and of IS added to blank serum (Figure 1A) as well as that of post-administrative TMPP injection solution (Figure 1D). There were two more peaks of un known significance in Figure 1C than that in Figure 1A and one more unknow n peak than that in Figure 1D. The serum TCR-derived component parameters of 4 spectra of UV, IR, MS and NMR (data not shown) are similar to that of TMP standard substance and the reference value. Thus it gives an evidence to reveal that this TCR-derived component is undoubtfully TMP. The TMP PK were calculated by 3P87 program (Chinese Pharmacological Association). The fitted figures from two compartment open model and their parameters are indicated in Table 5.
| Parameter | LW | LWandSW |
| α (h-1) | 1.928 ± 0.719 | 2.328 ± 0.719 |
| β (h-1) | 0.479 ± 0.205 | 0.479 ± 0.289 |
| Ka (h-1) | 19.58 ± 4.139 | 11.508 ± 2.821a |
| K10 (h-1) | 1.090 ± 0.502 | 0.788 ± 0.255 |
| K12 (h-1) | 0.470 ± 0.160 | 0.205 ± 0.092 |
| T1/2α (h-1) | 0.354 ± 0.101 | 0.298 ± 0.223 |
| T1/2β (h-1) | 1.448 ± 0.880 | 1.447 ± 0.901 |
| AUC (μg·h·m L-1) | 1.273 ± 0.255 | 0.836 ± 0.168b |
| VC/F (L/kg) | 9.665 ± 1.810 | 7.390 ± 1.089a |
The concept, terminology, essentials and significance, especially scientific evidences, of S- and R-PK hypothesis were firstly reported and theoretically elaborated in our previous papers[1-6]. These ideas were exclusively cited[22-26]. Besides the definition described in above method, we summarized the main elements as follows: ① TCR-derived components in vivo are possibly to be detected; ② their number are relatively restricted; ③ they could represent the therapeutic effect of the parent recipe; ④ their concentration and PK could be affected by the combination of TCM in TCR; ⑤ effects of new bioactive components related with those of their parent TCR; and ⑥ the syndrome state could affect their PK significantly. All the ideas of S- and R-PK exhibited the characteristics of combination of TCM theory and PK. The other two published papers only concerned the element 1[1-6]. So far, over 20 evidences in the available published literatures[2-8,12,13] supported the element 1.
It is necessary to develop scientific analytical method, mainly chromatography, for verifying the hypothesis of S- and R-PK. In general, western drug component (single chemical substance) in blood is only identified by the consistency of retention time of peak in chromatograms as compared with its standard substance de rived from the national and international authorized unit[27-29]. However, this method is not sufficient in analysing the serum TCR-derived component because the coherence of retention time may result from two possibilities, i.e., only a single component peak or an overlap of two or more component peaks according to the theory of chromatography[17].
We developed the above HPLC method, to determine the serum TMP PK after the administration of TMPP or TCR-LW. When TMPP solution (Figure 1D) was administered, determination of TMP qualitatively and quantitatively by HPLC is enough corresponding to the method for determining ordinary western drug[27-29]. As orally administering LW or LWandSM decoctions, the structure of TMP was firstly identified by HPLC in combining with UV, IR, MS and NMR (Figure 1C) and then its concentration and pharmacokinetic value were determined by HPLC. Thus, it is likely to avoid the analytical errors in serum TMP derived from LW or LW and SM in our present experiments[15-17]. Tables 1 and 2 indicated that these methods for the determinations of serum TMP and FA concentrations were simple, rapid, sensitive, accurate, specific and reduplicable, with high recoveries. All these indexes were sufficient in studying S- and R-PK.
S-PK hypothesis was advanced according to the theories of TCM and PK[1-6]. To verify this hypothesis, the animal models of spleen deficiency and blood stasis were chosen and their pharmacokinetic characteristics of TMP and FA were studied. One of these models, RMSDS, was induced by injecting reserpine, which h ad been used as an animal model for "spleen deficiency syndrome" (SDS) in TCM [30,31] because its physical signs were similar to those seen in the SDS.
The "spleen" digests food, transports, distributes and transforms nutrients and replenishes qi[32]. We inferred from the above "spleen theory" that the "spleen" should be also responsible for the absorption and disposal of drugs. So, syndrome state of "spleen" is likely to affect the PK. The aim described in the S-PK hypothesis is to demonstrate the pharmacokinetic differences between syndrome and non-syndrome. Our experimental design from S-PK hypothesis is that the poor absorption of drug in SDS state would decrease its blood concentration and bioavilability. On the contrary, Figure 2 and Table 3 show the results of the increases in TMP serum concentration and AUC in RMSDS. Moreover, the parameters of the distribution and elimination of TMP in RMSDS (Table 3) were slowish. The SDS state could markedly affect the absorption, distribution, metabolism and elimination of TMPP in rat, and the observed results from Figure 2 and T able 3 show that SJZD could restore the above abnormal PK of TMPP in SDS model rats. All these TMP pharmacokinetic characteristics in RMSDS and the normalizing effect of SJZD on PK of TMP in RMSDS provided evidences for S-PK.
The mechanism of forming TMP pharmacokinetic characteristics in RMSDS is unknown. It was reported that the gastric and intestinal motility[33] and the absorptivity of D-xylose[34] were all decreased in reserpine-induced RMSDS and the patients with SDS. These observations are paradoxical to the increased serum TMP concentration in RMSDS. This confusion should be settled by further studies. Figure 3 shows that at corresponding time points the FA concentration in RMBSS increased markedly (P < 0.05) as compared with normal rabbits. From Table 4 we also found that in RMBSS both the total volume of distribution (VB) and total elimination rate (CLB) decreased significantly while FA elimination half-life time (t1/2β) and AUC increased significantly. This phenomenon may be due to the impairment of microcirculation induced by HMWD infusion. HMWD can lead to abnormal blood rheology (dense, sticky, aggregative, coagulative). So, it is impossible for FA to be metabolized quickly and distributed widely. In conclusion, through this experiment we found a significant difference in the pharmac okinetic parameters between healthy rabbits and RMBSS. This results are coincident with S-PK hypothesis[18].
So far, the orthodox academic society has thought that TCR-derived chemical components in vivo and their PK are not detectable and enable to study because they or their components are too complicated, trace in quantity or unable to rep resent curative effect of their parent TCR[35-38]. Therefore, the article related to above problem is rarely seen in recent years, and resulted in hard to explore the basic pharmacodynamic substance of TCR. The R-PK hypothesis emerged in literature since 1991 holds the idea that TCR-derived chemical components in vivo and their PK are possibly to be determined[1-6]. However, t he qualitative and quantitative analysis of LW and LWandSM-derived TMP in vivo from Figure 1(C) and its PK from Figure 4 and Table 5 supported the R-PK hypothesis. Similarly, the determinations of over 20 TCR-derived chemical components in blood/urine and their PK[2-8,12,13,39] in published papers are consistent with the viewpoint of R-PK hypothesis. All these achievements help elucidate the pharmacology of TCM and TCR[39].
From the results provided in Figure 4 and Table 5 we found that the absorption (Ka), transport (K21) and distrubution (Vc/F) of LWandSM-derived TMP in rat serum were decreased significantly compared with those of LW-derived TMP. In Figure 4 and Table 5 it also shows that the concentration and AUC of LWandSM-derive d TMP in rat serum were lower than those of LW-derived TMP, which could explain why LW and SM are rarely used alone in a TCR. This viewpoint supported the hypo thesis that the combination of drugs in TCR could affect pharmacokinetic parameters of TCR-derived component in vivo. It is extremely urgent to explore which component play an important role in the field of interaction of LW or LWandSM derived components in vivo.
| 1. | Huang X, Ma Y, Jiang YP, Xia T, Ren P. The scientific evidence and prospect of traditional Chinese syndrome and recipe pharmacokinetics hypothesis. March of integration of TCM and WM towards 21st century. Beijing: China Medicine Pharmaceutics Science-technology Publisher 1991; 207-216. |
| 2. | Huang X, Jiang YP, Wen AD, Zang YM, Niu GB. Is it possible to study the pharmacokinetics of chemical component of herbal recipe. Chin J Intern Med. 1995;1:297-300. |
| 3. | Huang X. The concepts of recipe-derived component spectrum and target component in vivo/serum and their significance. Disi Junyi Daxue Xuebao. 1999;20:277-279. |
| 4. | Huang X, Zang YM, Xia T, Ren P. The hypothesis of traditional Chinese syndrome and recipe pharmacokinetics. Zhongyao Yaoli Yu Linchuang. 1994;10:43-44. |
| 5. | Huang X, Ren P. [Prevention and treatment of hypertension and coronary heart disease--therapeutic drug control]. Zhongguo Zhongxiyi Jiehe Zazhi. 1997;17:515-518. [PubMed] |
| 6. | Huang X, Chen KJ. The theory and practice of traditional Chinese syndrome and recipe hypothesis. Zhongyi Zazhi. 1997;38:745-747. |
| 7. | Tanaka S, Ihoko O, Shinichi T. Establishment of methods evaluating the "shyo" and effectiveness of Kampo formulae by measuring their blood concentration. J Med Pharmaceut Society for Wakan Yaku. 1986;3:276-277. |
| 8. | Kano Y, Sakurai T, Saito K. Pharmacological properties of Galenical preparation XII. Components of Chinese traditional prescription "Kanzobusito" in rat portal blood after oral administration. Shoyakugaku Zasshi. 1989;43:199-203. |
| 9. | Lemmer B. The cardiovascular system and daily variation in response to antihypertensive and antianginal drugs: recent advances. Pharmacol Ther. 1991;51:269-274. [PubMed] |
| 10. | Eradiri O, Midha KK. Comparison of diltiazem bioavailability from 3 marketed extended-release products for once-daily administration: implications of chronopharmacokinetics and dynamics. Int J Clin Pharmacol Ther. 1997;35:369-373. [PubMed] |
| 11. | Gries JM, Benowitz N, Verotta D. Importance of chronopharmacokinetics in design and evaluation of transdermal drug delivery systems. J Pharmacol Exp Ther. 1998;285:457-463. [PubMed] |
| 12. | Nishioka Y, Kyotani S, Miyamura M, Kusunose M. Influence of time of administration of a Shosaiko-to extract granule on blood concentration of its active constituents. Chem Pharm Bull (Tokyo). 1992;40:1335-1337. [PubMed] |
| 13. | Homma M, Oka K, Taniguchi C, Niitsuma T, Hayashi T. Systematic analysis of post-administrative saiboku-to urine by liquid chromatography to determine pharmacokinetics of traditional Chinese medicine. Biomed Chromatogr. 1997;11:125-131. [PubMed] |
| 14. | Ritschel WA, Forusz H. Chronopharmacology: a review of drugs studied. Methods Find Exp Clin Pharmacol. 1994;16:57-75. [PubMed] |
| 15. | Huang X, Ren P, Wen AD. [Pharmacokinetic characteristics of tetramethylpyrazine and study on hemorheology in rat model of spleen deficiency syndrome]. Zhongguo Zhongxiyi Jiehe Zazhi. 1994;14:159-61, 134. [PubMed] |
| 16. | Huang X, Wen AD, Jiang YP, Ren P, Zang YM. Determination of tetramethylpyrazine in serum by RP-HPLC after oral administration of boiled water extracts of Ligusticum chuanxiong to rats. Zhong Yao Cai. 1995;18:305-307. |
| 17. | Huang X, Xia T, Ren P. [Influence of combined Salvia miltiorrhiza and Ligusticum wallichii on pharmacokinetics of tetramethylpyrazine in rats]. Zhongguo Zhongxiyi Jiehe Zazhi. 1994;14:288-91, 261-2. [PubMed] |
| 18. | Wen AD, Huang X, Jiang YP, Fan YX. High-performance liquid chromatographic determination of free ferulic acid in serum of rabbits with blood stasis. Yaoxue Xuebao. 1995;30:762-767. [PubMed] |
| 19. | Shen ZY, Wang WJ. The reference standards of differential diagnosis of difficiency syndrome of traditional Chinese medicine. Zhongguo Zhongxiyi Jiehe Zazhi. 1986;6:598. |
| 20. | Liu XQ, Lou YC, Shi WZ. Study on the relationship between pharmacokinetics and pharmacodynamics of tetramethylpyrazine and effects of acute hepatic poisoning on its pharmacokinetics in rats. Beijing Yike Daxue Xuebao. 1991;23:185-189. |
| 21. | Liu XQ, Lou YC, Chen QT. The clinical pharmacokinetics studies of tetramethylpyrazine hydroch loride in normal volunteers and patients with acutecerebral ischemia disease (CID). Zhongguo Linchuang Yaoli Zazhi. 1991;7:32-36. |
| 22. | Zhang WH, Zha LL. Review and prospect of making blood stasis aminal model. Zhongguo Zhongxiyi Jiehe Zazhi. 1996;16:184-186. |
| 23. | Song DM, Su H, Wu MH, Huang XM. Effect of tetramethylpyrazine and radix salviae miltiorrhizae on collagen synthesis and proliferation of cardiae fibroblasts. Zhongguo Zhongxiyi Jiehe Zazhi. 1998;18:423-425. |
| 24. | Li M, Du LJ, Sun H. Survey of studies on pharmacokinetics of Chinese herbal combination. Zhongguo Zhongxiyi Jiehe Zazhi. 1998;18:637-639. |
| 25. | Ma W, Wang JH. A hypothesis of syndrome and treatment toxicology of chinese materia medica. Zhongyao Xinyao Yu Linchuang Yaoli. 1999;10:116-118. |
| 26. | Pan GY, Liu XD. The methods and prospects of studies on the recipe-pharmacokinetics of Chinese materia medica. Zhong Cao Yao. 1998;29:642-644. |
| 27. | Marzo A, Dal Bo L. Chromatography as an analytical tool for selected antibiotic classes: a reappraisal addressed to pharmacokinetic applications. J Chromatogr A. 1998;812:17-34. [PubMed] |
| 28. | Péhourcq F, Jarry C. Determination of third-generation cephalosporins by high-performance liquid chromatography in connection with pharmacokinetic studies. J Chromatogr A. 1998;812:159-178. [PubMed] |
| 29. | Tyrrell CJ, Denis L, Newling D, Soloway M, Channer K, Cockshott ID. Casodex 10-200 mg daily, used as monotherapy for the treatment of patients with advanced prostate cancer. An overview of the efficacy, tolerability and pharmacokinetics from three phase II dose-ranging studies. Casodex Study Group. Eur Urol. 1998;33:39-53. [PubMed] |
| 30. | Ren P, Song GZ, Xia T, Huang X, Zhang ZB, Hu JL. Relationship between diarrhea with spleen deficiency and motilin of plasma and intestinal tissue. Zhongguo Zhongxiyi Jiehe Zazhi. 1994;14:25-27. |
| 31. | Shen H, Guan CF. Description and application of an enzyme-linked immunosorbent assay for the detection of protein tyrosine kinase activity and the study on spleen deficiency syndrome. Zhongguo Zhongxiyi Jiehe Zazhi. 1998;18:243-245. |
| 32. | Xie ZF, Liao JZ. Traditional Chinese Internal Medicine. Foreign Languages Press, Beijing. Beijing: China Medicine Pharmaceutics Science-technology Publisher 1993; 37-39. |
| 33. | Ren P, Huang X, Jiang YP, Wen AD, Song GZ. Effect of Sijunzi, decoction on motilin pharmacokinetic characteristics of tetramethylpyrazine in rat model of spleen deficiency syndrome. Zhongguo Zhongxiyi Jiehe Zazhi. 1997;17:45-47. |
| 34. | Xiong DX. Surveying of bacteroides in human lower intestine. Weishengwu Xuebao. 1985;25:69-72. |
| 35. | Huang JC. Studies of the metabolism and pharmaceutics of active principles isolated from Chinese herbai medicine since the foundation of the people's republic of China. Yaoxue Xuebao. 1987;22:553-560. |
| 36. | Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1-7. [PubMed] [DOI] [Full Text] |
| 37. | Yano H, Mizoguchi A, Fukuda K, Haramaki M, Ogasawara S, Momosaki S, Kojiro M. The herbal medicine sho-saiko-to inhibits proliferation of cancer cell lines by inducing apoptosis and arrest at the G0/G1 phase. Cancer Res. 1994;54:448-454. [PubMed] |
| 38. | Chan K. Progress in traditional Chinese medicine. Trends Pharmacol Sci. 1995;16:182-187. [PubMed] |
| 39. | Huang X, Jiang YP, Zang YM, Niu GB. Advances in the study of metabolism and pharmacokinetics of chemical components in decoction of traditional Chinese medicine. Zhongcaoyao. 1995;26:546-549. |
Xi Huang MD and PhD graduated from Fourth Military Medical University as a postgraduate in 1995, worked as a postdoctoral research fellow in Laboratory of Cardiovascular Disease, Xiyuan Hospital, China Academy of Traditional Chinese Medicine from 1996 to 1998, now professor and director in Laboratory of Clinical Pharmacology of Chinese Medicine, majoring clinical pharmacology of Chinese medicine, having 50 papers published.
Edited by You DY
proofread by Sun SM