Published online Apr 15, 2000. doi: 10.3748/wjg.v6.i2.301
Revised: July 6, 1999
Accepted: July 14, 1999
Published online: April 15, 2000
- Citation: Lin XZ, Ma DL, Cui ZQ, Kang Y. Effects of rhubarb and the active ingredients of rhubarb on the cytoplasmic free calcium in INT-MNC of rabbits. World J Gastroenterol 2000; 6(2): 301-303
- URL: https://www.wjgnet.com/1007-9327/full/v6/i2/301.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i2.301
The recent studies have shown that rhubarb has not only the effect of removing stasis by purgation, but also intestinal barrier effects[1,2]. In order to further clarify the intestinal barrier mechanism of rhubarb, we studied the effects of rhubarb decoction and the active ingredients of rhubarb on the cytoplasmic free calcium in isolated intestinal mononuclear cells (INT-MNC).
Fura-2/Am was purchased from the Shanghai Institute of Physiology, the Chinese Academy of Sciences. Eagle’s MEM was purchased from Nissui Pharmaceutical Company, Japan. Collagenase (typeIV) and emodin (EMD) were products of Sigma Chemical Co. Sennosides (SEN) was provided by the Department of Plant Chemistry of the Tianjin Institute of Materia Medica. Rhubarb decoction (equal to 1 g/mL of protophyte) was provided by the Department of Pharmacology of the Tianjin Acute Abdominal Institute of Integrated Traditional Chinese and Western Medicine. Dithiothreitol (DTT) was purchased from Feibiochemi.
Rabbits weighing 2.2 kg ± 1.2 kg, in either sex, supplied by the An imal Department of Tianjin Medical University. They were randomly divided into control, rhubarb, EMD and SEN groups.
The rabbits were killed by a blow on the head and the abdomen was opened. Take 30 cm-40 cm of the ileum 10 cm from the ileocecal part, wash out the stool and mucoid on the surface of the mucosa with Hank’s solution (NaCl 137, KCl 5, Glucose 5.6 and Hepes 10 mmol/L, pH7.4) which is Ca2+ and Mg2+ free. Take the intestinal mucosa gently with coverglass and put it into a conical flask containing Dithiothreitol (DTT, 1 mmol/L) solution, swing and wash for 20 min at 37 °C, then put the mucosa into 5 mmol/L EDTA solution and swing for 30 min at 37 °C, and wash out the free epithelial cells using Hank’s solution. Repeat the procedure of EDTA for 3 times. Then put the intestinal mucosa into Hank’s solution containing collagenase. To digest it, swing for 3 h at 37 °C, filter 3 times with stainless sieve of 100 mesh. Put the cells into the lymphocyte separate solution and centrifuged at 500rpm for 5 min. Take the cells of the middle layer (INT-MNC), wash with ice-cold Ca2+ and Mg2+ free Hank’s solution and resuspend in Eagle’s MEM solution and dilute to a concentration of 1-2 × 108 mL/L.
Two mL cell suspension was incubated at super-thermostat water bath vibrator for 30 min in the presence of 5 μmol/L Fura-2/AM dissolved in dimethyl sulfoxide (DMSO) and 0.1% bovine serum albumin (37 °C, 40 rev/min, 95% O2+5% CO2). The Fura-2/Am-loaded INT-MNC was washed with Ca2+ and Mg2+ free Hank’s solution and centrifuged at 1000rpm for 10 min for three times. The INT-MNC was resuspended in 2 mL Ca2+ and Mg2+ free Hank’s solution until use.
The 2 mL Fura-2 loaded INT-MNC was incubated for 10 min at 37 °C for measurement. After the drugs were added and before measurement, the cell suspensions were blown and inspired 5-6 times with pipet to make the cell well distributed. Fura2/AM fluorescence was measured with RF-510 spectrofluorophotometer (Shimadzu, with thermostat). Emission wavelength (EM) was fixed at 490 nm. The excitation wavelength (EX) at 380 or 350 nm, the grating was 10 nm. The scanning speed was 100 nm/min. The changes of 350/380 nm fluorescence ratio were recorded by alternating rapidly the EX of 350 nm and 380 nm manually (the rotating was finished within 4 s - 6 s). The [Ca2+]i was calculated using the general formula[3] with Kd of 224 nmol/L: [Ca2+]i = Kd × (R-Rmin)/(Rmax-R) × S. Before calculation, the auto-fluorescence unloaded with Fura-2 should be subtracted.
The results were expressed as mean values of groups ¯x ± s. The Student t test was used for statistical comparison of difference of mean values between groups.
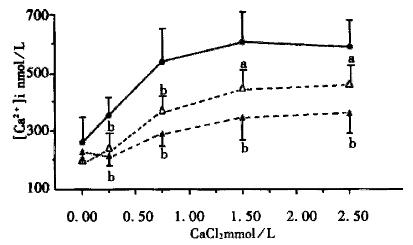
In the resting status, the INT-MNC[Ca2+]i was 252.55 nmol/L ± 42.54 nmol/L (n = 10) in Ca2+ free Hank’s solution containing ethyleneglycol-bis(β-aminoethyl ether)-N’,N’,N’,N’ tetraacetic acid (EGTA) 0.5 mmol/L. After adding CaCl2 (0.25 mmol/L, 0.75 mmol/L, 1.5 mmol/L and 2.5 mmol/L) to INT-MNC suspension sequentially, the [Ca2+]i levels were obviously elevated as compared with that at resting state (P < 0.01, Figure 1). This indicated that [Ca2+]i was increased with the extracellular calcium level.
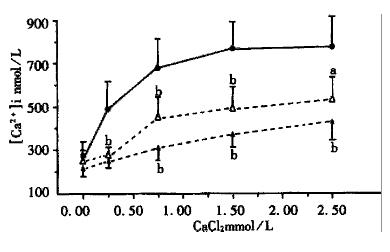
The INT-MNC was pretreated with rhubarb decoction (1 mg/L, 2 mg/L) or SEN (0.021 mmol/L, 0.084 mmol/L) for 15 min, in the resting state or after adding the above doses of CaCl2, the INT-MNC [Ca2+]i was more obviously lowered than the control groups (P < 0.01, Figure 1, Figure 2). The results showed that both rhubarb and SEN decreased the INT-MNC[Ca2+]i.
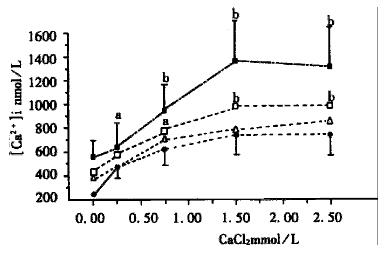
After pretreated with EMD (9.2 μmol/L, 18.5 μmol/L, and 37 μmol/L) for 15 min, in the resting status or adding the above doses of CaCl2, the INT-MNC [Ca2+]i was significantly increased as compared with that of the control groups (P < 0.01, Figure 3). The results showed that EMD promoted not only the release of intracellular Ca2+ but also the extracellular influx.
Intestine is one of the largest immune organs. Intestinal mucosa immune defence system is made up of correlative lymphoid tissues. INT-MNC mainly consists of macrophage and lymphocytes. The separated INT-MNC produces immunoglobulin (Ig) spontaneously in vitro[4]. It has an important effect on the immune regulation. Intracellular Ca2+ can regulate the endocrine system. When the Ca2+ level increases, Ca2+ produces physiologic effect as a stimulant. But when intracellular Ca2+ has overloaded, the function of cell arrests, eventually initiated the cascade of deleterious events leading to cell death. This study indicates that the INT-MNC pretreated with rhubarb decoction or SEN, decreased the [Ca2+]i dose-dependently as compared with the control groups (P < 0.01) when it is resting or is added with an equivalent amount of CaCl2. This is consistent with the previous results that the rhubarb has a Ca2+ channel blocking activity[5-7]. The antagonizing Ca2+ effects of rhubarb or SEN may be responsible for the intestinal barrier protective effect. On the contrary, the EMD promotes not only the release of intracellular Ca2+, but the extracellular influx. This shows that the EMD can improve body immune function. Both SEN and EMD are active ingredients of rhubarb. The different effects of rhubarb and the different active ingredients of rhubarb on the Ca2+ level suggest that rhubarb has many kinds of immunological regulatory effects on INT-MNC.
| 1. | Wu XC, Li SZ, Pei DK. Experimental research of the acute abdomen. The research series of integrated traditional Chinese and western medicine. Acute abdomen research. Shanghai: Shanghai Press of Science and Technology 1988; 185-193. |
| 2. | Wang SH. Experiences on the clinical application of Raixet Rhizoma Rhei. Zhongyi Zazhi. 1992;33:4-8. |
| 3. | Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440-3450. [PubMed] |
| 4. | Wu KC, Zhang XY, Mahida YR, Jewll DP. Altered immune regulation by intestinal macrophages in Crohn's disease. Zhonghua Xiaohua Zazhi. 1993;13:34-37. |
| 5. | Zhou JH, Wang JH. The protective effects of Da Cheng Qi decoction with effect of removing stasis by purgation on intestinal barrier function. The research series of basic and clinical study of Chinese materia medica: advances of pharmacological and clinical research of traditional Chinese medicines. Beijing: Military Medical Publishing House 1995; 254-261. |
| 6. | Lin XZ, Cui ZQ, Jin ZH, Ma DL. Effects of emodin on the cytoplasmic free calcium in the platelets. Zhongguo Zhongyao Zazhi. 1994;3:126-131. |
| 7. | Kang Y, Guo S, Wu XZ. [Da cheng qi tang on 45Ca content of the isolated colon smooth muscle from experimental colon obstruction rats]. Zhong Xi Yi Jie He Za Zhi. 1991;11:107-19, 70. [PubMed] |
Edited by Ma JY
Project supported by the National Natural Science Foundation of China, No.39170910