Published online Oct 15, 1999. doi: 10.3748/wjg.v5.i5.424
Revised: August 20, 1999
Accepted: September 14, 1999
Published online: October 15, 1999
- Citation: Chen XM, LaRusso NF. Human intestinal and biliary cryptosporidiosis. World J Gastroenterol 1999; 5(5): 424-429
- URL: https://www.wjgnet.com/1007-9327/full/v5/i5/424.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i5.424
Cryptosporidium parvum (C. parvum) is a coccidian parasite of the phylum Apicomplexa that infects the gastrointestinal, biliary and respiratory epithelium of humans and animals[1]. Early reports described a disease in humans characterized by protracted, watery diarrhea occurring in immunosuppressed patients, many with acquired immunodeficiency syndrome (AIDS). Recent epidemiologic studies indicate that cryptosporidiosis may also present as an acute, self-limited diarrheal disease in immunocompetent individuals and may account for 1%-10% of diarrheal disease worldwide[2,3]. Despite the magnitude and severity of cryptosporidial infection, the pathogenesis is poorly understood, and there is currently no effective therapy[3]. In this review, we provide a concise summary of what is known about cryptosporidial infection of the intestinal and biliary tract.
Cryptosporidium is a coccidian parasite and one of many genera of the protozoan phylum, Apicomplexa (class Sporozoea, subclass Coccidia). Six species are currently recognized on the basis of differences in host specificity, oocyst morphology and site of infection (C. parvum, C. muris, C. meleagridis, C. baileyi, C. serpentis and C. nasorum); only C. parvum causes diseases in humans[1,4].
C. parvum has a monoxenous life cycle, all stages of development (asexual and sexual) occurring in one host. The entire life cycle may be completed in as few as 2 d in many hosts, and infections may be short-lived or may persist for months. Once ingested, oocysts excyst in the gastrointestinal tract releasing infective sporozoites. The freed sporozoites attach to epithelial cells and become enclosed within parasitophorous vacuoles, developing attachment organelles (stages referred as trophozoites). The trophozoites then undergo asexual proliferation by merogony and form two types of meronts. Type I meronts form 8 merozoites that are liberated from the parasitophorous vacuole when mature; the merozoites then invade other epithelial cells where they undergo another cycle of type I merogony or develop into type II meronts. Type II meronts form 4 merozoites which do not undergo further merogony but produce sexual reproductive stages (called gamonts). Sexual reproduction occurs by gametogony and both microgamets (male) and macrogametocytes (female) are formed. Macrogametocytes are then fertilized by mature microgamets, and the resultant zygotes undergo further asexual development (sporogony) and form sporulated oocysts containing 4 sporozoites. Most oocysts are thick-walled and are excreted from the host in faecal material; some oocysts, however, are thin walled and have been reported to excyst within the same host leading to a new cycle of development[1]. The presence of these auto-infective oocysts and recycling type I meronts are believed to be the means by which persistent chronic infections may develop in hosts without further exposure to exogenous oocysts[1].
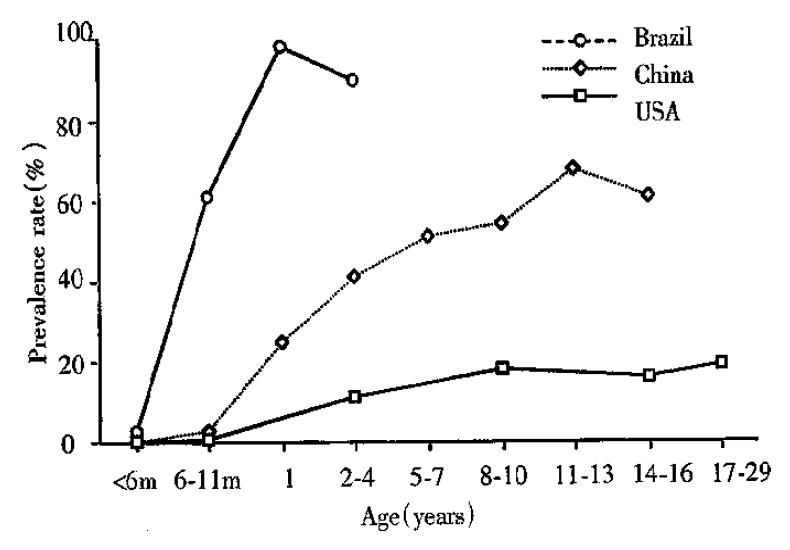
Infection of C. parvum in both immunocompetent and immunocompromised humans occurs worldwide. From prevalence studies, oocyst excretion rates are known to vary between 1%-3% in industrialized countries and 10% in less industrialized nations. Seroprevalence rates are much higher. In developed countries, they vary between 25%-35%, while in the developing world these rates are as high as 60%-90% (Figure 1)[3,5,6]. C. parvum infection was reported in 10%-15% of the children with diarrhea in the developing world. It has been reported that 10%-16% of AIDS patients with chronic diarrhea in North America and 30%-50% in the developing world are infected with C. parvum[7,8] while the in fection mainly occurs in the intestine in both immunocompetent and immunocompromised individuals, biliary cryptosporidiosis has only been reported in HIV-infected patients, being found in 20%-65% of the patients with so-called AIDS-cholangiopathy[9].
It has been reported that as low as 10 oocysts of C. parvum can cause human diseases. Many instances of human-to-human transmission have been recorded between household and family membranes, sexual partners (both heterosexual and homo sexual), hospital patients and staff, and children attending day care centers. While most cases of transmission involve oocysts derived from faecal samples, contaminated water is a source of infection among international travelers, and outbreaks have been associated with contamination of well water, surface water, swimming pools and public water supplies. C. parvum oocysts have been recovered from untreated surface waters, filtered swimming pool water, and most importantly, from treated drinking water. Homosexuals practicing oral-anal and/or anal-genital sex, veterinary personnel and animal handlers are particularly at risk[1]. Immunocompromised individuals with hypogammaglobulinaemia, organ transplanted recipients, patients undergoing bone marrow transplantation and patients on immunosuppressive drugs are also at high-risk[10].
Immunocompetent individuals who become infected generally experience a self-limited syndrome and become immune to reinfection. In contrast, severe chronic infections may develop in immunocompromised individuals with either congenital or acquired lymphocyte or γ-globulin deficiencies, suggesting both cell-mediated and humoral immune responses are involved in the resolution of infections and the development of immunity[1].
Immunoserological tests have detected specific IgG, IgM, IgA and even IgE antibodies in acute or convalescent sera from infected patients. Local and secretory antibodies have been detected in association with infections, including IgA antibodies in duodenal fluid[11]. Passively acquired antibodies have also been implicated in the prevention or control of infections. Several epidemiological studies recorded a lower prevalence of infections in breast-fed children than in bottle-fed children. However, the role of serum or secretory antibodies in the resolution of infections is unclear. Neutralizing antibodies against surface membrane determinants of free sporozoites and merozoites reduce infectivity, suggesting that some antibodies may neutralize intraluminal stages of the parasite. Whether these antibodies would be effective against intracellular developmental stages in which the parasite is covered by host membranes is unknown[1].
Experimental studies using immunocompr-omised SCID mice and nude mice have shown that resolution of a C. parvum infection requires B and/or T lymphocytes[12]. The identification of cryptosporidiosis as one of the opportunistic infections affecting individuals with AIDS also suggests that immunity to this parasite requires CD4+ T cells. CD4+ T cells of patients with AIDS infected with C. parvum show that fulminant and persistent disease only occurs in individuals with CD4+ counts < 50 cells/mm3. Individuals with CD4+ T counts > 200 cells/mm3 display only transient disease. Individuals with CD4+ T counts < 50 cells/mm3 have an increased incidence of biliary disease, an indicator of chronic cryptosporidiosis, and a decreased survival when compared to those individuals with higher CD4+ T cell counts[9]. IFNγ is important for resistance to C. parvum; the absence of this cytokine in mice with a targeted disruption of the IFNγ gene (gene knockout ) results in uncontrolled C. parvum infection[13].
Histological changes associated with intestinal cryptosporidiosis are relatively non-specific and include blunting of villi, hyperplasia of intestinal crypt cells, and infiltration of inflammatory cells into the luminal propria. Neutrophilic infiltrate, villus blunting, cryptitis, epithelial apoptosis and reactive epithelial changes in the intestine in AIDS patients with cryptosporidiosis correlate with the intensity of C. parvum infection[14]. Biliary cryptospor idiosis is also associated with a non-specific inflammatory response. Histologically, there is a periductal inflammatory response with interstitial edema, mixed inflammatory cell infiltrates, and hyperplasia and dilatation of the periductual glands. The fibrosis that develops around the portal tracts of AIDS patients with chronic cryptosporidial infection can mimic the histologic changes seen in primary sclerosing cholangitis. Autopsy reports and prospective studies have supported an etiologic role for the organism in biliary syndromes like sclerosing cholangitis and acalculous cholecystitis[15,16]. However, the pathophysiol ogical mechanisms underlying C. parvum infection of intestinal and biliary epithelia are not well understood, and at present our understanding of the pathogenesis is still limited to data from animal experimental studies.
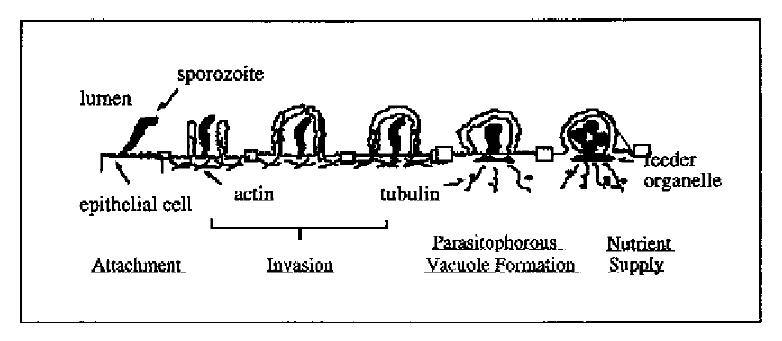
The process by which C. parvum sporozoites infect epithelial cells consists of two sequential steps: ① attachment of sporozoites to the plasma membrane of epithelial cells, a primary event in the initial host-parasite interaction and a prerequisite for the pathophysiological consequences; and ② invasion of sporozoites into host cells by invagination of the host cell plasma membrane, which engulfs and eventually completely surrounds the sporozoite to form a parasitophorous vacuole in which the organism remains intracellular but extracytoplasmic[17-19]. Electron microscopy has confirmed the intracellular but extracytoplasmic location of the organism within a parasitophorous vacuole formed by a continuous covering of microvillous membranes, and nearly all endogenous developmental stages of C. parvum are confined to the apical surface of epithelial cells. The parasite contains a unique “attachment” or “feeder” organelle which is prominent at the base of each parasitophorous vacuole. Originally, this feeder organelle was thought to be formed by repeated folding of parasite and host epithelial cell membranes. However, recent electron microscopic studies showed that the dense band of feeder organelle underlying the parasite attachment site represents modified host cell cytoskeleton. This organelle is thought to facilitate the uptake of nutrients by the parasite from the host cell.
C. parvum displays a clear predilection to infect only certain sites within the host. The parasite usually infects epithelia in the intestine, respiratory tract and bile ducts. In the human intestine, the stomach is rarely infected, and the upper small bowel, colon, and rectum are less affected than the mid small bowel[1]. Several cell lines from human or animal intestine are sensitive to C. parvum-infection in vitro, but the susceptibilities of those cells to infection differ[20]. Sporozoites attach to the cultured host cells by their anterior pole and attachment is dose, time, ion, pH and host cell-cycle dependent, and is inhibitable by antibodies against antigens on the sporozoite surface membrane[21,22]. Recently, we developed an in vitro system of biliary cryptosporidiosis, and demonstrated that the infection is both apical plasma membrane and liver cell specific (i.e., C. parvum can infect bile duct cells but not hepatocytes in vitro)[19]. These characteristics suggested specific molecules on the surface of both epithelial cells and C. parvum sporozoites are involved in the infection process. Previous studies did demonstrate the presence of a galactose-N-acetylgalactosamine (Gal-GalNAc) specific C. parvum sporozoite surface lectin which may mediate attachment of sporozoites to host cells[22,23]. Moreover, recent reports have shown that C. parvum sporozoite motility depends on the parasite cytoskeleton, and host cell cytoskeleton rearrangement might be involved in the biogenesis of parasitophorous vacuoles[19]. Nevertheless, the molecular mechanisms and specific molecules involved in the initial interaction between C. parvum sporozoites and epithelial cells remain obscure. Moreover, essentially nothing is known about how the organism actually invades cells and forms a parasitophorous vacuole, a complex organelle on which the life cycle and possibly the cytotoxicity of the organism is dependent. Based on our observations on the interaction of C. parvum with intestinal and biliary epithelial cells in vitro, we propose a molecular model for C. parvum infection of epithelial cells shown in Figure 2.
When microbes interact with host cells, the result is generally host cell dysfunction. Recent data in a variety of tissues infected with either parasite (such as Entamoeba histohytica, Schistosoma mansoni, Trypanosoma cruzi and Toxoplasma gondii), bacteria or viruses are consistent with the concept that microbial pathogens can kill host cells by an apoptotic mechanism. Experimentally, it has been shown that C. parvum infection of intestinal and biliary epithelial cell monolayers results in a functional disruption of the monolayers and release of LDH from the cell surface. Epithelial apoptosis and reactive epithelial changes in the intestine in AIDS patients with cryptosporidiosis have recently been shown to be associated with C. parvum infection[14]. We found that apoptosis occurs in the epithelial cells adjacent to C. parvum-infected biliary epithelia in vivo in the gallbladder of a patient with AIDS and biliary cry ptosporidiosis[19]. More recently, we found that C. parvum can induce apoptosis in the cultured human biliary epithelia[19,24]. These results suggest that C. parvum, like some other parasites, is directly cytopathic for epithelia via an apoptotic mechanism, a mechanism which is believed critical in liver diseases like primary biliary cirrhosis, primary sclerosing cholangit is, and hepatitis[25].
Release of cytokines and chemokine plays a critical role in the inflammation associated with microbial infection. Rapid upregulation of the C-X-C chemokine family was found in human intestinal epithelial cells infected with gram-negative or gram-positive bacteria. In an in vitro model of intestinal cryptosporidiosis, C. parvum induces IL-8 release from infected intestinal epithelial cell monolayers[26]. Release of cytokines and chemokines like IL-8 could be involved in the pathogenesis of inflammation in cryptosporidiosis. However, little is known about the early events following host-parasite interactions that influence the course of cytokine and chemokine upregulation and release.
The most common clinical manifestation of cryptosporidiosis is diarrhea, characteristically profuse and watery and often containing mucus but rarely blood or leucocytes. Other clinical signs observed include abdominal cramps, low grade fever, nausea and vomiting[10]. The duration and severity of clinical symptoms depend largely on the immune status of the infected individual. In immunocompetent individuals, the disease is usually self-limited. After an incubation period of 7-10 d, > 90% of infected cases present with watery diarrhea lasting approximately 2 weeks, 50% present with nausea, vomiting and cramp-like abdominal pain and 36% present with a febrile illness. In immunocompromised individuals, the disease is much more severe. In HIV-infected patients, the disease severity is related to the site of C. parvum infection and CD4+ cell count[27]. The diarrhea is watery and stool frequency can be up to 10 times a day with a mean volume of one litter. Individuals with AIDS infected with C. parvum can experience a 10% drop in body weight, and usually develop severe malabsorption. Most of them never clear the infection, and ultimately have a shorter survival than AIDS patients without cryptosporidiosis[28].
AIDS patients infected with C. parvum also develop extraintestinal disease, most frequently of the biliary tract[29]. Biliary cryptosporidiosis has only recently been recognized as a clinical entity, and is even less understood than the intestinal form of the disease. Although a number of pathogens account for opportunistic biliary tract infections in AIDS patients, C. parvum is the single most common identifiable pathogen and is found in 20%-65% of the patients with so-called AIDS-cholangiopathy[9]. These patients often have right upper quadrant pain, nausea, vomiting, fever and biochemical evidence of cholestasis. Strictures, narrowing and irregularities of the intrahepatic bile ducts, with dilatation of the common hepatic and common bile ducts and the thickening of the ductal walls, are often found by noninvasive and invasive imaging studies[30].
Indirect methods of diagnosing cryptosporidosis (by comparative symptomatology or clinical parameters) have proven unsatisfactory. Serological studies also have no place in diagnosis as many healthy individuals already have antibodies. At present, most cryptosporidial infections are diagnosed by the microscopic examination of host faecal material for the presence of C. parvum oocysts. Experimental studies have shown that oocyst excretion coincides well with the onset and duration of most clinical signs of disease. Most asymptomatic individuals can be screened to detect subclinical infections and even water samples can be examined for contamination by oocysts. C. parvum oocysts are much smaller than those of other coccidian parasites, and they differ in many of their staining and buoyancy characteristics. Thus, most conventional coprological techniques used in parasitology and microbiology laboratories are not entirely suitable for their detection. Many specialized staining procedures have been described to stain the oocysts and differential staining techniques are more desirable to avoid confusion. The technique of choice for many diagnostic laboratories has been acid-fast staining. Oocysts stain bright red whereas yeast, bacteria and other faecal debris only take up the counterstain. Several immunolabelling techniques have also been developed to detect oocysts. Both polyclonal rabbit antisera and monoclonal antibodies have been used to detect C. parvum oocysts in faecal and water samples by immunofluorescence and several diagnostic test kits are now commercially available[19,31,32].
When biliary disease is suspected, ultrasonography is the best initial diagnostic method. It will be suggestive in most cases by identifying biliary ductal wall thickening and/or gallbladder dilation or both. Computerized tomography might also be helpful. However, the most sensitive method to diagnose biliary tract disease in HIV-infected patients is endoscopic retrograde cholangiopancreatography (ERCP)[30]. If biliary disease is highly suspected and the patient has normal ultrasonography, ERCP should be performed. However, ERCP is not recommended to work-up suspected asymptomatic AIDS-cholangiopathy. The cholangiographic a ppearance of AIDS cholangiopathy is quite variable and has been described in different ways. Characteristically, the biliary tree appears irregular and distorted with focal dilation and narrowing in the intrahepatic and/or extrahepatic biliary tree. The most common cholangiographic pattern is papillary stenosis associated with intrahepatic sclerosing cholangitis, which occurs in approximately 50%-60% of patients[30,33,34]. Although occasionally diagnostic, percutaneous liver biopsy is rarely helpful and thus plays no role in the diagnosis of AIDS-cholangiopathy. Serum alkaline phosphatase is the most commonly elevated liver biochemical test with mean values in most series of 700 IU/L-800 IU/L. Mild increases in aminotransferases are common with values ranging from 65 to 123 IU/L, whereas hyperbilirubinemia is distinctly uncommon[30].
At present, there is no effective antimicrobial treatment available for cryptosporidiosis in man or animals. Gererally, immunocompetent individuals need no specific therapy. Supportive care with oral or intravenous fluids and electrolyte replacement is beneficial in alleviating the dehydration accompanying acute diarrhea while awaiting spontaneous recovery. In children, however, spiramycin may shorten the duration of oocyst excretion and diarrhea, although conflicting results have been obtained[35,36]. In AIDS patients without antiretroviral therapy, AZT therapy should be started. In these patients, a relationship between disease severity and CD4+ count has been documented. Paromomycin is the only agent so far that has been found to have efficacy in animals and humans in the treatment of intestinal crytosporidiosis[37-39]. It is an aminoglycoside anti biotic that is not significantly absorbed when given orally and is used for intestinal amoebiasis. In most studies, including a double blind trial, there was a good clinical and parasitological response[37-40]. However, after discontinuation of treatment, many patients relapse. In a well-documented study, paromomycin, 500 mg 4 times daily for 4 weeks and maintenance therapy of 500 mg twice daily, was used[41].
Therapy of biliary cryptosporidiosis in AIDS-cholangiopathy is primarily endoscopic[30]. For those patients with abdominal pain or cholangitis associated with papillary stenosis, endoscopic sphincterotomy may provide striking symptomatic relief as it facilitates drainage and decompression of the biliary system. Although survival is not prolonged, sphincterotomy may help improve the quality of life for those with papillary stenosis and pain. There is no evidence that sphincterotomy is beneficial for sclerosing cholangitis in the absence of papillary stenosis and CBD dilation, or in asymptomatic patients and it may be associated with a higher complication rate. Patients with diffuse intra- and extrahepatic sclerosing cholangitis alone have few specific treatment options. Paramomycin is not effective in biliary cryptosporidiosis in AIDS-cholangiopathy[40].
Since therapy remains difficult, prevention of the disease is critical. C. parvum infections are contracted by the ingestion or inhalation of oocysts, and therefore effective control measures must aim to reduce or prevent oocyst transmission. C. parvum have been proven remarkably resistant to chemical disinfection. Many commercial disinfectants (based on aldehyde, ammonia, alcohol, chlorine or alkaline compounds) are ineffective when used according to the manufacturers’ instructions and most conventional methods of water treatment do not effectively remove or kill all the oocysts from contaminated water[1]. Many recommendations have been made for the prevention and control of infections in specific locations; such as hospitals, laboratories, and day care centers. These recommendations have basically involved managerial practices designed to minimize further host contact with sources of infection. Isolation of infected individuals, careful handing and disposal of biohazardous waste, and heat treatment (boiling) of suspect contaminated water before consumption are helpful.
| 1. | O'Donoghue PJ. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:139-195. [PubMed] |
| 2. | Newman RD, Sears CL, Moore SR, Nataro JP, Wuhib T, Agnew DA, Guerrant RL, Lima AA. Longitudinal study of Cryptosporidium infection in children in northeastern Brazil. J Infect Dis. 1999;180:167-175. [PubMed] |
| 3. | Guerrant RL. Cryptosporidiosis: an emerging, highly infectious threat. Emerg Infect Dis. 1997;3:51-57. [PubMed] |
| 4. | Hoepelman IM. Human cryptosporidiosis. Int J STD AIDS. 1996;7 Suppl 1:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Ungar BL, Gilman RH, Lanata CF, Perez-Schael I. Seroepidemiology of Cryptosporidium infection in two Latin American populations. J Infect Dis. 1988;157:551-556. [PubMed] |
| 6. | Kuhls TL, Mosier DA, Crawford DL, Griffis J. Seroprevalence of cryptosporidial antibodies during infancy, childhood, and adolescence. Clin Infect Dis. 1994;18:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Colford JM, Tager IB, Hirozawa AM, Lemp GF, Aragon T, Petersen C. Cryptosporidiosis among patients infected with human immunodeficiency virus. Factors related to symptomatic infection and survival. Am J Epidemiol. 1996;144:807-816. [PubMed] |
| 8. | Sánchez-Mejorada G, Ponce-de-León S. Clinical patterns of diarrhea in AIDS: etiology and prognosis. Rev Invest Clin. 1994;46:187-196. [PubMed] |
| 9. | Vakil NB, Schwartz SM, Buggy BP, Brummitt CF, Kherellah M, Letzer DM, Gilson IH, Jones PG. Biliary cryptosporidiosis in HIV-infected people after the waterborne outbreak of cryptosporidiosis in Milwaukee. N Engl J Med. 1996;334:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 85] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Fayer R, Ungar BL. Cryptosporidium spp. and cryptosporidiosis. Microbiol Rev. 1986;50:458-483. [PubMed] |
| 11. | Laxer MA, Alcantara AK, Javato-Laxer M, Menorca DM, Fernando MT, Ranoa CP. Immune response to cryptosporidiosis in Philippine children. Am J Trop Med Hyg. 1990;42:131-139. [PubMed] |
| 12. | Theodos CM. Innate and cell-mediated immune responses to Cryptosporidium parvum. Adv Parasitol. 1998;40:87-119. [PubMed] |
| 13. | Griffiths JK, Theodos C, Paris M, Tzipori S. The gamma inter-feron gene knockout mouse: a highly sensitive model for evalua-tion of therapeutic agents against Cryptosporidium parvum. J Clin Microbiol. 1998;36:2503-2508. |
| 14. | Lumadue JA, Manabe YC, Moore RD, Belitsos PC, Sears CL, Clark DP. A clinicopathologic analysis of AIDS-related cryptosporidiosis. AIDS. 1998;12:2459-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Teare JP, Daly CA, Rodgers C, Padley SP, Coker RJ, Main J, Harris JR, Scullion D, Bray GP, Summerfield JA. Pancreatic abnormalities and AIDS related sclerosing cholangitis. Genitourin Med. 1997;73:271-273. [PubMed] |
| 16. | French AL, Beaudet LM, Benator DA, Levy CS, Kass M, Orenstein JM. Cholecystectomy in patients with AIDS: clinicopathologic correlations in 107 cases. Clin Infect Dis. 1995;21:852-858. [PubMed] |
| 17. | Rosales MJ, Mascaro C, Osuna A. Ultrastructural study of Cryptosporidium development in Madin-Darby canine kidney cells. Vet Parasitol. 1993;45:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Marcial MA, Madara JL. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane-mem-brane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology. 1986;90:583-594. |
| 19. | Chen XM, Levine SA, Tietz P, Krueger E, McNiven MA, Jefferson DM, Mahle M, LaRusso NF. Cryptosporidium parvum is cytopathic for cultured human biliary epithelia via an apoptotic mechanism. Hepatology. 1998;28:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 20. | Upton SJ, Tilley M, Brillhart DB. Comparative development of Cryptosporidium parvum (Apicomplexa) in 11 continuous host cell lines. FEMS Microbiol Lett. 1994;118:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 108] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Hamer DH, Ward H, Tzipori S, Pereira ME, Alroy JP, Keusch GT. Attachment of Cryptosporidium parvum sporozoites to MDCK cells in vitro. Infect Immun. 1994;62:2208-2213. [PubMed] |
| 22. | Joe A, Verdon R, Tzipori S, Keusch GT, Ward HD. Attachment of Cryptosporidium parvum sporozoites to human intestinal epithelial cells. Infect Immun. 1998;66:3429-3432. [PubMed] |
| 23. | Thea DM, Pereira ME, Kotler D, Sterling CR, Keusch GT. Identification and partial purification of a lectin on the surface of the sporozoite of Cryptosporidium parvum. J Parasitol. 1992;78:886-893. [PubMed] |
| 24. | Chen XM, Gores GJ, Paya CV, LaRusso NF. Cryptosporidium parvum induces apoptosis in biliary epithelia by a Fas/Fas ligand-dependent mechanism. Am J Physiol. 1999;277:G599-G608. [PubMed] |
| 25. | Patel T, Roberts LR, Jones BA, Gores GJ. Dysregulation of apoptosis as a mechanism of liver disease: an overview. Semin Liver Dis. 1998;18:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Laurent F, Eckmann L, Savidge TC, Morgan G, Theodos C, Naciri M, Kagnoff MF. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect Immun. 1997;65:5067-5073. [PubMed] |
| 27. | Flanigan T, Whalen C, Turner J, Soave R, Toerner J, Havlir D, Kotler D. Cryptosporidium infection and CD4 counts. Ann Intern Med. 1992;116:840-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 138] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Manabe YC, Clark DP, Moore RD, Lumadue JA, Dahlman HR, Belitsos PC, Chaisson RE, Sears CL. Cryptosporidiosis in patients with AIDS: correlates of disease and survival. Clin Infect Dis. 1998;27:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Cello JP. Human immunodeficiency virus-associated biliary tract disease. Semin Liver Dis. 1992;12:213-218. [PubMed] |
| 30. | Wilcox CM, Mönkemüller KE. Hepatobiliary diseases in patients with AIDS: focus on AIDS cholangiopathy and gallbladder disease. Dig Dis. 1998;16:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | MacPherson DW, McQueen R. Cryptosporidiosis: multiattribute evaluation of six diagnostic methods. J Clin Microbiol. 1993;31:198-202. [PubMed] |
| 32. | Aldras AM, Orenstein JM, Kotler DP, Shadduck JA, Didier ES. Detection of microsporidia by indirect immunofluorescence antibody test using polyclonal and monoclonal antibodies. J Clin Microbiol. 1994;32:608-612. [PubMed] |
| 33. | Ducreux M, Buffet C, Lamy P, Beaugerie L, Fritsch J, Choury A, Liguory C, Longuet P, Gendre JP, Vachon F. Diagnosis and prognosis of AIDS-related cholangitis. AIDS. 1995;9:875-880. [PubMed] |
| 34. | Farman J, Brunetti J, Baer JW, Freiman H, Comer GM, Scholz FJ, Koehler RE, Laffey K, Green P, Clemett AR. AIDS-related cholangiopancreatographic changes. Abdom Imaging. 1994;19:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Sáez-Llorens X, Odio CM, Umaña MA, Morales MV. Spiramycin vs. placebo for treatment of acute diarrhea caused by Cryptosporidium. Pediatr Infect Dis J. 1989;8:136-140. [PubMed] |
| 36. | Wittenberg DF, Miller NM, van den Ende J. Spiramycin is not effective in treating cryptosporidium diarrhea in infants: results of a double-blind randomized trial. J Infect Dis. 1989;159:131-132. [PubMed] |
| 37. | Armitage K, Flanigan T, Carey J, Frank I, MacGregor RR, Ross P, Goodgame R, Turner J. Treatment of cryptosporidiosis with paromomycin. A report of five cases. Arch Intern Med. 1992;152:2497-2499. [PubMed] |
| 38. | Fichtenbaum CJ, Ritchie DJ, Powderly WG. Use of paromomycin for treatment of cryptosporidiosis in patients with AIDS. Clin Infect Dis. 1993;16:298-300. [PubMed] |
| 39. | Scaglia M, Atzori C, Marchetti G, Orso M, Maserati R, Orani A, Novati S, Olliaro P. Effectiveness of aminosidine (paromomycin) sulfate in chronic Cryptosporidium diarrhea in AIDS patients: an open, uncontrolled, prospective clinical trial. J Infect Dis. 1994;170:1349-1350. [PubMed] |
| 40. | White AC, Chappell CL, Hayat CS, Kimball KT, Flanigan TP, Goodgame RW. Paromomycin for cryptosporidiosis in AIDS: a prospective, double-blind trial. J Infect Dis. 1994;170:419-424. [PubMed] |
| 41. | Bissuel F, Cotte L, Rabodonirina M, Rougier P, Piens MA, Trepo C. Paromomycin: an effective treatment for cryptosporidial diarrhea in patients with AIDS. Clin Infect Dis. 1994;18:447-449. [PubMed] |
Edited by Ma JY