Published online Jun 15, 1999. doi: 10.3748/wjg.v5.i3.263
Revised: April 22, 1999
Accepted: May 12, 1999
Published online: June 15, 1999
- Citation: Xia HHX, Fan XG, Talley NJ. Clarithromycin resistance in Helicobacter pylori and its clinical relevance. World J Gastroenterol 1999; 5(3): 263-266
- URL: https://www.wjgnet.com/1007-9327/full/v5/i3/263.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i3.263
The macrolide clarithromycin has emerged as the most important antibiotic in combined therapy for eradication of H. pylori infection[1,2]. However, concerns about increasing clarithromycin resistance in H. pylori and its impact on the efficacy of eradication therapy have been raised since its widespread acceptance in H. pylori therapy[3,4]. Here, we sought to review the geographic prevalence of clarithromycin resistance in H. pylori and its molecular mechanisms, and assess the clinical relevance of clarithromycin resistance.
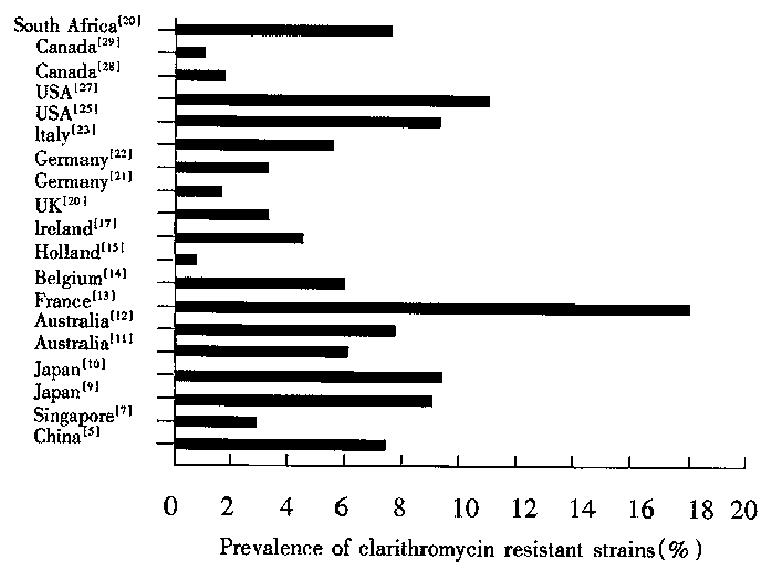
The worldwide, prevalence of primary (pre-treatment) clarithromycin resistance to H. pylori ranges from 0. 8% to 18% (Figure 1)[5-29]. The reported prevalence in China is between 4.8% and 7.5%, while the rate in Australia ranges from 6.1% to 7.8%[5,6,11,12].
Versalovic et al[30] were the first to identify an A→G transition mutation within a conserved loop of 23S rRNA of H. pylori, and its association with clarithromycin-resistance. The mutation occurs commonly at two gene posi tions cognate with positions 2058 and 2059 of Escherichia coli-23S rRNA, whichwere re-named 2143 and 2144, and now revised as 2142 and 2143, respectively[4,31]. Point mutations may occasionally occur at other positions, and can be a transition (A→G) or a transversion (A→C), but the transition is far more frequent[4,32-35]. Moreover, Versalovic et al[32] also obser ved that the A2142G mutation was associated with a high level of resistance (MIC > 64 mg/L) than the A2143G mutation[32]. These observations are s upported by others studies[33,36].
It has been reported that macrolide-resistance was not stable in some strains of H. pylori in vitro[17]. This phenomenon was also observed in vivo; i.e., strains developed resistance post-treatment and then reverted to being susceptible after a period of follow-up[17,30]. Versalovic et al[30] cultured five genotypically identical isolates subsequentially from one patient before and after treatment with clarithromycin alone. They observed that the first two post-treatment isolates with a low-level clarithromycin resistance had an A2143G mutation, which was not present in the susceptible pretreatment isolate or in the last two post-treatment isolates with reverted susceptibility[30]. This suggests that the mutation may be unstable[35]. However, Hulten et al[35] reported that clarithromycin resistance was stable after 50 subcultures in vitro, which is consistent with other studies[37].
Cross-resistance between macrolides in H. pylori has been observed[12,17,30]. Generally, H. pylori strains resistant to clarithromycin are also resistant to erythromycin, azithromycin and roxithromycin or vice versa. These observations have been confirmed at the molecular level[36].
The methods currently used for susceptibility testing of H. pylori to clarithromycin include agar dilution method, broth dilution method, disc diffusion test and the Epsilometer test (E-test)[17,38]. The agar dilution method determines the minimal inhibitory concentrations (MICs) of antibiotics against bacteria. This method is time consuming and not feasible for routine use. However, it is a reliable technique which is usually carried out as a reference method for other techniques[17,38,39]. Broth dilution method is rarely used because of the difficulty in growing H. pylori in broth. The disc diffusion test is the easiest and cheapest way of testing susceptibility. However, this test requires strict standardization before it can be used[39]. The E-test, developed in 1988, provides the MIC of a strain directly by using a diffusion-like method[40]. A plastic-coated strip contains a preformed antimicrobial gradient on one side and a scale on the other. The reading is taken at the point where the ellipse of growth inhibition intersects the strip. Standardization and correlation with the agar dilution method are also required prior to application. This method is now widely used by many investigators[12,13,15,16,18,22-28]. At present, no “gold standard” method has been proposed for testing H. pylori susceptibility to antibiotics including clarithromycin and metronidazole, as there is still a need for standardization regarding the appropriate medium, the supplementation, the size of the inoculum, the incubation atmosphere, the appropriate time to read the plates and the breakpoint differentiating resistance and susceptibility[38]. Since cross-resistance exists between macrol ides, erythromycin susceptibility testing may be useful in predicting (determining) clarithromycin resistant H. pylori strains[12,17]. Erythromycin susceptibility testing is well established in many microbiological laboratories, and it is much cheaper than clarithromycin susceptibility testing at present.
The association between point mutations on the 23S rRNA gene and macrolide resis tance in H. pylori potentially provides a new approach for diagnosing macrol ide resistant H. pylori strains. Although cycle DNA sequencing of the 23S rRNA gene amplicons is regarded as the reference method, simpler techniques have been developed[38]. These include polymerase chain reaction based restric tion fragment length polymorphism (PCR-RFLP), an oligonucleotide ligation assay (PCR-OLA), a DNA enzyme immunoassay (PCR-DEIA), a reverse hybridisation line probe assay (PCR-LiPA), and a preferential homoduplex formation assay (PCR-PHFA)[30,31,33,41-43]. The PCR-based molecular techniques are quicker than micro biological susceptibility testing, and more importantly, they can be performed directly on gastric biopsies and gastric juice[10,44,45].
Studies have shown that clarithromycin resistance in H. pylori substantially affects the success rate of eradication regimens containing clarithromycin (Table 1). Generally, dual therapy with an antisecretory agent (e.g., H2 antagonis t or proton pump inhibitor) and clarithromycin achieves eradication rates of 60% to 80% for susceptible strains, but less than 40% for resistance strains (Table 1). Triple therapy with an antisecretory agent, clarithromycin and another anti biotic (i.e., amoxycillin or metronidazole) increases the eradication rates to 80%-95% for susceptible strains, but the rates remain under 40% for resistant ones (Table 1). A preliminary study reported that a combination of ranitidine bismu th citrate and clarithromycin eradicated H. pylori at a rate of 98% and 92%, respectively, for both susceptible and resistant strains, but remains to be con firmed[13].
| Authors | Treatment regimens | Eradication rate (%) | Prevalence of resistant strains post-treatment (%)* | |
| Susceptible strains | Resistant strains | |||
| Liu et al[5] 1996 | LFC or BFC | 98 (45/46) | 0 (0/4) | 100 (5/5) |
| Suzuki et al[8] 1998 | LAC | 94 (66/70) | 0 (0/1) | 40 (2/5) |
| Miyaji et al[9] 1997 | OC or LC | 64 (9/14) | 0 (0/5) | 80 (20/25) |
| OAC or LAC | 85 (57/67) | 28 (2/7) | (Overall) | |
| OCM or LCM | 86 (68/79) | 38 (3/8) | ||
| Maeda et al[10] 1998 | LAC | 85 (29/34) | 40 (2/5) | 63 (5/8) |
| Megraud et al[13] 1997 | OC | 70 (33/47) | 38 (3/8) | 81 (17/21) |
| RbCC | 98 (42/43) | 92 (11/12) | (Overall) | |
| Debets-Ossenkopp et al[16] 1996 | RC | 81 (58/72) | 0 (0/1) | 73 (11/15) |
| Tompkins et al[18] 1997 | OC | 80 (101/127) | 0 (0/4) | 74 (14/19) |
| Moayyedi et al[19] 1998 | OCT | 91 (104/114) | 40 (2/5) | Nor reported |
| Schutze et al[24] 1996 | RC | 75 (21/28) | 20 (1/5) | 91 (10/11) |
| Laine et al[25] 1998 | AC | 35 (73/208) | 7.7 (2/2.6) | 53 (70/131) |
| OAC | 81 (153/190) | 27 (4/15) | (Overall) | |
| Yousfi et al[26] 1996 | RanMC | 87 (20/23) | 25 (1/4) | 67 (4/6) |
| Buckley et al[46] 1997 | OMC | 85 (71/84) | 0 (0/3) | 58 (7/12) |
Current anti H. pylori treatment regiments consisting of clarithromycin do not achieve an eradication rate of 100%. Emergence of clarithromycin-resistant strains during ineffective treatment has also been observed; the prevalence of clarithromycin-resistant strains cultured after treatment ranges between 40% and 100% (Table 1). This implies a likelihood of potential spread of clarithromycin-re sistant strains in the population. Thus, the prevalence of clarithromycin resist ance in H . pylori may exhibit a similar trend to the prevalence of metronida zole resistance in H. pylori. In Ireland, the prevalence of metronidazole re sistant strains was 7% in 1989, 34% in 1992 and 38% in 1996[17]. In Aust ralia, the prevalence of metronidazole resistance was 17% in 1988, but increased to 40% in 1995 and over 60% in 1998[11,47]. It is most likely that this increase is due to the use of metronidazole as a key agent in classic triple therapy (consisting of bismuth, metronidazole and tetracycline or amoxycillin), or increased use of this drug for other infections. Similarly, the current prevale nce of clarithromycin-resistant strains of 6%-8% in Australia is much higher than the rate of 1.9% reported four years ago in this country[11,12,48]. T his increase in the prevalence of clarithromycin resistance has been also report ed in Europe and the United States[14,20,27,49]. It is assumed that pres criptions of macrolides, especially the new members such as spiramycin, roxithro mycin, azithromycin and clarithromycin have been increased over the past years for the treatment of respiratory infection, sexually transmitted diseases and other infectious diseases. Thus, patients treated with any member of macrolides alo ne may select macrolide resistant H. pylori organisms (if infected), as cros s-resistance exists between macrolides.
Overall, H. pylori resistance to clarithromycin is of less clinical relevance as compared with resistance to metronidazole, mainly because of the low preval ence and the possible reversibility of resistance in some strains. Susceptibility testing is not routinely required before treatment because of the low prevalence of clarithromycin resistance (Figure 1). However, H. pylori should be cul tured and tested for clarithromycin susceptibility in patients who have failed the therapy containing clarithromycin (Table 1). Moreover, any previous use of ma crolides not aimed at anti-H. pylori infection should be also taken into account when clarithromycin is chosen for eradication of H. pylori.
The prevalence of clarithromycin resistant H. pylori is low, but appears to be increasing. Point mutations in the 23S rRNA gene, mainly at the positions 2142 and 2143 with a transition of A→G, are responsible for the resistance. Althou gh current triple therapies containing clarithromycin are able to eradicate up to 90% of susceptible strains, the eradication rates may be significantly reduced for resistant strains. Moreover, unsuccessful treatment with regimens containing clarithromycin can be associated with acquisition of resistance to the drug, which may explain the increasing rate of clarithromycin resistance.
| 1. | Graham DY. Clarithromycin for treatment of Helicobacter pylori infections. Eur J Gastroenterol Hepatol. 1995;7 Suppl 1:S55-S58. [PubMed] |
| 2. | Xia H H-X, Talley NJ. Prospects for improved therapy for Helicobacter pylori infection. Exp Opin Invest Drugs. 1996;5:959-976. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 3. | Xia HX, Buckley M, Hyde D, Keane CT, O' Morain CA. Effects of antibiotic-resistance on clarithromycin-combined triple therapy for Helicobacter pylori. Gut. 1995;37:A55. |
| 4. | Mégraud F. Epidemiology and mechanism of antibiotic resistance in Helicobacter pylori. Gastroenterology. 1998;115:1278-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Liu W, Lu B, Xiao S, Xu W, Shi L, Zhang D. Clarithromycin combined short-term triple therapies for eradication of Helicobacter pylori infection. Chin J Intern Med. 1996;35:803-806. |
| 6. | Hu P, Li Y, Chen M, Wu H, Cui J, Li Q. Clinical study of one week clarithromycin combination therapy for the treatment of H. py-lori infection. Chin J Dig. 1997;17:204-206. |
| 7. | Hua J, Ng HC, Yeoh KG, Ho KY, Ho B. Characterization of clinical isolates of Helicobacter pylori in Singapore. Microbios. 1998;94:71-81. [PubMed] |
| 8. | Suzuki J, Mine T, Kobayasi I, Fujita T. Assessment of a new triple agent regimen for the eradication of Helicobacter pylori and the nature of H. pylori resistance to this therapy in Japan. Helicobacter. 1998;3:59-63. [PubMed] |
| 9. | Miyaji H, Azuma T, Ito S, Suto H, Ito Y, Yamazaki Y, Sato F, Hirai M, Kuriyama M, Kato T. Susceptibility of Helicobacter pylori isolates to metronidazole, clarithromycin and amoxycillin in vitro and in clinical treatment in Japan. Aliment Pharmacol Ther. 1997;11:1131-1136. [PubMed] |
| 10. | Maeda S, Yoshida H, Ogura K, Kanai F, Shiratori Y, Omata M. Helicobacter pylori specific nested PCR assay for the detection of 23S rRNA mutation associated with clarithromycin resistance. Gut. 1998;43:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Xia HH, Kalantar J, Talley NJ. Metronidazole- and clarithromycin-resistant Helicobacter pylori in dyspeptic patients in western Sydney as determined by testing multiple isolates from different gastric sites. J Gastroenterol Hepatol. 1998;13:1044-1049. [PubMed] |
| 12. | Midolo PD, Bell JM, Lambert JR, Turnidge JD, Grayson ML. Antimicrobial resistance testing of Helicobacter pylori: a comparison of Etest and disk diffusion methods. Pathology. 1997;29:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Megraud F, Pichavant R, Palegry D, French PC, Roberts PM, Williamson R. Ranitidine bismuth citrate (RBC) co- prescribed with clarithromycin is more effective in the eradication of Helicobacter pylori than omeprazole with clarithromycin. Gut. 1997;41:A92. |
| 14. | De Koster E, Cozzoli A, Jonas C, Ntounda R, Butzler JP, Deltenre M. Six years resistance of Helicobacter pylori to macrolides and imidazoles. Gut. 1996;39:A5. |
| 15. | van Zwet AA, de Boer WA, Schneeberger PM, Weel J, Jansz AR, Thijs JC. Prevalence of primary Helicobacter pylori resistance to metronidazole and clarithromycin in The Netherlands. Eur J Clin Microbiol Infect Dis. 1996;15:861-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Debets-Ossenkopp YJ, Sparrius M, Kusters JG, Kolkman JJ, Vandenbroucke-Grauls CM. Mechanism of clarithromycin resistance in clinical isolates of Helicobacter pylori. FEMS Microbiol Lett. 1996;142:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Xia HX, Buckley M, Keane CT, O'Morain CA. Clarithromycin resistance in Helicobacter pylori: prevalence in untreated dyspeptic patients and stability in vitro. J Antimicrob Chemother. 1996;37:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Tompkins DS, Perkin J, Smith C. Failed treatment of Helicobacter pylori infection associated with resistance to clarithromycin. Helicobacter. 1997;2:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Moayyedi P, Ragunathan PL, Mapstone N, Axon AT, Tompkins DS. Relevance of antibiotic sensitivities in predicting failure of omeprazole, clarithromycin, and tinidazole to eradicate Helicobacter pylori. J Gastroenterol. 1998;33 Suppl 10:62-65. [PubMed] |
| 20. | Morton D, Bardhan D. A six-year assessment of tinidazole, metronidazole, clarithromycin, tetracycline and amoxicillin resistance in Helicobacter pylori- clinical isolates: a rising tide of antibiotic resistance. Gastroenterology. 1998;114:A907. |
| 21. | Adamek RJ, Suerbaum S, Pfaffenbach B, Opferkuch W. Primary and acquired Helicobacter pylori resistance to clarithromycin, metronidazole, and amoxicillin--influence on treatment outcome. Am J Gastroenterol. 1998;93:386-389. [PubMed] |
| 22. | Wolle K, Nilius M, Leodolter A, Müller WA, Malfertheiner P, König W. Prevalence of Helicobacter pylori resistance to several antimicrobial agents in a region of Germany. Eur J Clin Microbiol Infect Dis. 1998;17:519-521. [PubMed] |
| 23. | Piccolomini R, Di Bonaventura G, Catamo G, Carbone F, Neri M. Comparative evaluation of the E test, agar dilution, and broth microdilution for testing susceptibilities of Helicobacter pylori strains to 20 antimicrobial agents. J Clin Microbiol. 1997;35:1842-1846. [PubMed] |
| 24. | Schütze K, Hentschel E, Hirschl AM. Clarithromycin or amoxycillin plus high-dose ranitidine in the treatment of Helicobacter pylori-positive functional dyspepsia. Eur J Gastroenterol Hepatol. 1996;8:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Laine L, Suchower L, Frantz J, Connors A, Neil G. Low rate of emergence of clarithromycin-resistant Helicobacter pylori with amoxycillin co-therapy. Aliment Pharmacol Ther. 1998;12:887-892. [PubMed] |
| 26. | Yousfi MM, El-Zimaity HM, Cole RA, Genta RM, Graham DY. Metronidazole, ranitidine and clarithromycin combination for treatment of Helicobacter pylori infection (modified Bazzoli's triple therapy). Aliment Pharmacol Ther. 1996;10:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Vakil N, Hahn B, McSorley D. Clarithromycin-resistant Helicobacter pylori in patients with duodenal ulcer in the United States. Am J Gastroenterol. 1998;93:1432-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Best LM, Haldane DJ, Bezanson GS, Veldhuyzen van Zanten SJ. Helicobacter pylori: primary susceptibility to clarithromycin in vitro in Nova Scotia. Can J Gastroenterol. 1997;11:298-300. [PubMed] |
| 29. | Loo VG, Fallone CA, De Souza E, Lavallée J, Barkun AN. In-vitro susceptibility of Helicobacter pylori to ampicillin, clarithromycin, metronidazole and omeprazole. J Antimicrob Chemother. 1997;40:881-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Versalovic J, Shortridge D, Kibler K, Griffy MV, Beyer J, Flamm RK, Tanaka SK, Graham DY, Go MF. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996;40:477-480. [PubMed] |
| 31. | Taylor DE, Ge Z, Purych D, Lo T, Hiratsuka K. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother. 1997;41:2621-2628. [PubMed] |
| 32. | Versalovic J, Osato MS, Spakovsky K, Dore MP, Reddy R, Stone GG, Shortridge D, Flamm RK, Tanaka SK, Graham DY. Point mutations in the 23S rRNA gene of Helicobacter pylori associated with different levels of clarithromycin resistance. J Antimicrob Chemother. 1997;40:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Stone GG, Shortridge D, Versalovic J, Beyer J, Flamm RK, Graham DY, Ghoneim AT, Tanaka SK. A PCR-oligonucleotide ligation assay to determine the prevalence of 23S rRNA gene mutations in clarithromycin-resistant Helicobacter pylori. Antimicrob Agents Chemother. 1997;41:712-714. [PubMed] |
| 34. | Occhialini A, Urdaci M, Doucet-Populaire F, Bébéar CM, Lamouliatte H, Mégraud F. Macrolide resistance in Helicobacter pylori: rapid detection of point mutations and assays of macrolide binding to ribosomes. Antimicrob Agents Chemother. 1997;41:2724-2728. [PubMed] |
| 35. | Hultén K, Gibreel A, Sköld O, Engstrand L. Macrolide resistance in Helicobacter pylori: mechanism and stability in strains from clarithromycin-treated patients. Antimicrob Agents Chemother. 1997;41:2550-2553. [PubMed] |
| 36. | Wang G, Taylor DE. Site-specific mutations in the 23S rRNA gene of Helicobacter pylori confer two types of resistance to macrolide-lincosamide-streptogramin B antibiotics. Antimicrob Agents Chemother. 1998;42:1952-1958. [PubMed] |
| 37. | Debets-Ossenkopp YJ, Brinkman AB, Kuipers EJ, Vandenbroucke-Grauls CM, Kusters JG. Explaining the bias in the 23S rRNA gene mutations associated with clarithromycin resistance in clinical isolates of Helicobacter pylori. Antimicrob Agents Chemother. 1998;42:2749-2751. [PubMed] |
| 38. | Mégraud F. Resistance of Helicobacter pylori to antibiotics. Aliment Pharmacol Ther. 1997;11 Suppl 1:43-53. [PubMed] |
| 39. | Xia H, Keane CT, Beattie S, O'Morain CA. Standardization of disk diffusion test and its clinical significance for susceptibility testing of metronidazole against Helicobacter pylori. Antimicrob Agents Chemother. 1994;38:2357-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Xia HX, Daw MA, Beattie S, Keane CT, O'Morain CA. Prevalence of metronidazole-resistant Helicobacter pylori in dyspeptic patients. Ir J Med Sci. 1993;162:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Pina M, Occhialini A, Monteiro L, Doermann HP, Mégraud F. Detection of point mutations associated with resistance of Helicobacter pylori to clarithromycin by hybridization in liquid phase. J Clin Microbiol. 1998;36:3285-3290. [PubMed] |
| 42. | Van Doorn L J, Debets Ossenkopp YJ, Marais A, van Hoek K, Sanna R, Megraud F. Detection of 23S rRNA mutation associated to macrolide-resistance of Helicobacter pylori by PCR and a re-verse hybridization line probe assay. Gut. 1998;43:A7-8. |
| 43. | Maeda S, Yoshida H, Ogura K, Maysunaga H, Kawamata O, Shiratori Y. Detection of Helicobacter pylori 23S rRNA gene mutation as-sociated with clarithromycin resistance using preferential homoduplex formation assay (PCR-PHFA). Gut. 1998;43:A7. |
| 44. | Björkholm B, Befrits R, Jaup B, Engstrand L. Rapid PCR detection of Helicobacter pylori-associated virulence and resistance genes directly from gastric biopsy material. J Clin Microbiol. 1998;36:3689-3690. [PubMed] |
| 45. | Sevin E, Lamarque D, Delchier JC, Soussy CJ, Tankovic J. Co-detection of Helicobacter pylori and of its resistance to clarithromycin by PCR. FEMS Microbiol Lett. 1998;165:369-372. [PubMed] |
| 46. | Buckley MJ, Xia HX, Hyde DM, Keane CT, O'Morain CA. Metronidazole resistance reduces efficacy of triple therapy and leads to secondary clarithromycin resistance. Dig Dis Sci. 1997;42:2111-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Katelaris PH, Nguyen TV, Robertson GJ, Bradbury R, Ngu MC. Prevalence and demographic determinants of metronidazole resistance by Helicobacter pylori in a large cosmopolitan cohort of Australian dyspeptic patients. Aust N Z J Med. 1998;28:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Lian JX, Carrick J, Lee A, Daskalopoulos G. Metronidazole resis-tance significantly affects eradication of H. pylori infection. Gastroenterology. 1993;104:A133. |
Edited by Jing-Yun Ma