Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.179
Revised: January 19, 1999
Accepted: January 29, 1999
Published online: April 15, 1999
- Citation: Chen Q, Ye YB, Chen Z. Activation of killer cells with soluble gastric cancer antigen combined with anti-CD3 McAb. World J Gastroenterol 1999; 5(2): 179-180
- URL: https://www.wjgnet.com/1007-9327/full/v5/i2/179.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.179
There have been many reports on cancer therapy with lymphokine-activated killer (LAK) cells and interleukin-2 (IL-2), but the proliferative response and anti-cancer effect of LAK cells are dependent on IL-2 dose. Other methods to improve the anti-tumor activity of cytotoxic T cells by activation with anti-CD3 McAb in conjunction with IL-2 are being investigated in recent years. In this study, we attempted to explore the physiologic and biologic effects of T-killer cells (TAK) co-stimulated with soluble gastric cancer antigen, anti-CD3 McAb and IL-2.
Interlukin-2 was produced by Shanghai Bio-Chemical Institute and anti-CD3 monoclonal antibody was prepared from Tumor Institute, Chinese Academy of Medical Sciences. Medium 1640 was produced by Gibco Company of America.
Target cell: K562 and SGC-7901 were prepared by Radio biology Research Laboratory of our hospital, hepatocarcinoma cell line (SMC) was prepared by Tumor Research Laboratory of Fujian Medical University. All tumor cells were maintained in medium 1640 with 100 mL/L calf serum.
Tumor soluble antigen was extracted from SGC cells by salting-out method previo usly described by Chen et al[1] and stored at -20 °C. The mononuclear cells (MNC) were isolated from 50 mL venous blood of normal don or by centrifugation over a Ficol-lHypaque gradient and a final concentration of 1 × 109/L was obtained in each culture bottle. Three kinds of cytotoxic T cells by activation was maintained in medium 1640 with 750 kU/L IL-2 for LAK cells, 750 kU/L IL-2 and 100 mg/L CD3 McAb for CD3AK cells. TAK cells were stimulated with IL-2, CD3 McAb and the extracted tumor antigen at a dose of 100 mg/L of culture. All the cells were refed with new media every 2-3 days of incubation at 37 °C in air atmosphere containing 50 mL/L CO2 and were diluted to 1 × 109/L.
Expansion of the three kinds of killer cells was tested with typan blue staining. The cytotoxity of the three different killer cells were tested with MTT methods[2], and the ratio of killer cells and the target cells was 10:1.
Having cultured for 10 days, TAK cells were tested for CD3, CD4, CD8, NK and CD19 by Flow Cytometry.
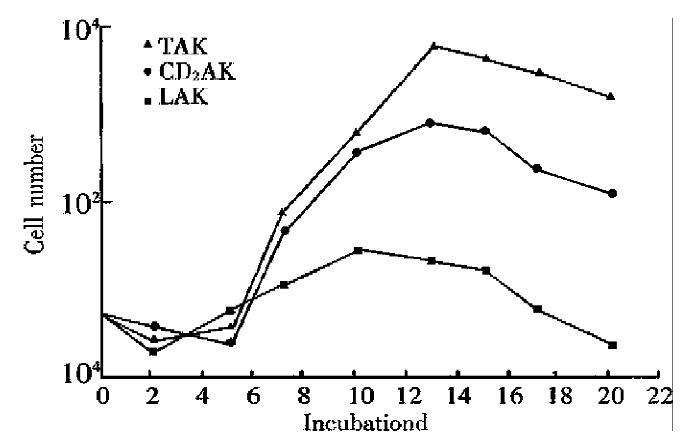
There was a similar growth tendency among the three kinds of killer cells. In LAK cells group, the maximum cell number of expansion was found about 10 days of culture, the cell number decreased rapidly on the 15th day and was fewer than the initial number on the 20th day. However, in TAK group and CD3AK group, the peaks of the cell expansion were found on th 13th day and the cell number was more than that in LAK group. The activity of cell expansion was TAK > CD3AK > LAK (Figure 1).
Table 1 shows that the killing activity of all the killer cells to K562 was low in the early stage of culture, but it increased as the rapid expansion occurred. On the 20th day, TAK and CD3AK cells maintained killing activity from the 15th day. On the other hand, the killing activity of TAK, CD3AK and LAK to SGC-7901 was 98.5%, 82.1%, and 62.1%, and was 74.9%, 51.3% and 52.4% to SMC respectively.
| Group | Incubation day | ||||||
| 3 | 7 | 10 | 12 | 15 | 17 | 20 | |
| TAK | 30.3 | 42.8 | 58.7 | 54.0 | 49.9 | 47.0 | 36.9 |
| CD3AK | 25.4 | 39.7 | 47.5 | 50.2 | 38.9 | 38.4 | 27.0 |
| LAK | 23.5 | 34.7 | 38.5 | 30.8 | 14.5 | 10.4 | 8.9 |
By flow cytometry, it showed that TAK cells were dominated by CD8 + T cells (Table 2).
| Incubation | CD3 | CD4 | CD8 | CD4/CD8 | NK | CD19 |
| 0 d | 52.80 | 49.40 | 26.40 | 1.87 | 23.30 | 7.20 |
| 10 d | 49.88 | 44.36 | 62.80 | 0.76 | 4.53 | 0.20 |
Anti-CD3 monoclonal antibody and IL-2 could co-stimulate the expansion of ly mphocytes, and make the cells become higher in anti-tumor activity (Table 3).
| Group | Stimulated by | Duplication of proliferation | Killing activity |
| 1 | IL-2 alone | 2.1 | 38.5% |
| 2 | IL-2 alone after costimulation | 3.8 | 40.1% |
| with anti-CD3 mAb for 48 h | |||
| 3 | Coexistence of anti-CD3 mAb | 5.3 | 58.7% |
| and IL-2 during incubation |
Under the inverted microscope, the killer cells were found to be round and bright large lymphocytes. They were aggregated into lumps with TAK lumps bigger than LAK lumps, and CD3AK lumps smaller. As the cell culture was continued, the shape of the killer cells changed and became irregular.
In vitro, we found that under the microscope the effect cells were aggregated onto the target cells after mixed cultivation for 1h, and that the targe t cells became enlarged after mixed culture for 6 h; and that cell debris could be seen after mixed culture for 15 h only in TAK group and CD3AK group .
Since Rosenberg et al[3] reported the anti-tumor therapy by LAK cell and IL-2 in 1985, it has been widely used. Its effective rate was 20%-35%. However, its adverse reaction was severe due to high doses of IL-2. For this reason, scientists are now trying to look for a certain kind of effect cells which has higher anti-tumor activity and requires lower doses of IL-2 to maint ain its activity.
In the study, CD8 + dominated cytotoxic T cells (TAK), generated from PBMC co-stimulated with soluble gastric cancer antigen (TSA) and anti-CD3 McAb, showed much higher cytotoxic activity against gastric cancer cell line from which TSA was extracted than that against hepatoma cell line (SMC). Moreover, we fou nd that the expansion of TAK in vitro required lower IL-2 concentration than CD-3AK did. It was encouraging that we have laid a foundation to solve the LAK activity which is dependent on IL-2.
As immune cells for treatment, the ratio between subtypes and its relative stability was of great importance. The results in flow cytometry showed that in the course of TAK culture, CD8+ cell number increased obviously, CD3+ decrea sed slightly and CD4+ had no change. It is suggested that TAK cells were do minated with CD8+ cells. We knew CD8+ T cells could be subdivided into Tc cells and Ts cells. If the number of Tc cells increa sed in the culture, the anti-tumor activity would be greatly enhanced. This may explain why CD8+ dominant TAK had strong anti-tumor activity. Further identification of Tc and Ts cells in the culture will be our next focus of research. Our results also showed that on the 10th day of culture, CD19+, CD16+ and CD56+ cells were rare, suggesting that anti-tumor effect of TAK cells was chiefly dependent on CD8+ T cells.
Tao et al[4] reported that stimulation of PBMC with anti-CD3 McAb and IL-2 for 48 h and then with IL-2 alone achieved a better anti-tumor activity. Our results showed that coexistence of anti-CD3 McAb and IL-2 during cell culture is of benefit to cell proliferation and cytotoxicity.
Our conclusion is that the immune effect cells generated from the PBMC stimulated with anti-CD3 McAb, IL-2 and TSA may be characterized by rapid proliferation in vitro, high cytotoxicity and low IL-2 dependence. Clinically, it is worth being considered.
| 1. | Chen YX, Shi W, Wan YX, Chen JD, Li YF, Zhai H. Soluble tumor antigens and anti-CD3 McAb costimulated tumor killer cells'T-AK cells. Chin J Microbiol Immunol. 1995;15:293-296. |
| 2. | Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods. 1990;131:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 412] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1963] [Cited by in RCA: 1839] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 4. | Tao JX, Zhang YK. Adoptive immunotherapy on cancer. Chin J Cell Biol. 1994;16:1-7. |
Edited by Xian-Lin Wang