Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.165
Revised: January 6, 1999
Accepted: January 23, 1999
Published online: April 15, 1999
- Citation: Liao SS, Li RC, Li H, Yang JY, Zeng XJ, Gong J, Wang SS, Li YP, Zhang KL. Long-term efficacy of plasma-derived hepatitis B vaccine among Chinese children: a 12-year follow-up study. World J Gastroenterol 1999; 5(2): 165-166
- URL: https://www.wjgnet.com/1007-9327/full/v5/i2/165.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.165
To evaluate long-term efficacy of a plasma-derived hepatitis B vaccine and provide evidence for decision-making on the vaccine booster doses, we conducted a prevalent follow-up study to examine serologic changes in hepatitis markers and vaccine efficacy in 350 children from the original cohort of 513 children who participated in a randomized, double-blind and placebo-controlled trial on a plasma-derived hepatitis B vaccine in Longan County, Guangxi Autonomous Region, C hina, in 1982. In this paper, we report the serologic changes in hepatitis markers and vaccine efficacy during 12 years after the initial vaccination in 350 children from the original cohort vaccinated in 1982.
Between October 1981 and April 1982, 789 children, ranging in age from 3 to 36 months (mean age, 21 months), were recruited from seven vaccination clinics in Longan County, Guangxi Autonomous Region, China, an endemic area for HBV infections. Participants at each clinic were randomly allocated into vaccine (n = 408) or control (n = 381) groups. The two groups were comparable in age, sex and pr evalence of hepatitis B virus (HBV) markers. The group allocation was unknown to the parents of the children and the staff. Children in the vaccine group receiv ed three 17. 5 μg doses of a plasma-derived hepatitis B vaccine (produced by Beijing Institute of Biol ogical Products, China) by intramuscular injection, according to the conventional 0.16 month schedule. Children in the control group were administered a placebo (vaccine diluent) at the same time. The blood specimens were collected before the administration of vaccine or placebo from each child for testing anti-HBs, antibody to hepatitis B core antigen (anti-HBc) and hepatitis B surface antigen (HBsAg).
Of 789 children, 513 (255 in vaccine group and 258 in control group) had no serologic markers of HBV infection (HBsAg, anti-HBc and anti-HBs) before the first vaccine/placebo dose. In this paper, we refer to the 513 children without HBV serologic markers before vaccination as the original study cohort.
In 1994, we obtained blood specimens from 350 (68%) of the orginal cohort of 513. Of the 350 participants, 167 were from the vaccinated group and 183 from the control group. Blood samples were tested for anti-HBs, anti-HBc and HBsAg. The loss to follow-up was attributed to temporary migration.
During the 12 years after vaccination, eight prevalent follow-up studies were conducted in the original cohort at 6 months, 1, 2, 3, 5, 8, 10 and 12 years after the first dose of vaccine/placebo. Each of the 350 children studied at the 12-year follow-up was observed for one to eight times, averaging 4.8 times in the vaccinated group and 4.9 in the control group during the 12 years.
| Time of testing | Methods and criteria of diagnosis | Manufecturer | ||
| HBsAg | Anti-HBc | Anti-HBs | ||
| Before and 6-month, | RPHA | PHA | RIA,S/Nratios | Beijing Institute of |
| 1-year after vaccination | ≥ 1:8 | ≥ 1:8 | ≥ 2.1 | BiologicalProducts |
| 2 to 10 years | RIA, S/N ratios | RIA, inhibitory | RIA,S/Nratios | Beijing Institute of |
| after vaccination | ≥ 2.1 | ratios ≥ 75% | ≥ 2.1 | BiologicalProducts |
| 12 years after | RIA, S/N ratios | RIA, inhibitory | RIA,S/Nratios | Abbot, |
| vaccination | ≥ 2.1 | Ratios ≥ 75% | ≥ 2.1 | USA |
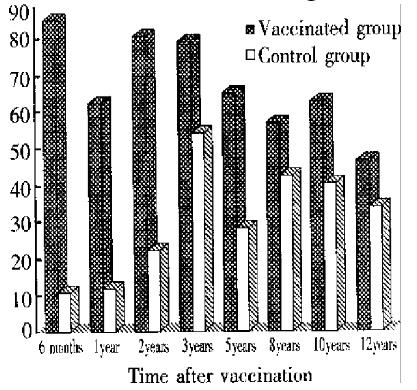
In 1994, 46.1% of vaccinated children had a protective level of anti-HBs, compared with 33.9% of controls (P < 0.05).
Among the vaccinated children with detectable anti-HBs (S/N ratios ≥ 2.1), the titers of anti-HBs declined gradually during the 12-year period. At 6 m onths after the first dose, the median S/N ratio of anti-HBs in the vacci nated group was 143, and 33 at one year after, but at the 12-year follow-up, the median S/N-ratio of anti-HBs in the vaccinated group was lower than that of controls (15 vs 36, P < 0.0001).HBV infections during 12-year follow-up(Table 2).
| Time since first dose | % of HBsAg | % of anti-HBc | ||||
| Vaccinated group | Control group | Efficacy (%) | Vaccinated group | Control group | Efficacy (%) | |
| 6 months | 2.9 | 7.4 | 60.8 | 5.9 | 10.8 | 45.4 |
| 1 year | 3.4 | 3.9 | 2.5 | 13.4c | 81.3 | |
| 2 years | 1.2 | 8.6a | 86.0 | 2.5 | 24.7d | 89.9 |
| 3 years | 1.0 | 10.4c | 90.4 | 12.2 | 29.6b | 58.8 |
| 5 years | 0.0 | 14.6c | 100.0 | 1.3 | 24.0d | 94.6 |
| 8 years | 5.9 | 13.6 | 56.6 | 7.8 | 23.7b | 67.1 |
| 10 years | 1.3 | 14.6c | 91.1 | 1.3 | 14.6c | 91.2 |
| 12 years | 1.8 | 10.9c | 83.5 | 9.6 | 37.7d | 74.5 |
Table 2 shows the HBV infections and protective efficacy of the vaccine against HBsAg during the 12 years.
At 12 years, 14 chronic HBsAg carriers were detected in the control group, whereas only one carrier was noted in the vaccinated group (7.6% vs 0.6%, P < 0.0001), which corresponded to a protective efficacy of 92%. The only HBsAg carrier in the vaccinated group had been HBsAg positive since 6 months after the first dose of vaccine. This finding suggests that infection was present before protection could be elicited. In the control group, however, 4 of the 14 chronic carriers were identified as HBsAg positive from six months after the first dose of placebo, and 8 carriers had been infected since or after one year. In the other 2 control children who were chronic carriers, the time of infection could not be established.
We found in this study that protective efficacy against HBsAg at the 12 years af ter vaccination was 83% and the efficacy against chronic HBsAg carriage was 92%. The immunogenecity of the vaccine, which was a vaccine on field trial, was slightly lower and the level of anti-HBs decreased more rapidly in our study than those used by Wainwright and Coursaget[1,2]. However, the medium and long-term protection against HBV infection in our study did not differ greatly fr om that in these studies. Coursaget et al[2] studied the efficacy of the hepatitis B vaccine in three groups of children: nonvaccinated, vaccinated with a booster dose at school-age, and vaccinated without a booster dose. At 9 to 12 years after vaccination, they reported a protective efficacy against HBsAg of 88%, with no difference in efficacy between groups with and without a booster dose. Xu et al[3] reported a protective efficacy against HBsAg of 77% in preschool and school-age children at six years after vaccination. But none of these studies reported the protective efficacy against chronic HBsAg carriage status. Vaccine protection did not parallel the decrease in titres of anti-HBs. This finding indicates the persistence of immunologic memory and supports the recommendation that administration of booster doses should not be based only on the level of anti-HBs but also on the measure of protection against HBV infe ctions[4]. Because of the protection of the vaccine against HBV infections shown in this study, we do not recommend administration of booster doses of the hepatitis B vaccine for at least 12 years after the initial vaccination.
| 1. | Wainwright RB, McMahon BJ, Bulkow LR, Parkinson AJ, Harpster AP. Protection provided by hepatitis B vaccine in a Yupik Eskimo population. Seven-year results. Arch Intern Med. 1991;151:1634-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Coursaget P, Leboulleux D, Soumare M, le Cann P, Yvonnet B, Chiron JP, Coll-Seck AM, Diop-Mar I. Twelve-year follow-up study of hepatitis B immunization of Senegalese infants. J Hepatol. 1994;21:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Xu HW, Sui XF, Men BY. Evaluation of efficacy of plasma de-rived hepatitis B vaccine at six years after vaccination. J Xi'an Med Univ. 1995;16:368-371. |
| 4. | Hadler SC. Are booster doses of hepatitis B vaccine necessary. Ann Intern Med. 1988;108:457-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
This study was supported by grant from China Medical Board, New York, Grant No. 93-582.
Edited by Jing-Yun Ma