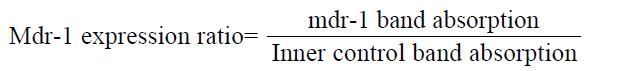Published online Feb 15, 1999. doi: 10.3748/wjg.v5.i1.53
Revised: November 27, 1998
Accepted: December 16, 1998
Published online: February 15, 1999
AIM To detect the congenital expression patterns of mdr-1 gene in commonly encountered malignant tumors in clinic, and the relationship between the expression of mdr-1 gene and the prognostic morphology in esophageal carcinomas.
METHODS A total of 151 resected samples of malignant tumors without preoperative treatment were taken from Anyang City Tumor Hospital. The congenital expression of their mdr-1 gene was detected with reverse transcripti on polymerase chain reaction (RT-PCR) and was compared with each other. The positive incidence of mdr-1 gene in 46 samples of esophageal carcinoma was compared with their differentiated grades, TNM stages and macroscopic types, and the precautions and advantages of RT-PCR were evaluated.
RESULTS All the 151 samples were confirmed to be malignant histopathologically, including cancers of stomach and gastric cardia (n = 51), esophagus (n = 46), colorectum (n = 16), breast (n = 15), thyroid (n = 10), lung (n = 9) and uterine cervix (n = 24). The positive expression rate of their mdr-1 gene was 33.3%, 37%, 31.3%, 13.2%, 40%, 55%, and 0% respectively. All the 46 samples of esophageal carcinoma were pathologically confirmed to be squamous cell carcinoma. The total expression rate of their mdr-1 gene was 37% (17/46), 35% (6/17), 40% (8/20), and 33% (3/9) for differentiation grade I, II and III respectively. The expression rate of TNM classification was 33% (6/18), 40% (5/12) and 37% (6/16) in stage IIa, IIb and III. The expression rate was 33% (3/9) in ulcerous type, 37% (3/8) in constrictive types, 33% (5/15) in fungoid types, and 40% (6/14) in medullary types. No statistically significant difference was found.
CONCLUSION Compared with other methods, RT-PCR is more simple, reliable and accurate in detecting mdr-1 gene expression in tissues of tumor. The overexpression of mdr-1 gene in these neoplasms suggested that cases should be handled differently for chemotherapy with rational use of drugs. Excision is the chief treatment for carcinoma of esophagus. The expression of mdr-1 gene in tissues of esophageal cancer is correlated with the parameters of tumor molecular biology which are independent of histopathological mor phology.
-
Citation: Zhang LJ, Chen KN, Xu GW, Xing HP, Shi XT. Congenital expression of
mdr -1 gene in tissues of carcinoma and its relation with pathomorphology and prognosis. World J Gastroenterol 1999; 5(1): 53-56 - URL: https://www.wjgnet.com/1007-9327/full/v5/i1/53.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i1.53
Multidrug resistance (MDR) of malignant tumor cell has aroused widespread interest. It has been shown that MDR is present in many malignant tumors. One of its molecular bases is mdr-1 gene amplification and its expression product. Failure of chemotherapy was chiefly due to drug resistance of tumor cells[1-9]. It is very important to detect-MDR in choosing reasonable treatment, espec ially in using effective chemotherapeutic drugs for a specific patient. Others[10] believe that mdr-1 gene expression in cancer tissue is a malignant biological indicator for neoplasms. Few research reports have been found in the literature on mdr-1 gene expression in tissues of esophageal carcinoma, and on its relation with the morphological parameters of esophageal carcinoma. For these reasons, a primary study was made.
One hundred and fifty-one specimens were taken from the Surgical Department of Anyang City Tu-mor Hospital, Henan Province soon after they were excised. Of the 151 cases, 78 were male and 73 female. They were aged from 21 to 80 years, averag-ing 52.1. All the cases were confirmed to be malignant tumors without preoperative treatment. Of them, 51 were cancers of stomach and gastric car-dia, 46 cancers of esophagus, 16 cancers of colorec-tum, 15 cancers of breast, 10 cancers of thyroid, 9 cancers of lung and 4 cancers of uterine cervix. The 46 patients of esophageal carcinoma were all permanent residents of Anyang citizenship, 26 male and 20 female. The site of cancer was found in the up-per, middle and lower thor acic segment in 8, 30, and 8 cases respectively according to 1987 UICC cri-teria. Eighteen cases were in stage IIa, 12 in stage IIb, and 16 in stage III according to TNM classifica-tion. All the cases were squamous cell carcinomas. Of them, 17 were in grade I, 20 in grade II, and 9 in grade III according to SUN Shao-Qian’s grading system for squamous cell carcinoma. Nine were found to be ulcerous type, 6 constrictive type, 15 fungoid type, and 16 medullary type according to WU Ying-Kai’s gross pathological typing method.
Reverse Transcription Polymerase Chain Reaction (TT-PCR) Reagent Kit was supplied by Beijing Jinghai Biological Engineering Company. Bio-RAD Gene CyclerTM (Gene Amplifier) was made in Japan. LG15-w high-speed centrifuge was made by Beijing Medical Centrifuge Factory. SA-U94.11 ul-traviolet transilluminator was made by Shanghai Zhongya Biological Institute.
The sequences of mdr-1 gene primers
5’ACCCATCATTGCAATAGCAG3’
5’TGTTCAAACTTCTGCTCCTG3’
The sequences of inner control β2-microglobulin gene primers
5’ATGGCTCGCTCGGTGACCCTAC3’
5’TCATGATGCTTGATCACATGTCTCG3’
Methods for determining mdr-1 gene expression[1] was used with minor modifications. Major steps were extraction of total tumor RNA by guanidine isothiocyanate, synthesis and amplification of com-plementary DNA (cDNA) to mdr-1 gene by RT-PCR. The products were separated by electrophore-sis on agarose gel containing EB. DNA bands were made visible by transillumination with ultraviolet.
Only one 300 bp band was found in the negative results. Inner control band and mdr-1 gene 170 bp band were found in the positive results. Gene ex-pression was calculated on a concentration scanner by the relative yield of the mdr-1 gene to the β2 in-ner control gene. Its formula is expressed as:
Math 1
The ratio < 0.1 means negative expression, > 0.4 high expression, 0.1-0.4 moderate expression. The observed parameters included mdr-1 gene ex-pression positively in macro- and micro-scopicmor-phology of the specimens, and comparison mdr-1 expression in various groups.
Chi-square test was used, and P value less than 0.05 stands for statistical significance.
The expression of mdr-1 gene in the studied samples was over 30%, except that in cancer of uterine cervix and breast (Table 1).
| Site of tumors | n | H | M | L/N | H&M |
| Esophagus | 46 | 10.9 | 26.1 | 63.0 | 37.0 |
| Gastric cardia | 35 | 17.1 | 11.9 | 71.0 | 29.0 |
| Stomach | 16 | 25.0 | 12.5 | 72.5 | 27.5 |
| Colorectum | 16 | 18.8 | 12.5 | 68.7 | 31.3 |
| Lung | 9 | 22.0 | 33.0 | 45.0 | 55.0 |
| Breast | 15 | 6.6 | 6.6 | 86.8 | 13.2 |
| Thyroid | 10 | 20.0 | 20.0 | 60.0 | 40.0 |
| Uterine cervix | 4 | 0 . 0 | 0.0 | 100.0 | 0.0 |
| Total | 151 | 15.2 | 18.6 | 66.2 | 33.8 |
The total expression rate of mdr-1 gene was 37% (17/46) in the 46 cases of esophageal carcino-ma with no relation to the morphological parameters of the tumor. It was an independent molecular bio-logical characteristic of the tumor (Tables 2, 3 and 4).
| TNM stage | n | No. of positive cases | Positive rate (%) |
| IIa | 18 | 6 | 33 |
| IIb | 12 | 5 | 40 |
| III | 16 | 6 | 37 |
| Grade | No. of positive cases | Positive | rate (%) |
| II | 6 | 3 | 5 |
| II | 8 | 4 | 0 |
| III | 3 | 3 | 3 |
| Types | n | No. of positive cases | Positive rate (%) |
| Ulcerative | 9 | 3 | 33 |
| Constrictive | 8 | 3 | 37 |
| Fungoid | 15 | 5 | 33 |
| Medullary | 14 | 6 | 40 |
It is believed that mdr-1 gene is one of the normal sequences of human genome. Nevertheless, its ex-pression and expressive level are decided by differ-ent cell type and environmental factors. The mdr-1 gene expression can be investigated with several molecular methods including evaluation of protein expression and mRNA. Protein may be detected by Western blot analysis and immunohistochemical techniques. Immunohistochemical staining is commonly used, but it is not suitable for quantitative determination of protein due to its complicacy and in-fluence of experimental conditions. Since all organ-isms store their genetic information in nucleic acid, methods of direct detection of mdr-1 at mRNA level have advantages of high efficiency, sensitivity, and specificity. Traditional methods for mRNA such as S1 nuclease test, RNA slot blots, RNA protection assays, in situ hybridization and Northern blot analysis are greatly limited due to their overlaborate procedure and poor sensitivity. RT-PCR was used in present study. After cDNA of mdr-1 gene was syn-thesized according to the transcribed mRNA of mdr-1, it was detected by PCR in vitro. Compared with other gene measure ments, RT-PCR is one of the most sensitive, specific, reproducible, effective, simple, and time-saving methods[2]. We believe that the prospects of its application in clinic are quite broad.
One of the mechanisms of drug resistance to cancer cells is called MDR which is known as the resistance to lipophilic drugs such as daunorubicin, adriamycin, vincristin, and colchicin. It is very un-favourable to chemotherapy, because once tumor cells develop resistance to one of the these drugs, they will develop resistance to all lipophilic drugs[5-7]. How to evaluate multidrug- resistant tumor cells is a problem demanding prompt solution. Recently, there were several papers on the mecha-nism, evaluation and reversion of MDR[5-8]. Most of them focused on mdr-1 and its products, p-170[2-6]. p-170 (P-gp) was considered as an ATP-dependent drug molecular pump, which would lead to failure of chemotherapy as a result of drugs being pumped out from cells. This fact has been proved in many researches of diseases such as leukocythemia, breast can cer and melanoma. These recearches were of no significance in clinical practice because they were usually focused on a certain kind of tumors and their results were obtained from malignant cell line with different methods. In the present study, the expression of mdr-1 gene in commonly encountered malignant tumors was synchronically studied by the same technicians with same instruments, experi mental methods and reagents. The results showed that all the detected neoplasms except cervical carcino-ma, expressed mdr-1 gene in different degrees (Table 1). Breast cancer also had a low expression of mdr-1. The positive number of mdr-1 gene in the other tumor tissues was more than 1/3 except that in breast and cervical cancers. This kind of expression was congenital because these tumors had not received chemotherapy when they were detected. It indicated tumor carried mdr-1 gene and its product-Pgp from the development of tumor. Clinicians should pay great attention to the mechanisms of its drug resistance if they are tenable. They should differentiate the subgroups of malignant tumors from molecular level in addition to taking other clinical indexes such as tissue differentiation, TNM staging system, and tissue type into consideration, in order to avoid unsuitable chemotherapy and use of MDR-drugs especially lipophilics. Combined treatment with reverser of mdr-1 gene and supressor of P-gp should be used to improve curative effect if it has been proved to be effective. Some researchers held that MDR of tumors could not be completely explained by mdr-1 gene and its P-gp system[10], and further research should be made. In our phase II study, a control study will be made on the difference of mdr-1 gene expression in cancer and normal tissues as well as in those before and after chemotherapy. We believe that the theory of MDR will be an important reference index for chemother-apy of tumors as a result of our better understanding of it.
Surgical treatment is the commonly used treat-ment for esophageal carcinoma. Its curative effect is chiefly decided by TNM stages, histological differ-entiation and types. Generally speaking, it is better for the early and well differentiated squamous cell carcinoma than the late and poorly differentiated adenocarcinoma or the undifferentiated one. However, exceptions in clinic suggest that further research at the molecular level of gene is needed in esophageal carcinoma which has its unique biological characteristics independent of its morphological parameters as other malignant tumors.
Since the finding of mdr-1 gene and its product P-gp (P-glycoprotein, p17 0), they have been applied to researches on their relations to cytotoxic chemotherapeutic drugs, especially to the lipophilic drugs. Many of these researches were in the field of hematic malignancies, and new ways were explored to reverse MDR. However, attention was seldom paid to the congenital expression of mdr-1 gene in tissues of esophageal carcinoma and its relation with morphological parameters. It was found in this study that the congenital expression of mdr-1 gene was 37% in tissues of the 46 cases of esophageal car-cinoma without chemoth erapy before operation. It was much higher than that reported in leukemi-a[4,9-13], melanoma[6], and breast cancer[1,3,5,7]. This is one of the possible reasons why no progress has been achieved in the curative effect of chemotherapy for esophageal carcinoma in the past years. It indicates that only by attaching importance to the selection of chemotherapeutic drugs and suitable chemotherapy for esophageal carcinoma, can its curative effect be achieved. It also suggests that surgical treatment for it at present should be stressed. Many researches have demonstrated that cancer is a genetic disease. Besides traditional mor-phological indexes, the following factors were found to be related with the prognosis of esophageal carcinoma such as antioncogene, oncogene and their abnormal products as well as others at the level of gene molecules, and have been taken into account in clinic. Overexpression of mdr-1 gene was believed to be an index of drug resistance and further malig-nization of the histogical behavior of cancer cells[10]. The theory of drug resistance of tumors was advanced by Goldie and Codman[8] in 1979 in the light of gene change. It held that drug resistance was resulted from gene mutation of tumor cells produced in frequency of the tumor, and that the larger the tumor, the more the frequency of proliferation and the stronger the drug resistance. This theory also indicates that drug resistance of tumors has a positive correlation with the stage of tumors. No significant difference was found in the expression of mdr-1 gene in tissues of esophageal carcinoma on the basis of its morphology, differentiati on and TNM. It was suggested that expression of mdr-1 gene is a molecular parameter independent of surgical patho-morphological indexes. This results show that surgical treatment is the first choice for esophageal carci-noma at present due to its drug resistance.
It was held that expression of mdr-1 gene was a protective mechanism of cells, which was found in some normal tissue cells in addition to cancer cells[10]. The results in our study seemed to support it. Long follow-up study is needed to decide whether overexpression of mdr-1 gene in esophageal carcinoma is an index of its further malignization, because no comparison was made for its difference in cancer and normal tissues as well as before and after its chemotherapy.
Although surgical treatment of esophageal car-cinoma is destructive and will exert some influence on the quality of life of the patients, it remains the first choice before a breakthrough is made in chemotherapy. In order to improve the curative effect of chemotherapy, the reverse mechanism of MDR should be further studied while the drug-resis-tant mechanism is comprehensively researched.
| 1. | Charpin C, Vielh P, Duffaud F, Devictor B, Andrac L, Lavaut MN, Allasia C, Horschowski N, Piana L. Quantitative immunocytochemical assays of P-glycoprotein in breast carcinomas: correlation to messenger RNA expression and to immunohistochemical prognostic indicators. J Natl Cancer Inst. 1994;86:1539-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Chen K, Xing H, Cheng B. [Expression of mdr-1 gene in cancer tissue and its association with morphological indexes of esophageal carcinoma]. Zhonghua Yixue Zazhi. 1998;78:462-463. [PubMed] |
| 3. | Goldstein LJ. MDR1 gene expression in solid tumours. Eur J Cancer. 1996;32A:1039-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Bertolini F, de Monte L, Corsini C, Lazzari L, Lauri E, Soligo D, Ward M, Bank A, Malavasi F. Retrovirus-mediated transfer of the multidrug resistance gene into human haemopoietic progenitor cells. Br J Haematol. 1994;88:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Benchekroun MN, Schneider E, Safa AR, Townsend AJ, Sinha BK. Mechanisms of resistance to ansamycin antibiotics in human breast cancer cell lines. Mol Pharmacol. 1994;46:677-684. [PubMed] |
| 6. | Berger W, Elbling L, Minai-Pour M, Vetterlein M, Pirker R, Kokoschka EM, Micksche M. Intrinsic MDR-1 gene and P-glycoprotein expression in human melanoma cell lines. Int J Cancer. 1994;59:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Veneroni S, Zaffaroni N, Daidone MG, Benini E, Villa R, Silvestrini R. Expression of P-glycoprotein and in vitro or in vivo resistance to doxorubicin and cisplatin in breast and ovarian cancers. Eur J Cancer. 1994;30A:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Gottesman MM. How cancer cells evade chemotherapy: sixteenth Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1993;53:747-754. [PubMed] |
| 9. | Rowinsky EK, Adjei A, Donehower RC, Gore SD, Jones RJ, Burke PJ, Cheng YC, Grochow LB, Kaufmann SH. Phase I and pharmacodynamic study of the topoisomerase I-inhibitor topotecan in patients with refractory acute leukemia. J Clin Oncol. 1994;12:2193-2203. [PubMed] |
| 10. | Beck J, Niethammer D, Gekeler V. High mdr1- and mrp-, but low topoisomerase II alpha-gene expression in B-cell chronic lymphocytic leukaemias. Cancer Lett. 1994;86:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Yamashita T, Watanabe M, Onodera M, Shimaoka K, Ito K, Fujimoto Y, Itoyama S, Sugawara I. Multidrug resistance gene and P-glycoprotein expression in anaplastic carcinoma of the thyroid. Cancer Detect Prev. 1994;18:407-413. [PubMed] |
Edited by Jing-Yun Ma