Published online Apr 15, 1998. doi: 10.3748/wjg.v4.i2.165
Revised: February 22, 1998
Accepted: March 28, 1998
Published online: April 15, 1998
AIM: To obtain greater antigenicity of HCV NS3 protein.
METHODS: The HCV NS3 cDNA fragment was amplified by reverse transcription polymerase chain reaction from the sera of the HCV infected patients. The DNA sequence was determined by dideoxy-mediated chain termination method using T7 polymerase. HCV NS3 protein was expressed in E. coli.
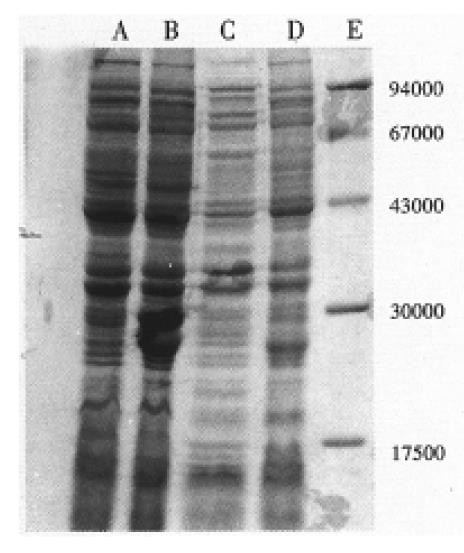
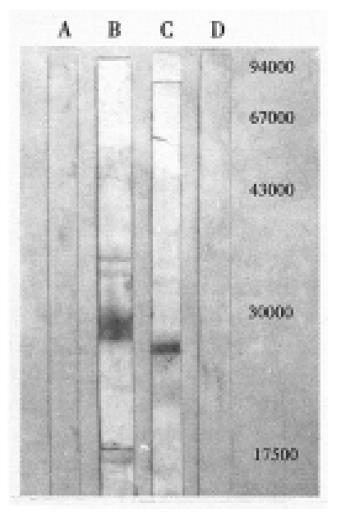
RESULTS: Sequence analysis indicated that the HCV isolate of this study belongs to HCV-II; SDS-PAGE demonstrated an Mr 23800 and an Mr 22000 recombinant protein band which amount to 14% and 11% of the total bacterial proteins separately. Western blotting and ELISA showed NS3 protein possessed greater antigenicity.
CONCLUSION: Recombinant HCV NS3 protein was expressed successfully, which provided the basis for developing HCV diagnostic reagents.
- Citation: Zhu FL, Lu HY, Li Z, Qi ZT. Cloning and expression of NS3 cDNA fragment of HCV genome of Hebei isolate in E. coli. World J Gastroenterol 1998; 4(2): 165-168
- URL: https://www.wjgnet.com/1007-9327/full/v4/i2/165.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i2.165
Since HCV (hepatitis C virus) was identified as the major cause of post-trans fusional non-A, non-B hepatitis by Choo et al[1] in 1989, the harm of this infectious disease to the human health has been gradually realized. Up to 50%-60% of the HCV hepatitis cases turn chronic, developed into cirrhosis and hepatocellular carcinoma. Due to lack of effective therapeutic methods, it is exceptionally important to prevent the transmission of HCV. As HCV is mainly transmitted through blood sources, the key to preventing the transmission of HCV is to screen the blood donors using specific and sensitive HCV diagnostic reagents. HCV NS3 antigen is necessary in anti-HCV diagnostic reagents because of the lower divergence in NS3 region of HCV genome and the strong antigenicity of NS3 protein. In addition, because of the early appearance and high incidence of the antibody against NS3 antigen. In this report, the HCV NS3 gene fragment was cloned and expressed in E. coli. The obtained NS3 antigen showed strong antigenicity, which provided the basis for developing HCV ELISA reagents.
Serum for cDNA synthesis was derived from a patient who comes from Hebei Province, which is po-sitive for anti-HCV and HCV RNA. HCV RNA was extracted by single step of guanidinium-phenol-chloroform method[2]. Ninety-four serum specimens for ELISA were screened from 460 sera derived from patients with different types of hepatitis, which are anti-HCV positive tested by Abbott second generation HCV ELISA, provided by the Institute of Hepatitis, Youan Hospital.
Expression vectors are pRX vectors with trpE promotor[3] and pMY vector with pRpL promotor. pRX vector expresses fusion protein with 18 amino acid at N-termini of the protein induced by IPTG. pMY vector expresses non-fusion protein induced by heat at 42 °C.
HCV NS3 protein antigenicity analysis was conducted with computer according to HCV-BK[4] sequence. Refered to the analysis results, the conservative region was selected and the primers were designed as follows: Outer primer: F1 5’GTTGCGAAGGCGGTGGACTT3’ R1 5’GTCGTCTCAATGGTGAAGGT3’ Inner primer: F2 5’GGAATTCTCCGGCTGCATATGCA3’ R2 5’CCATCGATAGGTATAGCCCGTCAT3’
To facilitate subcloning, EcoR I and Cla I restriction endonuclease sites were added at the 5’-termini of the inner primer. The outer reverse primer was used in both cDNA synthesis and cDNA amplification.
According to the methods by Widell and Cristiano[5,6], before reverse transcription, RNA template was denatured at 94 °C for 5 min, then on ice promptly. Reverse transcription and first round of PCR were run in the same buffer at the same tube. Reaction volume was 100 μL, containing AMV 1.6 U and Taq DNA polymerase 2.5 U. The reaction order is reverse transcription at 42 °C for 30 min, inactivation of reverse transcriptase 94 °C for 3 min, and PCR consisting of 94 °C 55 s, 42 °C 1 min, 72 °C 1.5 min for 5 cycles, and 94 °C 55 s, 55 °C 1 min, 72 °C 1.5 min for another 30 cycles. One tenth of the first round of PCR products were used at template to conduct the second round of PCR, the conditions were the same as the last 30 cycles of the first round of PCR. Finally, PCR products were analyzed on 15 g/L agarose gel electrophoresis.
The cDNA fragment was subcloned into M13 mp 18/19 vectors and single stranded DNA template was prepared. Sequencing reaction was performed using T7 sequencing kit. Nucleotide and amino acid homology analysis were conducted using Goldkey program.
cDNA fragments and vectors were cleavaged by restrictive endonuclease, ligated with T4 DNA ligase, the E. coli HB101 was transformed. The recombinant plasmids were identified through restrictive endonuclease digestion[7]. E. coli HB101 carrying recombinant plasmid were inoculated in M9 media. After incubation at 30 °C for about two hours, the 3β-indolyl acrylic acid was added to the final concentration of 10 mg/L, and the bacteria were cultured for 5 h to express the protein. E. coli HB101 carrying recombinant plasmid pMYNS3 were cultured at 30 °C for about two hours, then transferred to 42 °C for another 5 h to express the recombinant protein.
Twelve percent SDS-PAGE and Western blotting were used to identify the expressed proteins. The sample was prepared as follows: removing 1 mL of induced culture, centrifuging to collect the pellet, resuspending the pellet and boiling for 5 min to lyse the bacteria with 2 × SDS loading buffer. After the electrophoresis, the polyacrylamide gel was split into two pieces. One piece of gel was stained with Coomassie Brilliant Blue R-250, and scanned to analyze the expression level of the recombinant protein. The other piece was blotted to nitrocellulose membrane. Blotting condition was 0.8 m·cm2·1.5 h. The blotted membrane was incubated with blocking solution containing 10 ml/L fatal bovine serum for one hour, then incubated with anti-HCV positive serum (diluted 1: 100) at 4 °C overnight. The following day, the membrane was incubated with sheep anti-human IgG-HRP (diluted 1:500) at room temperature for 30 min, finally stained with substrate 3, 3’-diaminobenzidine.
The recombinant protein expressed by pMYNS3 was purified by ion-exchange chromatography, and the purified protein was used as antigen to test 94 control positive sera by ELISA.
The PCR products were analyzed on 15 g/L agrose gel electrophrosis, and the expected 634 bp cDNA fragment was clearly seen. Sequencing results revealed that the cDNA fragment is 634 bp, and proved to be HCV NS3 fragment in comparison of the sequence of cDNA fragment and the corresponding region of HCV-BK. Its nucleotide and amino acid homology with genotype II isolate HCV-BK, genotype I isolate HCV1, genotype III isolate HCV-J6 and genotype IV isolate HCV-J8 were 90.8% and 95%, 80% and 94.9%, 73.2% and 88.2%, 73.5% and 86.7%, respectively[8-10]. These results indicated that the isolate in this study belongs to HCV-II. In addition, we also compared its homology with the sequence of corresponding region of HCV isolates derived from the patients who came from Hebei and Taiwan separately, the nucleotide and amino acid sequence homology were 90.4% and 95.4%, 90.8% and 93.8%, respectively[11,12]. SDS-PAGE analysis demonstrated that pRXNS3 plasmid expressed a protein with a molecular weight of about Mr 23800 which amounts to 14% of the total bacterial proteins (Figure 1). pMYNS3 plasmid expressed a molecular weight of about Mr 22000 protein, which covers 11% of the total bacterial proteins (Figure 1). Two forms proteins strongly reacted with anti-HCV anti-bodies in the sera in western blotting test (Figure 2). Using the purified pMYNS3 expressed recombinant protein as an antigen to test 94 control anti-HCV positive sera, 85 (90.4%) of 94 were detected. Among the 94 sera samples, 28 (96.6%) of 29 derived from patients with known transfusion history were detectable. Other sera derived from patients diagnosed clinically as having acute, chronic hepatitis and cirrohosis, the detection rates were 83.3% (10/12) for acute hepatitis, 86.8% (33/38) for chronic hepatitis and 93.3% (14/15) for cirrhosis, respectively.
In this report, the NS3 fragment of HCV genome was reversed transcribed and amplified successfully from the serum of a patient who comes from Hebei Province, and the cDNA fragment was sequenced and expressed with high level in E. coli. The results of western blotting and ELISA showed greater antigenicity of the recombinant protein, which demonstrated good prospects of using the protein as an antigen to detect anti-HCV antibodies. The nucleotide and amino acid homology analysis indicated that the HCV isolate in this study should belong to HCV-II. Another HCV isolate derived from the same area is also HCV-II[11], which suggested in some extent that the endemic HCV isolates in this region might be HCV-II. The sequence comparison of NS3 region of HCV between different isolates showed that NS3 region is the relative conservative region in HCV genome, in addition, the amino acid homology is higher than nucleotide homology, so using NS3 protein as antigen to detect anti-HCV antibodies will be characterized by a wide coverage of HCV infection. The prokaryotic expression vector pRX and pMY were used to express HCV NS3 protein. pRX vector with trpE promotor is induced by 3-indolyl acrylic acid and expresses a fusion-protein with 18 amino acid at N-termini of the protein. pMY vector with pRpL promoter is induced by heating at 42 °C and expresses non-fusion protein. We found that the expression level of fusion protein is higher than that of non-fusion protein. The possible explanation is that the proteinase of host bacteria will not recognize the recombinant fusion protein as non-self protein to lyse it. The fusion of bacterial protein to recombinant protein increases the stability of protein and raises the expression level. The comparison between the fusion and non-fusion protein in antigenicity showed no obvious difference. Because pMYNS3 is induced easily by heat, we used the protein expressed by pMYNS3 as coating antigen in ELISA. This study estimated the diagnostic value of NS3 recombinant antigen using 94 control anti-HCV positive sera which are detectable with Abbott second generation ELISA. The results showed that NS3 antigen can detect 85 (90.4%) of 94 of control sera, among these, 28 (96.6%) of 29 sera derived from hepatitis patients with known transfusion history were detected. These results consistent with the results of Abbott’s ELISA test. The successful expression of HCV NS3 protein has provided an ideal basis for developing anti-HCV diagnostic reagents.
| 1. | Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4996] [Cited by in RCA: 4669] [Article Influence: 126.2] [Reference Citation Analysis (1)] |
| 2. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40518] [Cited by in RCA: 39206] [Article Influence: 1005.3] [Reference Citation Analysis (0)] |
| 3. | Rimm DL, Pollard TD. New plasmid vectors for high level synthesis of eukaryotic fusion proteins in Escherichia coli. Gene. 1989;75:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Takamizawa A, Mori C, Fuke I, Manabe S, Murakami S, Fujita J, Onishi E, Andoh T, Yoshida I, Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991;65:1105-1113. [PubMed] |
| 5. | Widell A, Månsson AS, Sundström G, Hansson BG, Nordenfelt E. Hepatitis C virus RNA in blood donor sera detected by the polymerase chain reaction: comparison with supplementary hepatitis C antibody assays. J Med Virol. 1991;35:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Cristiano K, Di Bisceglie AM, Hoofnagle JH, Feinstone M. Hepatitis C viral RNA in serum of with chronic non-A, non-B hepatitis: detection by the poly-merase chain reaction using multiple primer sets. Hepatology. 1991;14:51-55. [RCA] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Sambroo KJ, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed, New York:. Cold Spring Harbor Larbor Laboratory. 1989;3-101. |
| 8. | Choo QL, Richman KH, Han JH, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby R, Barr PJ. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:2451-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1136] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 9. | Okamoto H, Okada S, Sugiyama Y, Kurai K, Iizuka H, Machida A, Miyakawa Y, Mayumi M. Nucleotide sequence of the genomic RNA of hepatitis C virus isolated from a human carrier: comparison with reported isolates for conserved and divergent regions. J Gen Virol. 1991;72:2697-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 323] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Okamoto H, Kurai K, Okada SI, Yamamoto K, Lizuka H, Tanaka T et al. Full length sequence of a hepatitis C virus genome having poor homology to reported isolates: comparative study of four distinct genotypes. Virology. 1992;188:331-341. [RCA] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 385] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Bi SL, Bai XH, Cong ME, Tian HW, Sun DG, Margolis HS et al. Primary structure and variation of Chinese hepatitis C virus genome. Acta Virol. 1993;9:114-127. |
| 12. | Chen PJ, Lin MH, Tai KF, Liu PC, Lin CJ, Chen DS. The Taiwanese hepatitis C virus genome: sequence determination and mapping the 5' termini of viral genomic and antigenomic RNA. Virology. 1992;188:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 3.9] [Reference Citation Analysis (0)] |