Published online Apr 15, 1998. doi: 10.3748/wjg.v4.i2.125
Revised: March 25, 1998
Accepted: April 2, 1998
Published online: April 15, 1998
AIM: To investigate the mechanisms of codon 249 mutation of p53 gene in the formation of hepatocellular carcinoma (HCC).
METHODS: Codon 249 mutation accompanied by loss of heterozygosity (LOH) and its effect on translation and transcription were studied using SSCP, IHC and RT-PCR/slot hybridization.
RESULTS: Codon 249 mutations were detected in 32.9%, LOH detected in 68.4% among the HCC patients. Mutations of condon 249 were accompa-nied by LOH in 90%. The positive rates of p53 protein and mRNA were 91.3% and 95.7%, in mutational group, both were significantly higher than those in the non-mutational group (91.3% vs 19.1% and 95.7% vs 40.4%, respectively, both P < 0.01). The translation of p53 gene was strongly related to its transcription by correlation analysis (r = 0.8208).
CONCLUSIONS: LOH might play an important role in hepatocarcinogenesis of codon 249 mutation, which could increase both transcription and translation of p53 gene. The increased expression of p53 protein mainly depend on the increased transcription of p53 gene.
- Citation: Peng XM, Peng WW, Yao JL. Codon 249 mutations of p53 gene in development of hepatocellular carcinoma. World J Gastroenterol 1998; 4(2): 125-127
- URL: https://www.wjgnet.com/1007-9327/full/v4/i2/125.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i2.125
HCC is one of the most common human tumors in certain areas of Africa and Asia including southern China. However, the mechanisms, especially the molecular mechanisms of hepatocarcinogenesis are still not well understood. Since condon 249 mutations of p53 gene were observed in 45%-50% of HCCs from southern Africa and Qidong, China, it has been believed that mutations of codon 249 plays an important role in hepatocarcinogenesis[1-3]. But now mutation causes HCC, is still unclear. Mutant p53 proteins with much longer half-life are often overexpressed in HCC. They can inactivate the wild proteins through dominant negative in vitro[4-7]. Losses of heterozygosity are often observed in HCC. They are favorable for mutations to enact[8-10]. Nevertheless, the effect of codon 249 mutations on transcription or translation of p53 gene and the relationship between these mutations and LOH are still unclear. Therefore, in this study, we investigated these questions using surgical specimens of HCC from southern China where the incidence of HCC is moderate to high.
Specimen Seventy surgical specimens of HCC were collected from the First Affiliated Hospital and Cancer Hospital, Sun Yat-Sen University of Medical Sciences. Cancerous and pericancerous tissues were separated by pathologists.
Reagents Restriction enzyme Hae III was purchased from GIBco BRL. p53 protein monoclonal antibody (DO-7) and LSAB system were purchased from DAKO company. Dig detection kit was from B. M. Company.
Examination of codon 249 mutation using RFLP analysis Exon 7 was amplified using primers 5’-GGCGACAGAGCGAGATTCCA-3’ (sense) and 5’-GGGTCAGCGGCAAGCAGAGG-3’ (anti-sense). Twenty μL amplified DNA product (286 bp) was digested with 2.5 U-restriction enzyme Hae III. DNA fragments with and without digestion were analyzed in 2% agarose gel.
Examination of p53 gene LOH using SSCP analysis Exon 3-4 was amplified using primers 5’-AAATTCATGGGACTGACTTT-3’ (sense) and 5’-AATGCAGGGGGATACGGCCAGC-3’ (anti-sense). PCR product was precipitated at -20 °C for 1 h by adding 2.5 vol of alcohol and 1/10 vol of 4 M sodium acetate. Pellets were resuspended in 10 μL Formamide dye mixture (95% formamide, 20 mmol/L EDTA, 0.05% bromphenol blue). Samples were heated at 95 °C for 5 min, chilled on ice and immediately loaded (5 μL) on 6% acry-lamide/TBE-gel. fGels were run at 40 W for 4 h at room temperature. Silver-staining was used to visualize the bands. LOH of p53 gene was ensured by comparing the band pattern from cancerous tissue and pericancerous tissue of the same patient.
Detection of p53 proteins using immunohistochemistry p53 proteins in tissues were detected using immunohistochemistry (IHC). Semi-quantification was carried out in 5 groups of patients divided according to the percentage of positive cells (< 1%, 1%-5%, 5%-25%, 26%-50% and > 50%).
Detection of p53 mRNA using RT-PCR Total cell RNA was extracted using Chomczynski’s method. DNA fragments of 555 bp were amplified using RT-PCR with primers 5’-TACTCCCCTGCCCTCAA-3’(sense) and 5’-GTTGGGCAGTGCTCGCT-3’ (anti-sense)[11]. RT-PCR products were transferred to nitrocellulose membrane. p53 mRNA was detected by hybridization with the Dig-probes which were labeled with PCR, and then visualize the dots with Dig detection kit. The degree of dot color was classified into 5 grades.
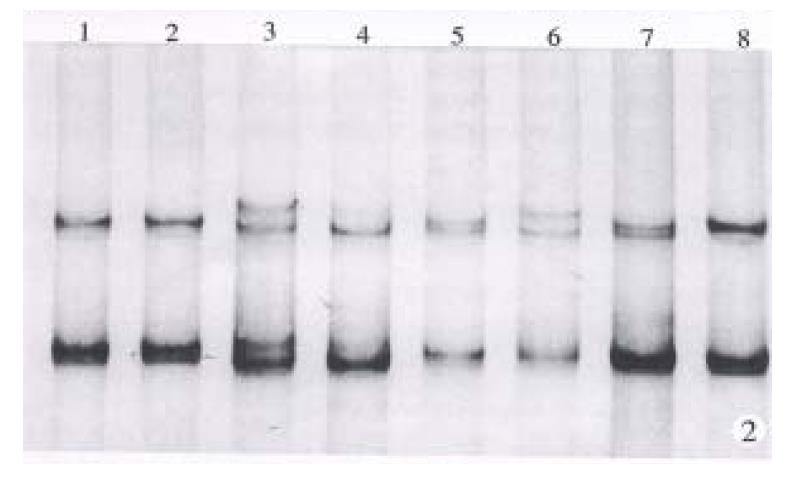
Exon 7 of cancerous and pericancerous tissues from 70 cases of HCC were all amplified satisfactorily. The number of base pairs of PCR product was as the same as designed. Mutations of codon 249 were detected in 23 of these cases, with a positive rate of 32.9%. One of them showed partial mutation, which was confirmed to be an allele mutation through SSCP analysis. Typical results are shown in Figure 1.
Exon 4 of p53 gene has a heterogeneous location (CGC/CCC) at condon 72. The number, however, of band pattern in this study was more than 4. It might suggest that there were two or more heterogeneous locations in DNA fragment amplified from exon 3 to 4. The increased heterogeneous locations were favorable to LOH analysis. Heterozygous form was found in 57 of 70 (81.4%) cases of HCC and LOH was detected in 39 of the 57 (68.4%) cases which carried heterozygous alleles. Heterozygous alleles were found in 20 of 23 cases with mutant codon 249, 18 of which (90%) were mutant allele accompanied by LOH. Typical results are shown in Figure 2.
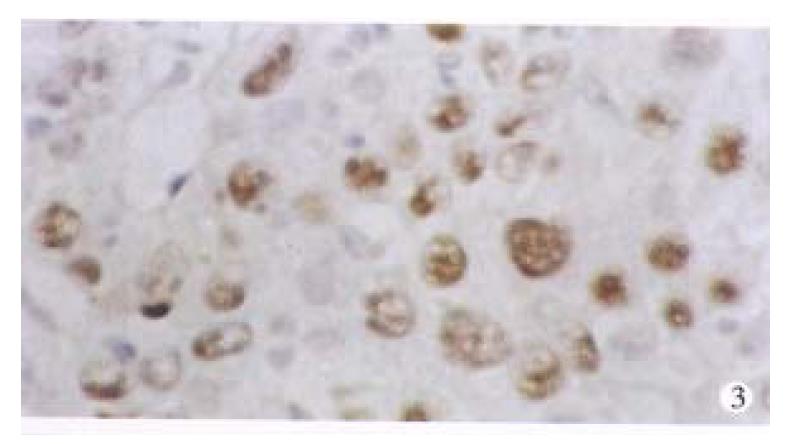
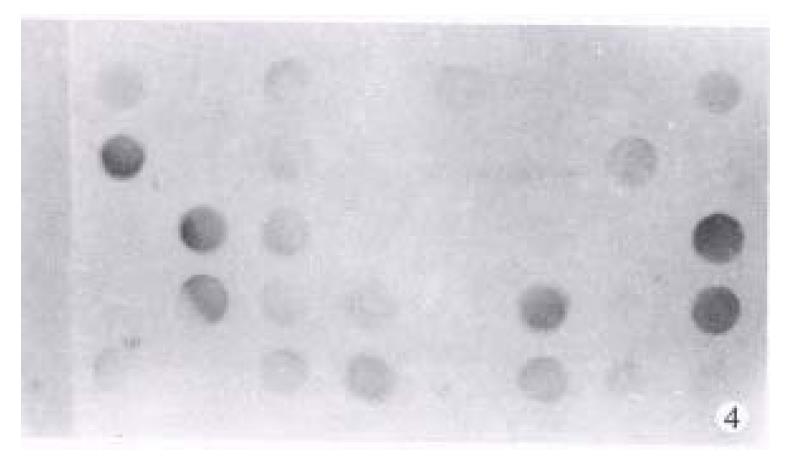
p53 proteins were detected only in cancerous tissues of HCC. Positive cells 7% were found in 30 cases, with a rate of 42.9%. Typical positive stain is shown in Figure 3. The relationship between the expression of p53 proteins and codon 249 mutations is shown in Table 1 (Figure 4, Figure 5).
The relationship between the expression of p53 proteins and the expression of p53 mRNA among 23 cases carrying codon 249 mutation is shown in Figure 5. Grade correlation analysis demonstrated that they were strongly correlated (correlation coefficient r = 0.8208).
Tumor suppressor p53 gene often shows mutational hotspot of codon 249 in HCC, i.e., selective G to T mutation usually occurs in the third base of codon 249. This unique mutation has been reported to be involved in the carcinogenesis, differentiation and metastasis of HCC[1-6]. How this mutation causes HCC, however, is unclear. It is believed that some codon mutations of p53 gene can prolong the half-life of p53 proteins. Thus, the mutant p53 proteins often had overexpression in HCC, and inactivated its antitumor effect in combination with wild p53 proteins[4-7]. However, the effect of mutation on the transcription and translation of p53 gene is unclear. This study showed that the p53 mRNA expression in mutational group was significantly higher than that of non-mutational group. The expression of p53 proteins was strongly correlated to the expression of p53 mRNA. These results suggested that codon 249 mutation could increase p53 mRNA expression, and increased mRNA was an important factor in the overexpression of mutant p53 proteins.
When one allele mutated, it was difficult to a-mass enough cells to allow the other allele to mutate[7]. Therefore, it is important to study whether LOH exists in the other allele. Our results showed that 90% codon 249 mutations were accompanied by LOH of the other allele, suggesting that LOH of p53 gene was also important in hepatocar-cinogenesis as mutations. LOH might be favorable for mutations to enact.
In conclusion, HCC will be prevented if we take some measures to avoid recurrent hepatic damage, and reduce the occurrence of LOH, and the clinical outcome of HCC will be much better if the transcription of p53 mRNA can be suppressed by chemical or gene therapy.
| 1. | Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991;350:427-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 976] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 2. | Peng XM, Peng WW, Yao JL, Zhou YP. The relationship between hepatitis c and bvirus infection and aberration of p53 gene. Chin J Hepatol. 1997;5:124-125. |
| 3. | Soini Y, Chia SC, Bennett WP, Groopman JD, Wang JS, DeBenedetti VM, Cawley H, Welsh JA, Hansen C, Bergasa NV. An aflatoxin-associated mutational hotspot at codon 249 in the p53 tumor suppressor gene occurs in hepatocellular carcinomas from Mexico. Carcinogenesis. 1996;17:1007-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 80] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Hayashi H, Sugio K, Matsumata T, Adachi E, Takenaka K, Sugimachi K. The clinical significance of p53 gene mutation in hepatocellular carcinomas from Japan. Hepatology. 1995;22:1702-1707. [PubMed] |
| 5. | Qin L, Tang Z, Liu K. [The relation between p53 mutations and tumor invasiveness of human hepatocellular carcinoma]. Zhonghua Zhongliu Zazhi. 1995;17:405-408. [PubMed] |
| 6. | Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49-53. [PubMed] |
| 7. | Ng IO, Lai EC, Chan AS, So MK. Overexpression of p53 in hepatocellular carcinomas: a clinicopathological and prognostic correlation. J Gastroenterol Hepatol. 1995;10:250-255. [PubMed] |
| 8. | Rogler CE, Chisari FV. Cellular and molecular mechanisms of hepatocarcinogenesis. Semin Liver Dis. 1992;12:265-278. [PubMed] |
| 9. | Yumoto Y, Hanafusa T, Hada H, Morita T, Ooguchi S, Shinji N, Mitani T, Hamaya K, Koide N, Tsuji T. Loss of heterozygosity and analysis of mutation of p53 in hepatocellular carcinoma. J Gastroenterol Hepatol. 1995;10:179-185. [PubMed] |
| 10. | Lasko D, Cavenee W, Nordenskjöld M. Loss of constitutional heterozygosity in human cancer. Annu Rev Genet. 1991;25:281-314. [PubMed] |
| 11. | Shieh YS, Nguyen C, Vocal MV, Chu HW. Tumor-suppressor p53 gene in hepatitis C and B virus-associated human hepatocellular carcinoma. Int J Cancer. 1993;54:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Project supported by the China Medical Board (CMB) of New York, Grant No. 93-582.