Published online Apr 15, 1998. doi: 10.3748/wjg.v4.i2.112
Revised: February 20, 1998
Accepted: March 20, 1998
Published online: April 15, 1998
AIMS: To assess the protective effect of diallyl disulfide (DADS) and its combined use with N-acetyl-cysteine (NAC) on acetaminophen (APAP) hepatotoxicity in C57BL/6N (B6) mice pretreated with β-naphthoflavone (BNF).
METHODS: B6 mice were divided into six groups and all compounds used were injected intraperitoneally. Except for control and APAP group (receiving APAP only), the other groups received an injection of APAP (350 mg/kg) 48 h after BNF (200 mg/kg) and either of DADS (200 mg/kg), or NAC (500 mg/kg) or both DADS and NAC. DADS was given 2 h before APAP and NAC was injected with APAP. The mean survival time was recorded and livers were examined histologically. Hepatic glutathione (GSH) levels and plasma ALT were also determined at different time points. To evaluate the effect of DADS or NAC on hepatic P450 induction by BNF, liver microsomes were prepared and 7-ethoxyresorufin O-dealkylase (ERD) activity was determined using spectrofluorometrical methods. In vitro effect of DADS or NAC on ERD activity was assayed by directly incubating microsomal suspension with DADS or NAC of different concentrations.
RESULTS: APAP was not toxic to mice without BNF pretreatment, but caused severe liver necrosis and death of all BNF-treated mice in 4 h. A sharp depletion of GSH (approximately 62% of its initial content at 2 h and 67% at 4 h) and a linear elevation of ALT levels (536.8 ± 29.5 Sigma units at 2 h and 1302.5 ± 74.9 at 4 h) were observed. DADS and NAC given individually produced mild protection, resulting in prolonged survival, a slower decline of GSH level and a less steeper elevation of ALT level. All mice died eventually. Co-administration of DADS and NAC completely protected mice. GSH level in this group lowered by about 35% and 30% at 2 and 4 h, and ALT was 126 ± 18 and 157.5 ± 36.6 Sigma units at 2 and 4 h. ERD activity in BNF-treated mice was about 5 times that of the constitutive level determined in normal mice. Neither DADS nor NAC inhibited P450 1A1/1A2 induction as determined by their effect on the induction of ERD activity. In vitro assay indicates that DADS, but not NAC, was a potent inhibitor of ERD activity (IC50 = 4.6 μM).
CONCLUSIONS: A combined use of both DADS and NAC produced full protection in BNF treated mice against APAP hepatotoxicity. The mechanism is that DADS inhibits P450 1A1/1A2 activity, but not induction, which substantially reduces production of NAPQI, while NAC enhances liver detoxifying capability via serving as a precursor of GSH and stimulating GSH synthesis.
- Citation: Zhao C, Duquet S, Zhou YX. Effects of combined use of diallyl disulfide and N-acetyl-cysteine on acetaminophen hepatotoxicity in β-naphthoflavone pretreated mice. World J Gastroenterol 1998; 4(2): 112-116
- URL: https://www.wjgnet.com/1007-9327/full/v4/i2/112.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i2.112
Acetaminophen (APAP) has been widely used for decades, especially as an over the counter analgesic in North America. It has been long known that an overdosed APAP can cause severe damages to the liver or even death of the experimental animals and individuals who have ingested large quantities of APAP accidentally or in an attempt to commit suicide. In the liver, APAP is bioactivated and converted by cytochrome P450 to its metabolite, N-acetyl-p-benzoquinoneimine (NAPQI), which has been shown to be toxic to animals in vivo[1] and to cultured hepatocytes[2,3]. Although the precise mechanism of APAP hepatotoxicity is not well understood, a number of studies have suggested that NAPQI exerts a cytotoxic effect through its covalent binding to cytosolic or microsomal proteins and membrane components[4-6], inhibition of mitochondrial respiration, depletion of ATP[7,8], etc.
For bioactivation of APAP, several P450 iso-forms are implicated, including P450 1A1, 2A1, 2B1, 2C11, 2E1, 3A1 and 3A2[9,10]. These enzymes are inducible by cigarette smoking, alcohol consumption, drugs, and some chemical compounds used daily at home for cleaning or as a pesticide. Elevation of P450 activity by these inducers can markedly enhance APAP hepatotoxicity in humans[11] and experimental animals[12]. For instance, alcohol is an inducer of P450 2E1 and therefore alcoholics are more susceptible to and at higher risk for development of APAP hepatotoxicity[11]. More attention should be paid to these risk factors because these individuals may develop hepatotoxicity at the rapeutic doses. In recent years, several compounds have been tested for their protective effects on overdose APAP. However, information about the possible treatment for APAP toxicity in P450-elevated animal models or human subjects is very limited. Diallyl sulfide (DAS) is a P450 inhibitor specifically for 2E1. Recently, a study showed that DAS prevented APAP hepatotoxicity in normal rats[13]. The current work investigated the role of diallyl disulfide (DADS) and its combined use with N-acetyl- cysteine (NAC) in protecting APAP-caused liver damage in mice in which P450 levels were induced by β-naphthoflavone (BNF) pretreatment. To our knowledge, this is the first investigation on the inhibitory effect of DADS on hepatic P450 1A1/1A2 induced by BNF in mice and the protective effect of the use of DADS and NAC in combination on APAP caused-hepatotoxicity.
Animals and animal treatment. C57B/6N (B6) mice (4-6 weeks of age, 18 g-20 g in body weight) were purchased from Harlan Sprague Dawley (Indianapolis, USA). The mice were divided into six groups (10 mice each) and treatment for each group is shown in Table 1. All compounds used were purchased form Sigma, USA (except indicated otherwise) and injected intraperitoneally. BNF was injected at dose of 200 mg/kg body weight (in corn oil) 48 h prior to administration of APAP (350 mg/kg body weight in saline) as described previously[14]. DADS (200 mg/kg body weight, purchased from Aldrich, USA) was diluted in 0.2 mL corn oil and given 2 h before APAP challenge. NAC of 500 mg/kg body weight was injected at the same time when APAP was given. For control, corn oil and saline were used in replace of BNF and APAP, respectively. The animals were then kept in warm environment under close observation.
| APAP | BNF + APAP | DADS | NAC | DADS + NAC | |
| BNF | 200 | 200 | 200 | 200 | |
| DADS | 200 | 200 | |||
| APAP | 350 | 350 | 350 | 350 | 350 |
| NAC | 500 | 500 |
Evaluation of APAP hepatotoxicity. After APAP challenge, the survival time for each group was recorded, calculated and expressed as mean ± SD. APAP-induced hepatotoxicity in each group was also evaluated histologically and enzymatically. For histology, immediately after death of the animal or sacrifice of the mice surviving longer than 48 h, the liver was removed, fixed in 10% neutral buffered formalin, washed with phosphate buffer saline (PBS), dehydrated in an increasing concentration of ethanol, and finally embedded in paraffin. Sections of 6 μm in thickness were stained with hematoxylin and eosin and examined under light microscopy. The extent of liver necrosis was evaluated semi-quantitatively using a scale of 0-4 according to the scoring system introduced by Mitchell et al[15]. Score 0, indicates no evidence of necrosis; score 1, less than 6%; score 2, 6%-25%; score 3, 26%-50%; and score 4, more than 50% of the liver cells are necrotic.
Blood samples were collected at 2, 4 and 6 h after APAP administration and plasma ALT levels were determined using Sigma GPT Kit (fol-lowing the manufacturer’s instruction). The values for each group were expressed as mean ± SD. ALT less than 20 Sigma units is considered normal and a range of 20-35 units is a border line according to the manufacturer’s instruction.
Another set of groups of B6 mice was used in order to determine the effects of DADS and NAC on hepatic GSH levels. After the same treatment as shown in Table 1, the mice were sacrificed at 2, 4, 6, 8 and 24 h after APAP administration. The liver was homogenized in 4 volumes of 0.1 M PBS and the homogenate was mixed with an equal volume of 4% sulfosalicylic acid. After centrifugation, 0.5 mL supernatant was added to 4.5 mL 0.1 mM bis-(3-carboxy-4-nitrophenyl) disulfide in 0.1 M PBS (pH8.0). The mixture was incubated at 25 °C for 60 min in the dark. GSH concentration was determined at absorbance 412 nm using a Shimadzu UV-Visible Recording Spectrophotometer UV-160.
To explore the mechanism of DADS protection against APAP hepatotoxicity, we further investigated the effect of this compound on hepatic P450 1A1/1A2 activity induced by BNF. Five groups of B6 mice were used and treated as control, BNF-APAP, DADS, NAC, and DADS + NAC groups as summarized in Table 1, except that APAP was omitted in all of these groups and NAC was given 47 h after BNF. Forty-eight hours after BNF, the mice were killed and livers were immediately removed for determination of activity of hepatic microsomal 7-ethoxyresorufin (ER) O-dealkylase by the method introduced by Burke et al[16]. Briefly, livers were homogenized in 0.1 M Tris buffer containing 0.25 M sucrose (pH7.5). The supernatant fractions from the centrifuged liver homogenate (13500 × g for 20 min) were recentrifuged at 105000 × g for 60 min. The pellets were washed and resuspended in 0.1 M PBS and used for assay immediately. The reaction mixture contained 2 mL PBS, 20 μL microsomal suspension, and 10 μL ethoxyresorufin (50 μM). A baseline of fluorescence was recorded at an excitation wavelength of 510 nm and an emission wave-length of 586 nm using a Shimadzu fluorospec-trometer RF-540. After addition of 200 μL NADPH to the reaction mixture, the increase in fluorescence was recorded in air at room temperature. The specific activity of the enzyme was calculated by comparing with a standard curve.
Assessment of direct effects of DADS or NAC on induced P450 1A1/1A2 activity was conducted by incubating microsomal suspension prepared from BNF-treated mouse liver with different concentrations of DADS or NAC, respectively. Incubation was carried out at room temperature for 30 min and followed by the measurement of 7-ethoxyresorufin dealkylase activity as described above.
Statistical significance was analyzed using statistical software StatView 4.1 (Jandel Scientific Inc., USA). One-way ANOVA and Student t test were used for difference among multiple values or between two values, respectively.
APAP group showed no sign of APAP toxicity. However, BNF + APAP mice suffered from severe toxicity and all died eventually. The difference was statistically significant between the APAP group and BNF + APAP group in terms of mortality and mean survival time (P < 0.001). The use of either DADS or NAC alone prolonged the mean survival time (P < 0.01) but did not affect the mortality rate when comparing DADS group and NAC group with BNF + APAP group. The use of DADS and NAC in combination completely protected the animals, and the mortality rate was reduced to 0 (P < 0.001), (Table 2).
| Control | APAP | BNF + APAP | DADS | NAC | DADS + NAC | |
| Mean survival time (h) | > 48 | 3.17 ± 0.18 | 6.29 ± 0.49 | 7.0 ± 0.40 | > 48 | |
| Mortality (%) | 0 | 0 | 100 | 100 | 100 | 0 |
Histological evaluation of the liver necrosis revealed that mild damage (Score 1) was found in 10% of mice receiving APAP only, and 90% of this group appeared normal morphologically. However, extremely severe hepatic necrosis was observed in BNF + APAP group, with Mitchell’s score 4 for 9 mice and 3 for 1. The necrosis mainly occurred in the lobular center and in some cases, the normal lobular structures were destroyed. Although the use of either DADS or NAC (Group 4 and 5) could significantly prolong the mean survival time, Mitchell’s scores of these mice were still recorded as high as 3 for 9 mice of DADS group and 8 mice of NAC group, respectively. Seven animals of DADS + NAC group were recorded histologically as score 1 (mild damage), and three as score 2. There was a statistical significance between DADS + NAC group and BNF + APAP group (P < 0.01).
As shown in Figure 1, hepatic GSH in BNF + APAP group sharply declined by 60% of its initial concentration in first two hours after APAP and continued to drop until all mice died at 4 h. In DADS, NAC and DADS + NAC groups, GSH levels also dropped by about 35% in first two hours, though significantly higher than that of BNF + APAP group (P < 0.05). At 4 and 6 h, GSH in DADS + NAC group maintained at a significantly higher level (P < 0.01, as compared with DADS or NAC group) and all mice survived whereas liver GSH in either DADS or NAC group continued to decline and all died about 7 h after APAP. Plasma ALT levels of these groups are illustrated in Figure 2. ALT in BNF + APAP mice increased almost linearly, and DADS or NAC group showed moderate elevation while ALT in DADS + NAC group went up only mildly. Statistical significance (P < 0.05) was found between BNF + APAP group and either DADS or NAC group and DADS + NAC group, but not between DADS and NAC groups (P > 0.05).
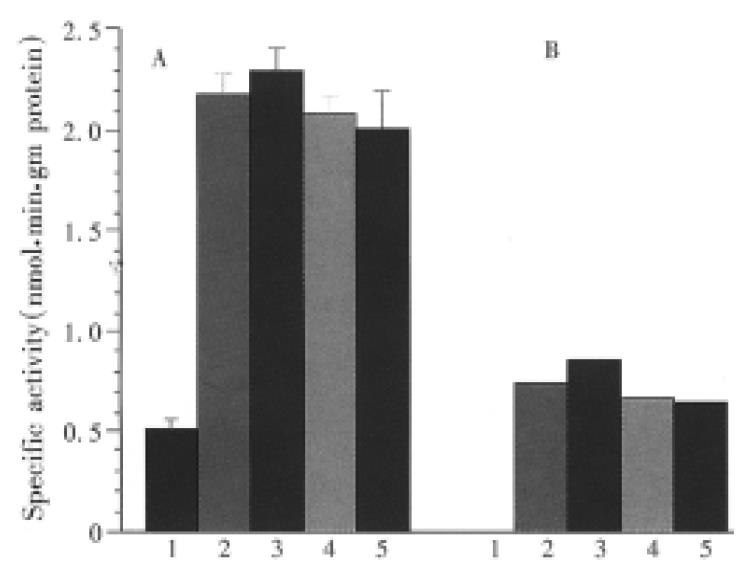
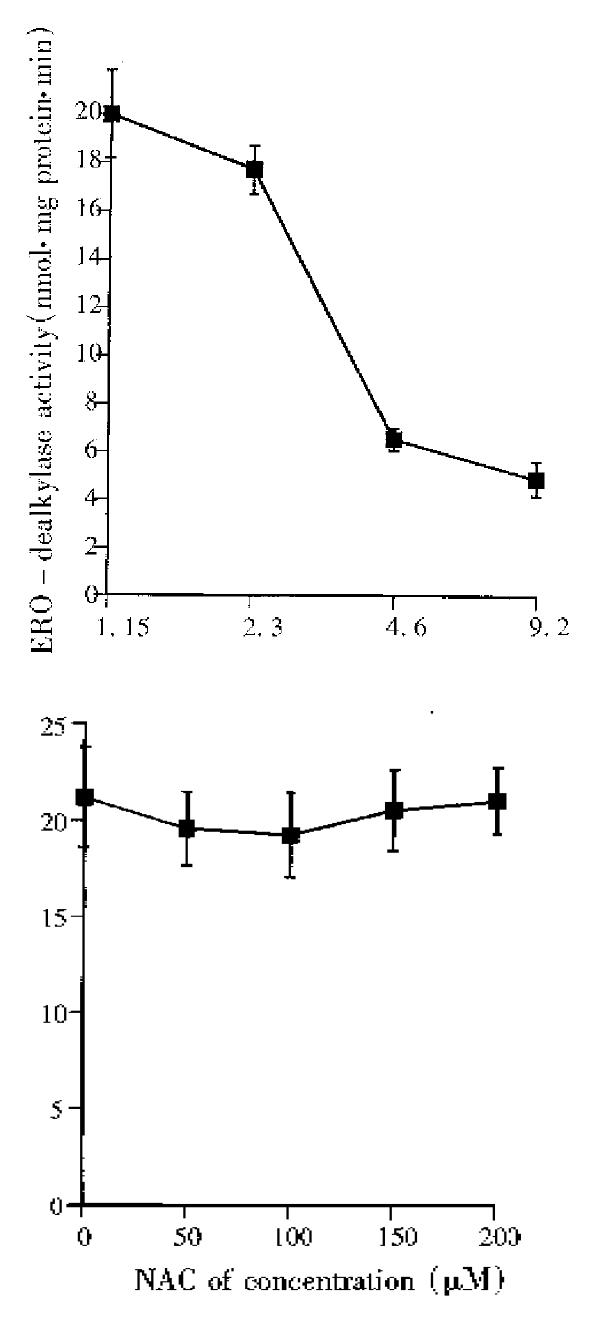
In vivo study on effects of DADS, NAC or DADS and NAC used in combination on hepatic P450 1A1/1A2 are shown in Figure 3. BNF can significantly induce P450 1A1/1A2, as determined by ERD activity, and compared with the constitutive activity in the control mice (P < 0.01). There was no significant difference in ERD activity between BNF, DADS, NAC, and DADS + NAC groups (P > 0.05). However, in vitro study by direct incubation of microsomal suspension prepared from BNF-treated mouse liver demonstrated that DADS can significantly inhibit P450 1A1/1A2 activities induced by BNF, IC50 being approximately 4.6 μM of DADS, whereas NAC showed no direct inhibitory effect on the enzyme activity (Figure 4 a and b).
Studies on APAP hepatotoxicity remain a very active area since much is still uncertain despite continuous efforts worldwide. We have previously demonstrated the inductibility of P450 1A1/1A2 by BNF in B6 mouse hepatocytes in vivo[14]. The cur-rent study showed that BNF-pretreated mice developed severe liver necrosis and all mice died in a few hours after challenged with APAP. However, when APAP of the same dosage was applied to mice without BNF pretreatment, the animals tolerated it well. These findings indicate that P450 induction by BNF in hepatocytes markedly enhanced APAP hepatotoxicity and, therefore, is a critical step in the development of liver necrosis. P450 1A1/1A2 is probably the major isoform involved in this case, which is in agreement with the study by Snawder, et al[12].
In treatment of APAP poisoning, compounds which inhibit P450 activity and drugs which increase hepatic GSH pooling (i.e. enhancing liver detoxifying capability) are expected to protect animals against APAP cytotoxicity since NAPQI is converted from APAP by P450 enzymes and normally and mainly detoxified by conjugation with GSH. The re-action increases the solubility of NAPQI and facilitates its elimination through the kidneys. DADS is attractive because of its nature as an extract derived from garlic[13]. Recently, Hu[13] reported that DAS (another compound derived from garlic) given intra-gastrically at a dose of 200 mg/kg body weight reduced the mortality from 40% to 0% in rats challenged with APAP at dose of 750 mg/kg body weight. The protective action of DAS was thought to be related to its inhibitory effects on the hepatic P450 activity, especially for P450 2E1[16,17]. In the current study, we observed that DADS given in-traperitoneally prolonged the survival time of B6 mice in which P450 activity had been induced by BNF. However, this regimen did not improve the histological evaluation and the total mortality rate, although hepatic GSH content and plasma ALT levels showed signs of improvement. NAC has been clinically used as the mainstay of APAP poisoning. Nevertheless, NAC used individually in this study did not provide satisfactory protection. The combination regimen we tested produced a full protection evidenced by a significant reduction in severity of liver necrosis and the mortality rate reduced to zero. Significant improvement in plasma ALT and liver GSH was also observed. Therefore, this regi-men may have a clinical potential and may be the choice of treatment for APAP intoxication in subjects with elevated P450 enzyme activity.
The mechanism of protection by DADS against APAP hepatotoxicity is more likely to be associated with its inhibitory action on hepatic P450 1A1/1A2 activity since in vitro enzyme assay demonstrated that DADS is a potent P450 1A1/1A2 inhibitor, although it does not affect induction of this enzyme by BNF[18]. DADS was markedly suppressed, but was unable to completely eliminate the elevated hepatic P450 activity, and the non-suppressed portion of P450 enzyme can still produce an amount of NAPQI sufficient to cause liver necrosis and animal death. On the other hand, NAC does not directly detoxify NAPQI but serves as a precursor of GSH and stimulates hepatic GSH pooling. Hence, effectiveness of measures in enhancing liver detoxifying capability depends upon how potent these prodrugs are and how quickly they can be converted into forms that can be used to detoxify NAPQI. By combination regimen, DADS reduces generation of NAPQI by inhibiting the key enzyme and the NAPQI generated by non-suppressed P450 can be substantially trapped and conjugated by increased GSH due to administration of NAC. Therefore, the combination regimen acts on two steps in the metabolic pathway of APAP to provide the full protection.
Interestingly, the hepatic GSH depletion and plasma ALT elevation in either DADS or NAC group were much less severe than those of BNF group but the death of animals was not avoided. It is likely that adequate maintenance and/or rapid restoration of liver GSH by combination regimen contributes, at least in part, to the full protection. Moreover, plasma ALT level, a commonly used liver damage marker, may not be parallel with the extent of hepatic necrosis and may not truly reflect the severity of intoxication if DADS or NAC had been given. Therefore, plasma ALT level may not be used as a solely reliable parameter to assess the severity of liver damage and to predict the prognosis.
We would like to thank Dr. M. Kur-pakus for the use of the laboratory facilities and to Dr. D. Shi for his assistance in statistics.
| 1. | Miner DJ, Kissinger PT. Evidence for the involvement of N-acetyl-p- quinoneimine in acetaminophen metabolism. Biochem Pharmacol. 1979;28:3285-3290. [PubMed] |
| 2. | Harman AW, Kyle ME, Serroni A, Farber JL. The killing of cultured hepatocytes by N-acetyl-p-benzoquinone imine (NAPQI) as a model of the cytotoxicity of acetaminophen. Biochem Pharmacol. 1991;41:1111-1117. [PubMed] |
| 3. | Miller MR, Wentz E, Blair JB, Pack D, Hinton DE. Acetaminophen toxicity in cultured trout liver cells. I. Morphological alterations and effects on cytochrome P450 1A1. Exp Mol Pathol. 1993;58:114-126. [PubMed] |
| 4. | Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, Pumford NR, Hinson JA. Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol. 1991;138:359-371. [PubMed] |
| 5. | Birge RB, Bulera SJ, Bartolone JB, Ginsberg GL, Cohen SD, Khairallah EA. The arylation of microsomal membrane proteins by acetaminophen is associated with the release of a 44 kDa acetaminophen-binding mouse liver protein complex into the cytosol. Toxicol Appl Pharmacol. 1991;109:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Holtzman JL. The role of covalent binding to microsomal proteins in the hepato-toxicity of acetaminophen. Drug Met Rev. 1995;25:395-451. |
| 7. | Esterline RL, Ray SD, Ji S. Reversible and irreversible inhibition of hepatic mitochondrial respiration by acetaminophen and its toxic metabolite, N-acetyl-p-benzoquinoneimine (NAPQI). Biochem Pharmacol. 1989;38:2387-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Donnelly PJ, Walker RM, Racz WJ. Inhibition of mitochondrial respiration in vivo is an early event in acetaminophen-induced hepatotoxicity. Arch Toxicol. 1994;68:110-118. [PubMed] |
| 9. | Harvison PJ, Guengerich FP, Rashed MS, Nelson SD. Cytochrome P-450 isozyme selectivity in the oxidation of acetaminophen. Chem Res Toxicol. 1988;1:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Lee CA, Thummel KE, Kalhorn TF, Nelson SD, Slattery JT. Inhibition and activation of acetaminophen reactive metabolite formation by caffeine. Roles of cytochromes P-450IA1 and IIIA2. Drug Metab Dispos. 1991;19:348-353. [PubMed] |
| 11. | Pezzano M, Richard C, Lampl E, Pelletier G, Fabre M, Rimailho A, Auzépy P. [Hepatic and renal toxicity of paracetamol in chronic alcoholic patient]. Presse Med. 1988;17:21-24. [PubMed] |
| 12. | Snawder JE, Roe AL, Benson RW, Roberts DW. Loss of CYP2E1 and CYP1A2 activity as a function of acetaminophen dose: relation to toxicity. Biochem Biophys Res Commun. 1994;203:532-539. [PubMed] |
| 13. | Hu JJ, Yoo JS, Lin M, Wang EJ, Yang CS. Protective effects of diallyl sulfide on acetaminophen-induced toxicities. Food Chem Toxicol. 1996;34:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Zhao C, Zhou YX. Induction of cytochrome P450 1A1/1A2 in mouse hepatocytes by β-naphthoflavone: an immunocytochemical study. Chin J New Gastroenterol. 1996;4:334-335. |
| 15. | Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185-194. [PubMed] |
| 16. | Burke MD, Mayer RT. Ethoxyresorufin: direct fluorimetric assay of a microso-mal O-dealkylation which is preferentially inducible by 3-methylcholanthrene. Drug Metabol Disposition. 1974;2:583-588. |
| 17. | Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, Guengerich FP, Yang CS. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol. 1993;6:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 279] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Brady JF, Li DC, Ishizaki H, Yang CS. Effect of diallyl sulfide on rat liver microsomal nitrosamine metabolism and other monooxygenase activities. Cancer Res. 1988;48:5937-5940. [PubMed] |