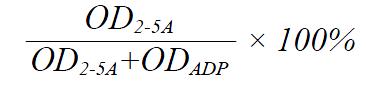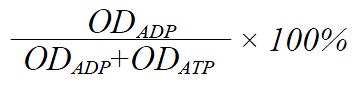Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.70
Revised: July 20, 1997
Accepted: September 2, 1997
Published online: February 15, 1998
AIM: To establish a nonradioactive assay for 2’-5’ oligoadenylate synthetase (2-5 AS) and to measure the 2-5AS in peripheral blood mononuclear cell (PBMC) extracts of patients with chronic hepatitis C before IFN-α injection, 24 h and one month after the first injection.
METHODS: 2-5AS in cell extracts of PBMCs from 10 normal persons and 15 chronic hepatitis C patients were determined with PEI cellulose thin-layer chromatography.
RESULTS: The assay of 2-5AS in human PBMC was found to be rapid, sensitive, specific and reliable. The 2-5AS activity of PBMC in normal persons was in a quite low level (2.0%), and it was increased about ten-folds after stimulation of IFN (19.7%), (P < 0.01). In 15 chronic hepatitic C patients, the basal levels of 2-5AS before IFN treatment were higher than those of normal persons, being much higher in the group showing poor response to IFN treatment, but 24h after the first injection of IFN-α the 2-5AS level showed a more rapid and much greater rise in those patients with a good response.
CONCLUSION: 2-5AS may be a useful parameter of biological response during the IFN therapy.
- Citation: Tong WB, Zhang CY, Feng BF, Tao QM. Establishment of a nonradioactive assay for2’-5’ oligoadenylate synthetase and its application in chronic hepatitis C patients receiving interferon-α. World J Gastroenterol 1998; 4(1): 70-73
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/70.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.70
The double-stranded RNA (dsRNA) dependent enzyme, (2’-5’) oligoadenylate synthetase (2-5AS), which was first discovered in interferon (IFN)-treated cells, could polymerize ATP into (2’-5’) oligoadenylate (2-5A). These were probably related to the antiviral activity and perhaps antiproliferative effects of IFN, called 2-5AS system. Treatment of cells (such as peripheral blood mononuclear cells, PBMC) with IFN led to the de novo synthesis of 2-5AS; this enzyme could produce oligoadenylates from ATP in the presence of double-stranded RNA (ds RNA) according to the equation: (n + 1) ATP→PPP5’A(2’P5’A)n+nPPi (n≥ 1). (2’-5’) oligoadenylates greater than the dimeric form could activate an endoribonuclease (RNase L) which was able to cleave mRNA, leading to aninhibition of viral protein synthesis[1].
Recently, some researchers determined 2-5AS level of PBMC in patients with chronic viral hepatitis as a biological response parameter during the therapy with IFN or in the diagnosis of diseases related to the IFN system. Now, lots of radioactive methods have been reported for measuring 2-5AS in EAT cells, Hela cells, Wish cells, rabbit reticulocytes and lymphocytes[2]. In this paper, we deve-lopeda non-radioactive assay for determining the 2-5AS in human PBMC: PEI-cellulose thin-layer chromatography.
Rabbit reticulocytes were prepared from adult male New Zealand rabbits (from Animal Laboratory of Beijing Medical University) by a modified method of Borsook et al[3]. One ml of a neutralized 2.5% aqueous solution of phenylhydrazine hydrochloride was injected subcutaneously each day, together with 0.05 mg of folic acid and 0.05γ of vitamin B12 were injected intramuscularly daily, reticulocyte counting was performed on smears of blood drawn from ear veins and stained with brilliant cresyl blue in 0.15 mol/L NS. On the 7th or 8th day, blood samples were drawn while over 90% of the circulating red cells were then reticulocytes.
The blood samples were added to a flask containing heparin, washed three times with 0.01 mol/L phosphate buffer solution (PBS) pH 7.4. After centrifugation, the supernatant was removed completely and the cells were treated with equal volume of lysis buffer (20 mmol/L Tris-HCl pH 7.6, 5 mmol/L magnesium acetate, 30 mmol/L 2-mercaptoethanol, 1 mmol/L EDTA, 10% glycerol, 0.5% Nonidet P-40) at 4 °C for 30 min. After centrifugation (15000 ×g, 10 min at 4 °C), the 2-5 AS containing supernatant was collected and frozen at -70 °C for use.
Human PBMC of 10 normal persons were isolated from heparinized whole blood (5-10 mL) by Ficoll-Hypaque sedimentation (Lymphocyte Separation Medium). Then 1 × 107 washed PBMC per mL were incubated 24 h (37 °C, 5%CO2) in RPMI-1640 supplemented with 20% fetal calf serum with or without adding IFNα (INTRON, Schering, NJ) at 1000 U/L. After washing three times with PBS, PBMC were sedimented at 9000 ×g for 1 min and then lysed by lysis buffer (1 × 107 cells per ml), and extracts were prepared by centrifugation of the lysed cells at 15000 ×g for 10 min at 4 °C, then were stored at -70 °C for use.
PBMC extracts from fifteen chronic hepatitis C patients were prepared as stated above, before IFN-α injection, 24 h and also 1 month after the first injection of IFNα. They were stored at -70 °C for use.
Because 2-5AS can catalyze the synthesis of 2-5A, the detection of 2-5A might be a reflection of the 2-5AS activity. In this study, we used the PEI-cellulose thin-layer plates (Sigma, USA) to separate 2-5A from residual ATP. The plate can specially separate the chemical compound with over 3 molecule-phosphate residues from those with less than 3 molecule-phosphate residues by thin-layer chromatography. First, the residual ATP not polyme-rized into 2-5A was digested with digestion buffer (Hexokinase 2.52 g/L, 30 mmol/L glucose monohydrate, 10 mmol/L magnesium acetate) to transform it into ADP (with 2 molecule-phosphate residues), then the chromatography was carried out, so the 2-5A was separated from ADP. The results of chromatography could be seen under ultraviolet lamp.
Poly (I) : poly (C) agarose beads were washed with buffer A (20 mmol/L Tris-HCl pH 7.6, 5 mmol/L magnesium acetate, 30 mmol/L 2-mercaptoethanol, 1mmol/L EDTA, 10% glycerol) three times, then suspended in buffer A (1 + 1 by vol) and 40 μL aliquots were transferred into Eppendorf tubes. After centrifugation and removal of supernatant, 400 μL PBMC extracts (or 0.8 ml rabbit reticulocytes lysates) were added into the tubes, reaction continued for 1 h at 4 °C. Following this binding reaction, the agarose-beads were washed four times with 800 μL buffer A. The 2-5AS reaction was started by addition of 25 μL buffer B (buffer A + ATP 3 mmol/L, poly (I) : poly (C) 50 mg/L, creatine phosphokinase 0.1 g/L, creatine phosphate 6 mmol/L), then incubated overnight at 37 °C, the reaction was stopped by heating the mixtures (3-5 min at 95 °C). After 2-5AS reaction, 20 μL of incubation mixture supernatants was transferred into wells of microtiter plates, 5 μL of digestion buffer were added (30 min at room temperature). Then all the samples were spotted onto a PEI-cellulose thin-layer plate (20 cm × 20 cm) 2 cm from the bottom. After drying the spots, the plate was immersed in methyl alcohol for 10 min with continuous shaking. After drying the plate, the chromatography was developed in chromatographic buffer (0.75 mol/L potassium phosphate tribasic anhydrous, pH 3.5) and developed to the top of the plate in this solvent, the procedure took about 10 min. The plate was then dried and exposed to ultravioletray: there was only one ultraviolet absorbing spot of “ADP” to the PBMC sample without 2-5AS activity, but there were two spots (one for “ADP”; another for “2-5A”) to the sample with different 2-5AS level. Under UV analyzer, each spot was then cut out with scissors, to be immersed into 0.5 ml soaking buffer (20 mmol/L Tris-HCl pH 7.6, 1 mmol/L EDTA, 1.5 mol/L NaCl) overnight, and absorbance at 259 nm was determined by the supernatant of each sample. The 2-5AS activity was calculated from the percentage of ATP turnover, according to the following formula:
Math 1
Study 1: Each batch of rabbit reticulocyte lysate was subject to examination sixteen times.
Study 2: PBMC extracts from 10 normal persons were measured for 2-5AS with or without induction by IFNα, and the results were used for determining the varying responses in different normal persons to IFNα.
Study 3: Fifteen patients with chronic hepatitis C (11 male, 4 female; the average age for 40.7 years) who were treated with IFNα were studied. All the patients were positive for anti-HCV and HCV-RNA, but negative for anti-human immuno-deficiency virus (HIV) and anti-hepatitis A, B and D, their diseases had lasted more than one year, and all the fifteen patients had elevated serum alamine aminotransferase (ALT) activities of at least twice the upper limit of normal before IFNα therapy.
The patients were given IFNα intramuscularly for 3 months, at a dose of 3 mega units (MU) daily during the first 4 weeks and then thrice weekly for 8 weeks. Determinations for 2-5AS of PBMC were performed in samples obtained pre-treatment, 24 h and 1 month after the first injection. At the same time, the fifteen patients’ serum samples were examined every two weeks for ALT and anti-HCV, HCV-RNA. This study was to determine the relation between 2-5AS level and the curative effect to IFNα for chronic hepatitis C patients.
Statistical analysis was made with t test.
To 25 μL of the buffer B was added different volumes 0 μL-5 μL per sample) of digestion buffer. After reaction, the incubated mixtures were spotted onto a plate and the chromatography was developed, the digestive percentage of ATP with different volumes of digestion buffer is shown in Table 1.
| The volume of digestion buffer (μL) | 0 | 1 | 2 | 3 | 4 | 5 |
| Digestive percentage of ATP (%) | 0 | 3.1 | 18.3 | 34.1 | 100 | 100 |
The digestive ratio of ATP was analyzed according to the similar formula of the turnover percentage of ATP:
Math 2
The results of Table 1 suggested that when 4 μL of digestion buffer was added, the residual ATP in the incubation mixtures could be changed completely into ADP even if there was no 2-5AS activity in the sample. To ensure the accuracy of the assay, 5 μL of digestion buffer was added.
The results of rabbit reticulocyte lysate in the 16 determinations of a single sample for 2-5AS were 31.6 ± 0.02 (-x% ± s), with CV of 6.0%.
PBMC extracts were examined for 2-5AS in one batch; the average levels of the cells before (Group A) and after (Group B) induction by IFNα are shown in Table 2.
| Group A | Group B | |
| Before IFNα stimulation | After IFNα stimulation | |
| n | 10 | 10 |
| x (%) | 2.0 | 19.7 |
| s | 0.02 | 0.09 |
| P | < 0.01 |
In the fifteen patients, after IFNα therapy, the ALT normalization rate was 53.5% (8/15) and the ALT reduction rate ( > 50% decrease of baseline ALT) was 33.3% (5/15), with a total improvement rate (normalization plus reduction rate) of 86.6% (13/15); clearance rate of HCV-RNA was 60.6% (9/15) and of anti-HCV was 20.0% (3/15).
Before IFNα therapy, the 2-5AS average activity in PBMC of 15 patients was 12.6%, significantly higher than that of the normal group (P < 0.01); the 2-5AS levels of patients 24 h and 1 month after the first injection was 64.9% and 40.4% respectively, an increase of 7.0 and 4.7 folds of that before IFNα injection.
These 15 patients were divided into two groups. In group A (9 patients), HCV-RNA disappeared after IFNα therapy, and group B (6 patients) HCV-RNA remained detectable after treatment. Their 2-5AS levels are shown in Table 3.
Chronic viral hepatitis is one of the major infectious diseases that endangers the public health seriously and thus is an important world wide problem. At present, there is no effective medication other than interferon ( IFN ). It is known that IFN has antiviral, antiproliferative, and immunoregulating properties, and its therapeutic effect depends to some extent on the dose, schedule and route of adiministration. So the determination of the optimal dose, schedule and route of administration is an important way to improve the response of patients treated with IFN. But the concentration of IFN in peripheral blood is very difficult to determine because of its low blood concentration and short half-life. Recently, some people determined the interferon-induced enzymes (such as 2’-5’ oligoadenylate synthetase, 2’-5’ oligoadenylate phosphodiesterase and protein kinase et al) as a biological response parameter of IFN[4] therapy.
Since the assay of 2-5AS was first reported in 1979, lots of methods had been introduced[5-8]. In this study, a new nonradioactive assay of 2-5AS was developed, the results of 2-5AS in rabbit reticulocyte lysates showed that this assay was a sensitive, rapid and specific method. The assay of 2-5AS in PBMC of 10 normal persons with or without IFN induction showed the following results: (1) The 2-5AS activity of PBMC in normal person was at a quite low level (2.0%), and the individual difference was obvious (0%-7.1%, s = 0.02); (2) The 2-5AS level increased about ten-fold after stimulation of IFN (19.7%), and the difference of 2-5AS levels before and after IFN stimulation was significant (P < 0.01); (3) There was much differences in the response of IFN stimulation in different persons (9.5%-36.6%, s = 0.09) and this might be a reason to explain a variable therapeutic effect of IFN in different patients.
Now, many reports have shown the relationship between 2-5AS level and the therapeutic effect of IFN in chronic hepatitis B patients[9,10], but we have not seen any report about chronic hepatitis C. In our study, the newly developed assay of 2-5AS (PEI-cellulose thin-layer chromatography) was used to determine 2-5AS in 15 chronic hepatitis C patients treated with IFN. The results showed that: (1) The 2-5AS activity of PBMC in patients with chro-nic hepatitis C before treatment was much higher than that of normal persons ( 12.6% vs 2.0%, P < 0.01). In patients with effective treatment (HCV-RNA disappeared after IFN therapy), the 2-5AS activity before IFN therapy was lower than that in patients with ineffective treatment (HCV-RNA remained detectable after IFN therapy) (9.3% vs 17.5%, P < 0.05). This might be associated with the endogenous IFN system, which has already been activated in patients with poor response to IFN the-rapy; the use of large doses of external exogenous IFN might inhibit the activated host IFN system. (2) Twenty-four after the first injection, the 2-5AS of all 15 patients increased in different degrees, compared with its basal levels (average 7.0 fold increase), but the 2-5AS level in patients with effective treatment increased more significantly than in patients with ineffective treatment (72.1% vs 54.1%, P < 0.05; 9.4 folds vs 3.4 folds, P < 0.01). This suggested that a greater increase of 2-5AS in PBMC 24 h after the first injection of IFN might be associated with the effectiveness of IFN therapy. (3) One month after the first injection, 2-5AS level was also higher than its basel level (average 4.7 fold increase), but there was no difference between patients with good response and those without (42.3% vs 37.5%, P > 0.05; 6.2 fold vs 2.4 fold, P > 0.05).
In summary, the results of our study suggest that: (1) The developed assay in this study (PEI-cellulose thin-layer chromatography) is a reliable way to determine 2-5AS of PBMC. (2) In patients with chronic hepatitis C, a less elevated basal level of 2-5AS of PBMC before IFN therapy and a rapid and greater increase in 2-5AS 24 h after the first injection of IFN may anticipate a better response to IFN therapy.
| 1. | Ferbus D, Justesen J, Bertrand H, Thang MN. (2'-5') Oligoadenylate synthetase in the maturation of rabbit reticulocytes. Mol Cell Biochem. 1984;62:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Bruchelt G, Beck J, Schilbach-Stückle K, Koscielniak E, Treuner J, Niethammer D. Methods for the determination of the interferon-induced enzyme 2'-5' oligoadenylate synthetase in mononuclear blood cells. J Clin Chem Clin Biochem. 1987;25:879-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 3. | BORSOOK H, DEASY CL, HAAGENSMIT AJ, KEIGHLEY G, LOWY PH. Incorporation in vitro of labeled amino acids into proteins of rabbit reticulocytes. J Biol Chem. 1952;196:669-694. [PubMed] |
| 4. | Merritt JA, Borden EC, Ball LA. Measurement of 2',5'-oligoadenylate synthetase in patients receiving interferon-alpha. J Interferon Res. 1985;5:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Johnston MI, Preble OT, Imai J, Jacobsen H, Torrence PF. A sensitive immunoenzymometric assay for 2',5'-oligoadenylate. Detection of elevated 2',5'-oligoadenylate synthetase in human peripheral mononuclear cells. J Immunol Methods. 1983;65:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Merlin G, Revel M, Wallach D. The interferon-induced enzyme oligo-isoadenylate synthetase: rapid determination of its in vitro products. Anal Biochem. 1981;110:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Miele MB, Liu DK, Kan NC. Fractionation and characterization of 2',5'-oligoadenylates by polyacrylamide gel electrophoresis: an alternative method for assaying 2',5'-oligoadenylate synthetase. J Interferon Res. 1991;11:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Schattner A, Merlin G, Wallach D, Rosenberg H, Bino T, Hahn T, Levin S, Revel M. Monitoring of interferon therapy by assay of (2'--5') oligo-isoadenylate synthetase in human peripheral white blood cells. J Interferon Res. 1981;1:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Furuta M, Akashi K, Nakamura Y, Matsumoto K, Yamaguchi H, Takamatsu S, Shimizu T. 2',5'-Oligoadenylate synthetase activity in peripheral blood lymphocytes as a clinical marker in interferon therapy for chronic hepatitis B. J Interferon Res. 1987;7:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Shindo M, Okuno T, Matsumoto M, Takeda M, Takino T, Sokawa J, Iwata A, Sokawa Y. Serum 2',5'-oligoadenylate synthetase activity during interferon treatment of chronic hepatitis B. Hepatology. 1988;8:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Project supported by the grant from China Medical Board (93-582) of New York, Inc.