Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.41
Revised: September 20, 1997
Accepted: October 19, 1997
Published online: February 15, 1998
AIM: To clone mouse anti-human gastric cancer mAb (3H11) variable genes and to construct 3H11 human-mouse chimericantibody.
METHODS: The entire VH and VL genes of anti-gastric cancer mAb 3H11 were cloned by RT-PCR method from 3H11 hybridoma cells, using 5’ primers for leader sequences. The 3H11 VL gene was then inserted into human-mouse chimeric light chain expression vector and transfected into murine Sp2/0 myeloma cells.
RESULTS: DNA sequence analysis indicated that the cloned genes included the whole leader sequences and the mature Ig variable region encoding sequences. Aftergene transfection, transient expression of chimeric light chain protein was detected.
CONCLUSION: DNA sequences and transient expression indicated that the cloned gene was functional. This work laid basis for constructing 3H11 human-mouse chimeric antibody in the future.
- Citation: Li J, Wang Y, Li QX, Wang YM, Xu JJ, Dong ZW. Cloning of 3H11 mAb variable region gene and expression of 3H11 human-mouse chimeric light chain. World J Gastroenterol 1998; 4(1): 41-44
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/41.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.41
The mouse mAb 3H11 was raised against human gastric cancer cells[1,2]. But 3H11 mAbs were derived from mouse hybridoma and their inherent immunogenicity in patients precluded its long-term use. In an attempt to circumvent this problem, chimeric antibodies in which the antigen-specific variable (V) regions of the mouse antibodies were joined to the constant (C) regions of human antibodies. These molecules should be much less immunogenic and hence be more suitable for application. In order to obtain the correct VL and VH genes, we designed 5’ primers according to the leader sequences and cloned the entire VL and VH genes by RT-PCR method. The transient expression of chimeric light chain protein indicated that the cloned gene was functional in expression.
Mouse 3H11 mAb hybridoma was provided by Biochemistry Department of Beijing Institute of Cancer Research. Chimeric light chain expression vector pAG4622 was a gift from S.L.Morrison (University of California, Los Angeles); γ-32P-ATP was from Beijing Ya Hui LtD. Fmol DNA sequencing system, pGEM-T vector system, Tag enzyme and restriction enzymes were purchased from Promega. Lopofectin was from Gibco BRL.
The 5’ primers for amplification of mouse V genes were synthesized according to Coloma et al[3]. An extra VH5’ primer was added according to newly published sequences data[4] (VHL4 in Table 1). The 5’primers were designed to hybridize to partially conserved sequences in the leader regions of VH and VL. The 3’primer for VL genes was located at V-C junction of VL. The 3’primer for VH genes was located at the boundary of VH and CH1, which was effective for IgG, IgG2a, IgG2b and IgG3 subclass. Restriction sites were incorporated into the primers as shown in Table 1 (R = A/G S = C/G Y = C/T M = C/A K = T/G H = A/C/T D = G/T/A).
| 5’Primers for VL Signal peptide |
| LL1: GGGGATATCCACCATGGAGACAGACACACTCCTGCTAT |
| LL2: GGGGATATCCACCATGGATTTTCAAGTGCAGATTTTCAG |
| LL3: GGGGATATCCACCATGGAGWCACAKWCTCAGGTCTTTRTA |
| LL4: GGGGATATCCACCATGKCCCCWRCTCAGYTYCTKGT |
| LL5: GGGGATATCCACCATGAAGTTGCCTGTTAGGCTGTTG |
| 3’Primer for VK spanning CK and JK1,2&4 |
| MVK: GGATACAGTTGGTGCAGTCGACTTACGTTTKATTTCCARCTT |
| 5’Primers for VH signal peptide |
| VHL1: GGGGATATCCACCATGGRATGSAGCTGKGTMATSCTCTT |
| VHL2: GGGGATATCCACCATGRACTTCGGGYTGAGCTKGGTTTT |
| VHL3: GGGGATATCCACCATGGCTGTCTTGGGGCTGCTCTTCT |
| VHL4: GGGGATATCCACCATGATRGTGTTRAGTCTTYTGTRCCTG |
| 3’Primer for VH spanning CH1 and JH |
| MVH: GACHGATGGGGSTGTYGTGCTAGCTGNRGAGACDGTGA |
Total RNA was isolated from 107 cells of 3H11 hydridoma cells using Trizol reagent from Gibco BRL. The VL and VH genes were amplified by RT-PCR method, purified by agarose gel electrophoresis and cloned with pGEM-1 system.
The VL and VH genes were sequenced by dideoxy-mediated chain-termination method, using fmol DNA sequencing system (Promega).
The pGEM-T vector containing 3H11 VL gene was digested with EcoR V and Sal I. The 3H11 VL gene was purifed by electrophoresis and electroelution and cloned into expression vector pAG4622. Transfection of DNA into Sp2/0 cells was accomplished by two methods: Lipofectin procedure: 20 μg plasmid DNA was mixed with 40 μL lipofectin and left at room temperature for 15 min. The mixture was added into 1.6 × 107 Sp2/0 cells in 2 mL serum-free culture medium drop by drop. The cells were then cultured at 37 °C in a CO2 incubator for 20 h and 2 mL RPMI1640 containing 20% fetal bovin serum was added. After further 72 h incubation, the supernatant was collected.
Electroporation[3]: 20 μg PvuII linearized plasmid DNA was added into 1.6 × 107 Sp2/0 cells. After 10 min in ice bath, the cells were electroporated at 960 μF, 2 kV/cm by GENE PULSER (Bio-RAD). After another 10 min in ice bath, the cells were transferred into culture flask that contained 10 mL RPMI1640 containing 20% fetal bovin serum. The cells were cultured at 37 °C in a CO2 incubator for 72 h and the supernatant was collected.
The expression of chimeric light chain was determined by ELISA. Goat anti-human IgG Fab (Sigma) at 2.3 mg/L in 0.05 M borate buffer, was absorbed overnight in a 96-well plate. Either a control or supernatant samples was added and incubated for 1 h. This was followed by a secondary antibody coupled to horseradish peroxidase (HRP) (Sigma) and developed with OPD (0.5 g/L) (Sigma) and absorbance at 492 nm. Between each addition, the plate was washed with phosphate-buffered saline with 0.05% Tween. The positive control was normal human blood serum, and the negative control was the supernatant of Sp2/0.
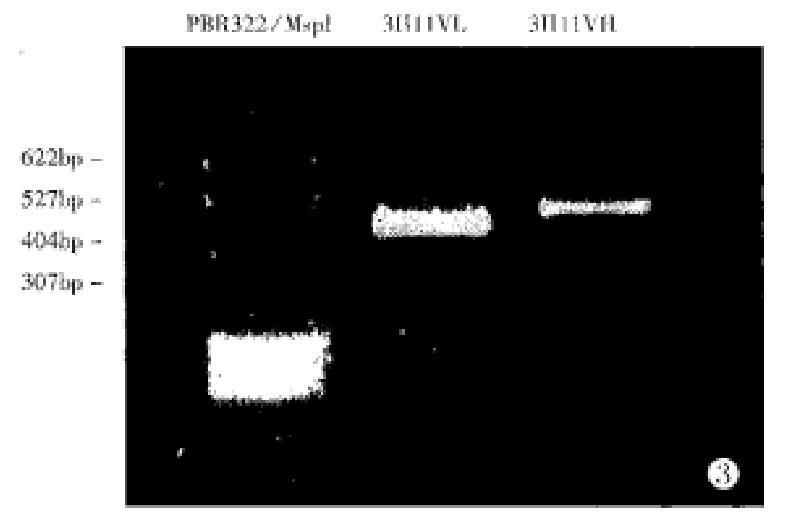
Total RNA was isolated from 3H11 cells and covered to cDNA. PCR amplification of VL and VH genes were done separately with sets of primer pairs. Agarose gelelectrophoresis showed that 3H11 VL gene was amplified with LL2 and VH gene with VHL1 (Figure 1)
The purified VL and VH PCR products were cloned into pGEM-T vector and sequenced with fmol DNA sequencing system. Comparison of the sequences with published antibody variable region data[4] indicated that the 3H11 VL gene contained a leader sequence of 57 nucleotides encoding leader peptide of 19 amino acid residues, while the 3H11 VH gene had a 66 bases long leader sequences that encoded a 22 residues leader peptide (Figure 2). The sequences of the mature VL and VH regions were the same as what we cloned previously using primers for framework region[5].
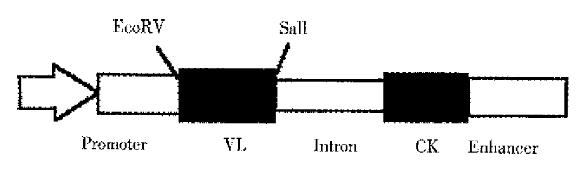
The 3H11 VL fragments in pGEM-T were digested with EcoR V and Sal I and ligated into pAG4622 vector (Figure 3). The resulting expression vector pAG4622-3H11 was verified by restriction enzyme analysis.
The expression vector pAG4622-3H11 was transfected into murine myeloma Sp2/0 cells by lipofectin or electroporation. Culture supernatant was harvested after the transfected cells were incubated at 37 °C for 72 h. Expression of chimeric 3H11 K chain was tested by ELISA. As shown in Table 2, human Ck was expressed in the culture supernatant of transfected cells indicated that the cloned gene was functional.
| ELISAsystem | Supernatant | Human Ig | Mouse Ig | ||
| 3H11 | Sp2/0 | Transfectant | |||
| Anti-human | 0.169 | 0.112 | 1.214 | 1.660 | 0.122 |
| Anti-mouse | 1.564 | 0.147 | 0.128 | 0.102 | 1.538 |
Monocloned antibodies (mAbs) are well-characte-rized highly specific reagents, and widely applied in vitro into immunochemical characterization and quantitation of antigens. They are being used clinically for both diagnosis and therapy increasingly. Their in vivo application is limited because most available mAbs are derived from mouse hybridomas, and their inherent immunogenicity in patients precludes their long-term administration. In an attempt to circumvent this problem, chimeric antibodies have been produced. Immunoglobulin V gene used to be obtained by genomic library which was tedious and time-consuming. Recent developments in PCR technology have greatly facilitated the cloning of variable region genes[6]. Two approaches have been used to design the 5’ primers. In one approach, degenerated primers for the 5’end are designed to recognize relatively conserved sequences within the 5’ end of framework 1. Because the primers are within the coding sequences of the mature antibody and the introduction of restriction sites, amino acid substitutions that change the Ag-binding specificity or the affinity of the cloned antibody may result[7]. An alternative approach takes advantage of the conservation of the leader sequences. Since the leader is not present in the mature antibody molecules, any sequence alternations introduced by the degenerated primers will not influence antibody function. The phenomenon occurred in our work, the deduced N-terminal sequence of mature 3H11 VH protein had 4 amino acid residue discrepancies between “leader primer” amplified VH gene in this work (QUQLWQS) and previous “framework 1 primer” amplified VH gene (LLELVQS). While in 3H11 VL gene there were 2 discrepant residues, QIVLT of authentic VL versus DIVMT from “framework 1 primer” amplified VL[8]. Indeed the changes had profound influence on the binding of engineered 3H11 Ab molecules to gastric cancer cells (manuscript in preparation). In order to maintain the original sequences of the C terminal of the V regions, Coloma et al[3] suggested that the V genes be first amplified with 3’primer located at constant regions. Then a second set of 3’ primers were designed according to the authentic sequences, and the V genes were PCR amplified again. Since there were only 4J segments for murine VL and VH genes respectively, we designed 3’primers for VL and VH genes that straddled the V-C junction and might at most cause one residue substitution in VL (Leu106→ Ile106 in JK5) or VH (Ser113→ Ala113) while the labour of cloning was greatly reduced.
Due to the diversity of Ab leader sequences, it would be more difficult to amplify V genes from leader by PCR[3]. There were some reports about RT-PCR method by primers for leader sequences abroad, but there is no report yet in our country. The facts that we had successfully amplified the variable genes not only of 3H11 but also of CD3 (manuscript in preparation) indicated that the primers were effective.
The amplified 3H11 VL gene was cloned into chimeric light chain expression vector and transfected into SP2/0 cells. Transient expression analysis demonstrated that the VL gene was functional, thus laid the basis for constructing 3H11 human-mouse chimeric antibody.
| 1. | Dong ZW, Wei SM, Zhang MY, Li ZF, Wan WH, Mou ZY et al. Application of the monoclonal antibody 3H11 against gastric cancer. Chin J Cancer Biother. 1995;Jun; 2:84-87. |
| 2. | Xu G, Zhang M, Liu B, Li Z, Lin B, Xu X, Jin M, Li J, Wu J, Dong Z. Radioimmunoguided surgery in gastric cancer using 131-I labeled monoclonal antibody 3H11. Semin Surg Oncol. 1994;10:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Coloma MJ, Hastings A, Wims LA, Morrison SL. Novel vectors for the expression of antibody molecules using variable regions generated by polymerase chain reaction. J Immunol Methods. 1992;152:89-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of proteins of immunological interest,5th edition. U.S. Department of Health and Human Service, Bethesda. 1991;. |
| 5. | Padlan EA. Anatomy of the antibody molecule. Mol Immunol. 1994;31:169-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 675] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 6. | Oi VT, Morrison SL. Chimeric antibodies. Biotechniques. 1986;4:214-219. |
| 7. | Orlandi R, Güssow DH, Jones PT, Winter G. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86:3833-3837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 437] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Fu YX, Ma SL, Wang Y, Yuan YH, Dong ZW. Cloning and sequence analysis of variable genes of anti-gastric cancer mAb 3H11. J Beijing Medical University. 1994;26:345-347. |
Project supported by the National 863 project of China, No. 863-102-09-01.