Published online Feb 14, 2026. doi: 10.3748/wjg.v32.i6.115941
Revised: November 19, 2025
Accepted: December 18, 2025
Published online: February 14, 2026
Processing time: 96 Days and 12.8 Hours
Filgotinib, a JAK1-preferential inhibitor, has demonstrated efficacy in pivotal clinical trials for moderate-to-severe ulcerative colitis (UC), leading to regulatory approvals. While emerging real-world studies provide short-term effectiveness data, comprehensive long-term evidence—particularly beyond 6 months—remains scarce, especially in heavily biologic-experienced populations.
To evaluate the 12-month effectiveness, safety, and predictors of response to filgotinib in a real-world cohort of patients with moderate-to-severe UC treated across multiple centers in Andalusia, Spain.
This multicenter, ambispective observational study (FILGUITO registry) included 104 adults with moderate-to-severe UC initiating filgotinib therapy. Demographic, clinical, laboratory, and endoscopic data were collected at baseline and at 8 weeks, 6 months, and 12 months. Effectiveness outcomes included clinical remission (Mayo partial score < 3 with no subscore > 1 and no rectal bleeding), clinical-biochemical remission (clinical remission plus fecal calprotectin < 250 μg/g), and steroid-free remission (clinical remission with no corticosteroid courses from week 8). Safety was assessed through adverse event monitoring.
The median age was 40.7 years (IQR: 29.5-51.0), with 56.7% male patients. The majority (79.8%) had prior advanced therapy exposure, with a median of 1 prior biologic (IQR: 1-3). Clinical remission rates were 60.8% at 8 weeks, 61.4% at 6 months, and 60.3% at 12 months (all P < 0.001 vs baseline). Clinical-biochemical remission reached 33.0%, 38.6%, and 31.0% at the same timepoints. Steroid-free remission was achieved in 60.0% at 6 months and 56.9% at 12 months. Median fecal calprotectin decreased significantly from 2000 μg/g at baseline (IQR: 755.8-2392.5) to 153 μg/g at 12 months (IQR: 21.3-569.0, P < 0.001). Treatment discontinuation occurred in 22.1% of patients by 12 months, primarily due to lack of response. Serious adverse events were rare (2.8%), with a favorable safety profile. Patients with only one prior biologic showed higher remission rates compared to those with multiple prior treatments.
Filgotinib demonstrates sustained clinical effectiveness and a favorable safety profile over 12 months in a real-world UC cohort with extensive prior biologic exposure. Effectiveness is optimized at earlier treatment lines, though meaningful benefit persists in heavily pre-treated populations.
Core Tip: This multicenter real-world study (FILGUITO) evaluated 12-month effectiveness and safety of filgotinib in 104 patients with moderate-to-severe ulcerative colitis, predominantly biologic-experienced. Clinical remission remained sustained at approximately 60% throughout follow-up, with clinical-biochemical remission reaching 38.6% at 6 months. Fecal calprotectin decreased significantly from 2000 μg/g to 153 μg/g at 12 months. Treatment discontinuation occurred in 22.1% of patients. Patients with fewer prior biologic failures showed superior outcomes. Filgotinib demonstrated sustained effectiveness and favorable safety over 12 months, with optimal benefit when used earlier in the treatment algorithm.
- Citation: Caballero-Mateos AM, Trigo-Salado C, Suárez-Toribio Á, Martín-Rodríguez MDM, Rodríguez-González FJ, Valdés-Delgado T, Pallarés-Manrique H, Trapero-Martínez AM, Olmedo-Martín R, Bailón-Gaona C, Benitez Cantero JM, Gros B, Sáez-Díaz A, Hernández-Martínez Á. Long-term clinical outcomes with filgotinib in ulcerative colitis: 12-month results from the FILGUITO study. World J Gastroenterol 2026; 32(6): 115941
- URL: https://www.wjgnet.com/1007-9327/full/v32/i6/115941.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i6.115941
The therapeutic landscape for moderate-to-severe ulcerative colitis (UC) has undergone significant evolution over the past two decades, with the introduction of biologic therapies representing a paradigm shift from conventional treatments including aminosalicylates, corticosteroids, and immunomodulators[1]. Despite advances in biologic therapy, including anti-tumor necrosis factor agents, vedolizumab, Ustekinumab and interleukin 23 inhibitors, a substantial proportion of patients with UC fail to achieve or maintain clinical remission[2]. This therapeutic challenge has driven the development of novel treatment approaches, particularly small molecule inhibitors targeting intracellular signaling pathways. JAK inhibitors represent an innovative class of oral small molecules that selectively target the JAK-STAT pathway, which plays a crucial role in cytokine signaling and immune cell activation[3]. Filgotinib, a JAK1 preferential inhibitor, was developed with the rationale of maintaining anti-inflammatory efficacy while potentially reducing safety concerns associated with broader JAK inhibition. This preference profile aims to preserve the beneficial immunomodulatory effects mediated through JAK1-dependent pathways while minimizing interference with JAK2-mediated hematopoiesis and JAK3-dependent adaptive immunity[4]. The SELECTION trial established the therapeutic potential of filgotinib in moderate-to-severe UC, leading to regulatory approvals by the European Medicines Agency, Health Canada, and the Japanese Pharmaceuticals and Medical Devices Agency, among others[5,6]. Despite the promising results from pivotal clinical trials, long term real-world evidence for filgotinib in UC remains limited, with only a handful of multicenter observational studies published to date[7]. This paucity of real-world data represents a significant knowledge gap in understanding the practical effectiveness, safety profile, and optimal positioning of filgotinib in diverse patient populations and routine clinical settings. To address this evidence gap, we established the FILGUITO registry to evaluate the effectiveness, safety, and predictors of response of filgotinib in Andalusian patients (Spain) with moderate-to-severe UC. This multicenter, observational study aims to evaluate clinical outcomes across multiple timepoints and identify factors that may guide optimal treatment management in routine clinical practice.
The FILGUITO registry is a multicenter, observational, ambispective study designed to assess the real-world effectiveness, safety, and predictors of response to filgotinib in adult patients with moderate-to-severe UC treated in routine clinical practice across twelve hospitals in Andalusia, Spain (GATEII group). This study reflects real-world treatment patterns in a geographically defined region with homogeneous healthcare protocols.
Patients included were adults (≥ 18 years) diagnosed with moderate-to-severe UC on clinical, endoscopic, and histological criteria, defined by a Mayo partial score ≥ 5 and/or the need for advanced therapy according to current clinical guidelines[2]. All eligible patients initiated filgotinib therapy according to local standard of care. Patients were included regardless of prior exposure to biologic agents or other JAK inhibitors, reflecting real-world treatment patterns. Patients were excluded if they were < 18 years old and had incomplete baseline data, less than 8 weeks of follow-up, poor treatment adherence or contraindications to filgotinib therapy according to prescribing information.
Between October 2023 and August 2025, patients treated with filgotinib were included and monitored. Demographic, clinical, biological, endoscopic, and treatment-related data were systematically collected from medical records at baseline and predefined timepoints. Data collection included baseline characteristics such as age, sex, disease duration, disease extent according to Montreal classification[8], smoking status, extraintestinal manifestations, and presence of other immune-mediated diseases. History of disease-related surgeries and previous treatments were documented including the number and type of prior biologic agents, JAK inhibitors, and conventional therapies. Clinical assessments comprised Mayo partial score and corticosteroid use patterns. Laboratory parameters included C-reactive protein (CRP), fecal calprotectin, complete blood count, cholesterol, triglycerides, lactate dehydrogenase, and liver function tests. Endoscopic findings were recorded using Mayo endoscopic score when available, and safety data encompassed adverse events, hospitalizations, and surgical interventions. Adverse events were categorized as serious (requiring permanent discontinuation, hospitalization, or resulting in death), moderate (necessitating temporary discontinuation), or mild (all others not meeting serious or moderate criteria). Follow-up assessments were performed at weeks 8, weeks 16, weeks 24, and weeks 52, with additional evaluations as clinically indicated.
Primary effectiveness outcomes were defined as clinical remission (Mayo partial score < 3 with no subscore > 1 and no rectal bleeding), clinical improvement (decrease > 1 point in Mayo partial score from baseline), and corticosteroid-free remission (Mayo partial score < 3 with no subscore > 1 and no rectal bleeding with no corticosteroid courses from week 8). Patients could be on corticosteroids at baseline; however, steroid-free remission specifically required discontinuation of systemic corticosteroids by week 8 and maintenance of this status through 12 months. Secondary effectiveness outcomes included combined clinical-biochemical remission (Mayo partial score < 3 with no subscore > 1 and no rectal bleeding and fecal calprotectin < 250 μg/g), endoscopic remission (Mayo endoscopic score < 2), endoscopic improvement (decrease ≥ 1 point in Mayo endoscopic score) and Urgency Numeric Rating Scale (NRS) as a patient-reported outcome (PRO)[9]. All adverse events were recorded and classified according to severity and relationship to study medication, with special attention paid to infections, malignancies, cardiovascular events, and laboratory abnormalities.
Study variables were described using medians and interquartile ranges for continuous variables, and frequencies and percentages for categorical variables. To assess changes over time in continuous variables, the Friedman test for related samples was used, complemented by post-hoc tests. Categorical variables were analyzed using McNemar’s test, suitable for comparing proportions in related samples. For comparisons between categorical variables, the χ2 test was applied, and the Mann-Whitney U test was used for comparisons between categorical and continuous variables. Steroid-free survival analysis was done using Kaplan-Meier. A modified non-responder imputation method was used, considering patients who discontinued treatment before the timepoint of analysis as “non-responders”, thus ensuring that calculated estimates maintained their efficacy. Statistical analyses were conducted using IBM SPSS Statistics 27.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, with protocol approval obtained from the Ethics Committee of Granada, Spain (Approval No. 1348-N-23). Given the observational nature of the study and ambispective data collection, informed consent requirements were waived according to local regulations, with patients informed through opt-out procedures where required. Patient confidentiality was maintained throughout the study with data anonymized before analysis, and all investigators adhered to applicable data protection regulations.
A total of 104 patients with moderate-to-severe UC initiating filgotinib therapy were included in the FILGUITO registry across eleven centers in Andalusia, Spain. Patient characteristics at treatment initiation are presented in Table 1.
| Characteristic | Value |
| Demographics | |
| Age (year) | 40.7 (29.5-51.0) |
| Male sex | 59 (56.7) |
| Disease duration (year) | 11.1 (5.0-14.0) |
| Disease characteristics | |
| Disease extent | |
| Extensive colitis | 54 (51.9) |
| Left-sided colitis | 39 (37.5) |
| Proctitis | 11 (10.6) |
| Smoking status | |
| Never smoker | 63 (60.6) |
| Former smoker | 29 (27.9) |
| Current smoker | 12 (11.5) |
| Extraintestinal manifestations | 32 (30.8) |
| Laboratory parameters, mean (IQR) | |
| C-reactive protein (mg/L) | 3.4 (1.1-8.9); median 5 (2-16) |
| Fecal calprotectin (μg/g) | 820 (450-1800); median 2000 (755.8-2392.5) |
| AST (U/L) | 22 (18-30) |
| ALT (U/L) | 24 (17-33) |
| Hemoglobin (g/dL) | 13.1 (11.7-14.3) |
| Leukocytes (× 103/μL) | 7.2 (5.8-9.8) |
| Neutrophils (× 103/μL) | 4.8 (3.5-7.1) |
| Platelets (× 103/μL) | 270 (230-310) |
The study population had a median age of 40.7 (IQR: 29.5-51.0) years, with 56.7% being male. The majority of patients had extensive disease (51.9%) or left-sided colitis (37.5%), and a mean disease duration of 11.1 years (IQR: 5.0-14). Approximately one-third (30.8%) presented extraintestinal manifestations. Laboratory parameters at baseline showed evidence of ongoing inflammation, with CRP levels of 3.4 mg/L (IQR: 1.1-8.9) and fecal calprotectin 820 μg/g (IQR: 450-1800). Other biochemical parameters are presented in Table 1. The mean number of prior advanced therapies was 1.9 ± 1.4, with a median of 1 (IQR: 1-3), including infliximab (74.0%), adalimumab (35.6%), vedolizumab (30.8%), ustekinumab (25.0%), and tofacitinib (16.3%). Most patients (79.8%) maintained 5-ASA therapy at filgotinib initiation. Over half (53.8%) had prior varicella-zoster virus vaccination. Concomitant corticosteroid and immunomodulator use at filgotinib start were 33.6% and 10.6%, respectively (Table 2).
| Treatment | n (%) |
| Infliximab | 77 (74.0) |
| Adalimumab | 37 (35.6) |
| Golimumab | 9 (8.7) |
| Vedolizumab | 32 (30.8) |
| Ustekinumab | 26 (25.0) |
| Tofacitinib | 17 (16.3) |
| Maintenance of 5-ASA | 83 (79.8) |
| Previous varicella zoster virus vaccination | 56 (53.8) |
| Concomitant corticosteroids | 35 (33.6) |
| Concomitant immunomodulators | 11 (10.6) |
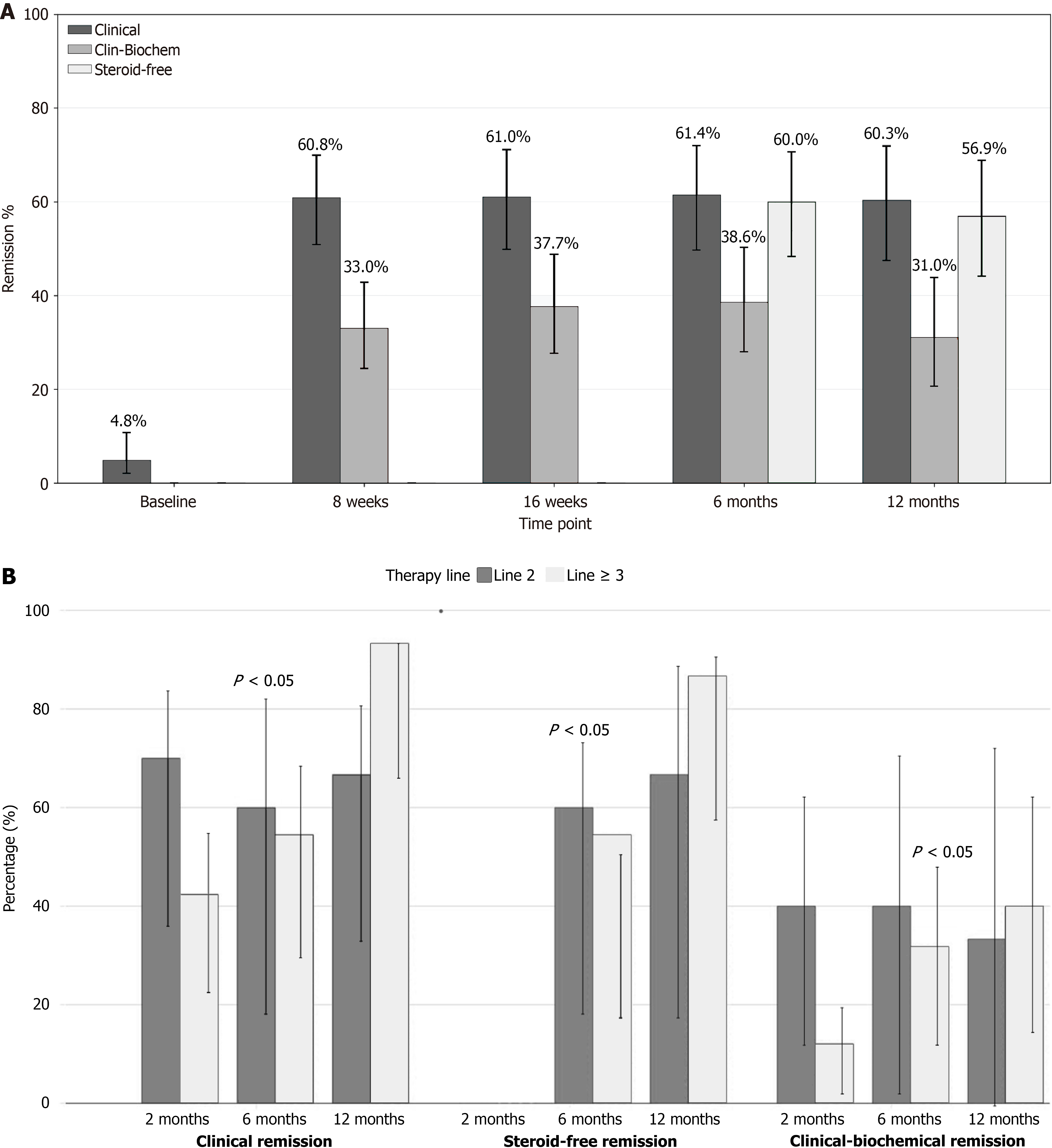
Clinical and biochemical outcomes: Effectiveness and safety results are shown in Table 3 and Figure 1A. The mean Mayo Partial Index significantly decreased from a median of 6 (IQR: 5-7) at baseline to 1 (IQR: 0.5-2.5) at 16 weeks, 2 (IQR: 1-3) at 8 weeks, 0 (IQR: 0-2.5) at 6 months, and 0 (IQR: 0-1) at 12 months (P < 0.001 for all the timepoint comparisons). Median CRP levels dropped from 5 mg/L (IQR: 2-16) to 2.2 mg/L (IQR: 1.0-6.1), 1.8 mg/L (IQR: 1-4.2), 1.7 mg/L (IQR: 1-4), and 1.0 mg/L (IQR: 0.2-3.3) at 8 weeks, 16 weeks, 6 months, and 12 months respectively (P < 0.05) for all the timepoint comparisons. Fecal calprotectin decreased from a median of 2000 μg/g (IQR: 755.8-2392.5) at baseline to 402.3 μg/g (IQR: 94.5-2000.0) at 8 weeks, 274 μg/g (IQR: 76.2-1365.3) at 16 weeks, 229.5 μg/g (IQR: 65.3-1066.3) at 6 months, and 153 μg/g (IQR: 21.3-569.0) at 12 months (P < 0.001). Clinical remission rates rose to 60.8% at 8 weeks, 61.0% at 16 weeks, 61.4% at 6 months, and 60.3% at 12 months, all showing significant improvement compared with baseline (P < 0.001). Clinical-biochemical remission increased to 33.0%, 37.6%, 38.6%, and 31.0% 8 weeks, 16 months, 6 months and 12 months, respectively (P < 0.001). Steroid-free remission was achieved in 60.0% at 6 months, and 56.9% at 12 months. Clinical improvement relative to baseline was observed in 77.9% at 8 weeks, 80.7% at 6 months, and 87.2% at 12 months. The number of patients who discontinued treatment increased over time, with 12 at 8 weeks, 19 at 6 months, and 23 at 12 months. Endoscopic reassessment was performed in 58 patients at baseline, 8 patients at 6 months, and 12 patients at 12 months. The mean Mayo endoscopic score decreased from 2.17 at baseline to 2.0 at 6 months and 1.71 at 12 months; these differences did not reach statistical significance. In terms of extraintestinal manifestations (30.8%), subjective improvement was reported by 9 out of 14 patients at 8 weeks (64.3%), 4 out of 13 at 16 weeks (30.7%), and 3 out of 8 at 12 months (37.5%). Significant reductions in the urgency scale (NRS) were observed compared with baseline values (median = 6, IQR: 3-8), with a median of 0 (IQR: 0-4) at week 24 (P = 0.003) and 0 (IQR: 0-1.5) at week 52 (P = 0.001). No statistically significant factors were identified in either univariate or multivariate analyses of 12-month steroid-free remission, except for a lower likelihood of remission associated with prior ustekinumab treatment in univariate analysis (OR = 0.3, 95%CI: 0.1-0.9, P = 0.037). This association did not persist after adjustment in multivariate analysis (Supplementary Table 1).
| Timepoint | Baseline (n = 104) | 8 weeks (n = 97) | 16 weeks (n = 77) | 6 months (n = 70) | 12 months (n = 58) | P value1 |
| Mayo Partial Index | 6 (5-7) | 2 (1-3); P < 0.0012 | 1 (0.5-2.5); P < 0.0012 | 0 (0-2.5); P < 0.0012 | 0 (0-1); P < 0.0012 | < 0.001 |
| CRP (mg/L) | 5 (2-16) | 2.2 (1.0-6.1); P = 0.0142 | 1.8 (1-4.2); P = 0.0242 | 1.7 (1-4); P = 0.0302 | 1 (0.2-3.3); P = 0.0042 | < 0.001 |
| Calprotectin (μg/g) | 2000 (755.8-2392.5) | 402.3 (94.5-2000.0); P = 0.0012 | 274 (76.2-1365.3); P < 0.001)2 | 229.5 (65.3-1066.3); P < 0.001)2 | 153 (21.3-569.0); P < 0.001)2 | < 0.001 |
| Clinical remission | - | 59 (60.8); P < 0.0013 | 47 (61.0); P < 0.0013 | 43 (61.4); P < 0.0013 | 35 (60.3); P > 0.0013 | |
| Clinical-biochemical remission | - | 32 (33.0); P < 0.0013 | 29 (37.6); P < 0.0013 | 27 (38.6); P < 0.0013 | 18 (31.0); P < 0.0013 | |
| Steroid-free remission | - | - | - | 42 (60.0); P = 0.0393 | 33 (56.9); P = 0.2113 | |
| Clinical improvement | - | 76 (77.9) | 57 (80.7) | 57 (80.7) | 51 (87.2) | |
| Urgency endoscopic scale | 6 (3-8) | 2 (0-4.8); P = 0.1183 | 0 (0-4); P = 0.0053 | 0 (0-4); P = 0.0033 | 0 (0-1.5); P = 0.0013 | < 0.001 |
| Treatment discontinuation | 12 (11.5) | 19 (18.2) | 19 (18.2) | 23 (22.1) |
When analyzing effectiveness by prior treatment exposure, both clinical remission and composite clinical-biochemical remission at 6 months were significantly higher in patients with only one previous biologic compared to those with two or more agents 89.7% vs 60.0% and 54.5% for clinical remission (P = 0.030); 55.2% vs 40.0% and 31.8% for composite remission (P = 0.038). This trend persisted at 12 months, without significant differences, though rates remained high, exceeding 85% for clinical remission in the one prior biologic group (Figure 1B).
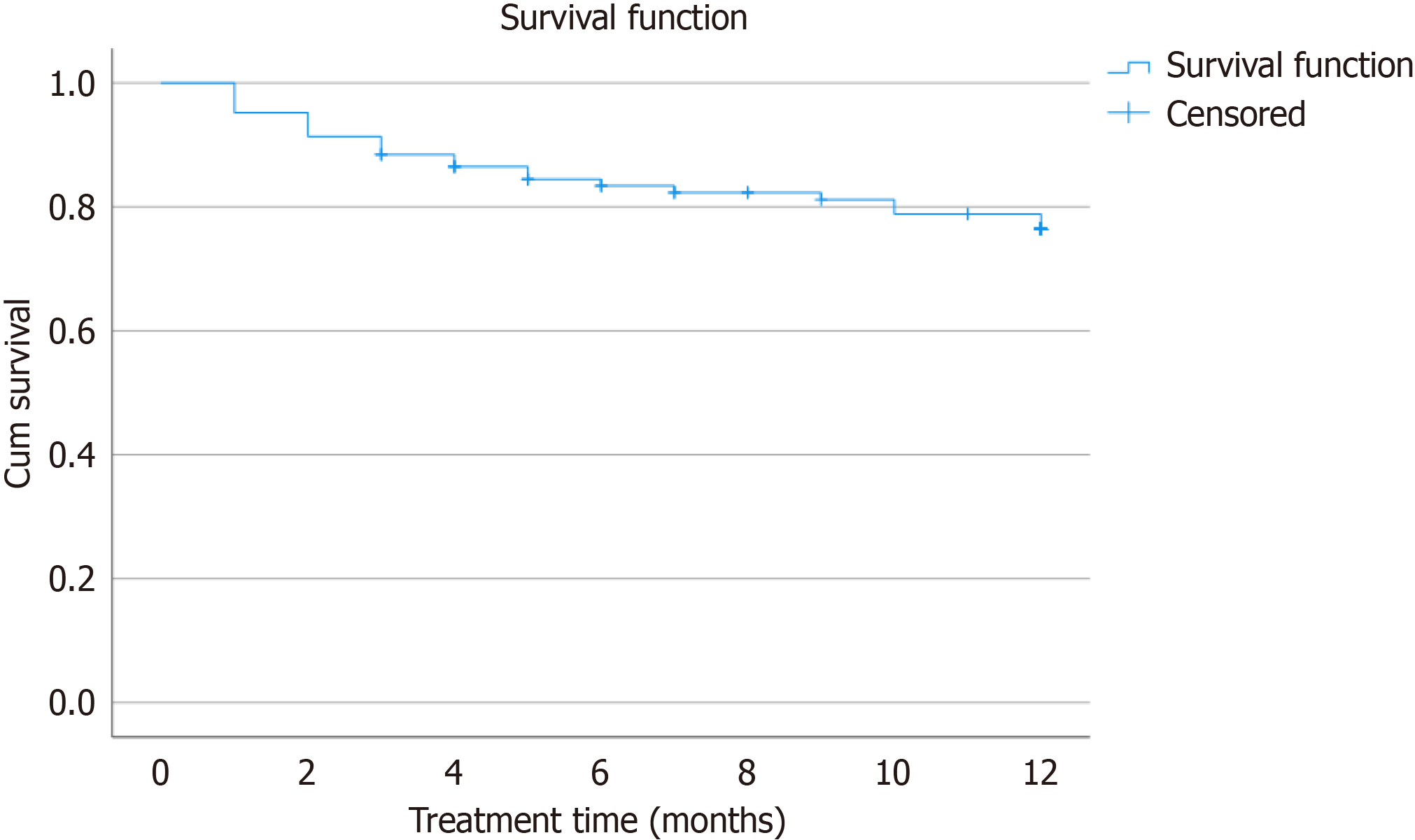
When evaluating steroid-free remission according to prior biologic treatment failure, outcomes at both 6 months and 12 months slightly favored patients who had previously failed vedolizumab, with a median of three prior failed treatments and steroid-free remission rates of 39.3% and 46.2%, respectively. Patients who had failed ustekinumab, with a median of four prior failed treatments, showed lower remission rates of 31.6% at 6 months and 35.3% at 12 months. Similarly, patients with prior failure to tofacitinib, also with a median of four failed treatments, had remission rates of 25.0% at 6 months and 35.7% at 12 months. At 12 months of follow-up, filgotinib was discontinued in 23 patients (22.1%), primarily due to lack of primary or secondary response (12 and 7, respectively; Figure 2). Adverse events leading to permanent discontinuation were infrequent and included one case each of fever, headache, and autoimmune thrombopenia. In one case, treatment was discontinued due to an unexpected pregnancy. Mild neutropenia and two cases of infection were observed but did not require treatment interruption. One patient experienced appendicitis, resulting in temporary suspension. Cholesterol levels increased from a median of 178 to 197 at one year (P < 0.001), while triglyceride levels showed no significant changes. No other serious adverse events were reported during the study period. Special attention was paid to infections, musculoskeletal and connective tissue disorders, embolism and thrombosis, and neoplasms, with no significant safety concerns identified in these categories.
This real-world study demonstrates that filgotinib achieves meaningful clinical effectiveness and maintains a favourable safety profile in patients with moderate-to-severe UC treated in a real-world setting. In our FILGUITO cohort, clinical remission rates reached 60.8% at 8 weeks and sustained at 60.3% at 12 months, accompanied by significant reductions in inflammatory biomarkers, with median fecal calprotectin decreasing from 2000 μg/g to 153 μg/g at one year (P < 0.001). These outcomes are particularly notable given that 79.8% of patients had prior exposure to advanced therapies, including 74.0% previously treated with infliximab and 30.8% with vedolizumab, representing a heavily biologic-experienced population that typically demonstrates lower response rates to subsequent treatments. Recent systematic reviews emphasize that real-world effectiveness data are essential for informing clinical decision-making, as controlled trial populations may not adequately represent the heterogeneous patient populations encountered in routine practice. Our findings contribute important evidence to this knowledge gap, demonstrating that the therapeutic benefit of filgotinib translates effectively from controlled trial settings to diverse real-world populations, including patients with complex treatment histories and those requiring rescue therapy after multiple biologic failures. In the SELECTION trial, filgotinib achieved 47.2% induction remission at week 10 in biologic-naive patients and 37.2% maintenance remission at week 58 in biologic-experienced patients[5]. The FILGUITO study achieved 60.8% clinical remission at week 8, 59.3% at week 24, and 60.3% at month 12. These rates are consistent with or marginally exceed those reported in similar real-world analyses: Nogami et al[10] observed 59.0% remission at week 24 with filgotinib; Gros et al[7] reported 61.5% at week 12 in a less-refractory United Kingdom NHS Lothian cohort; Young et al[11] documented 63.2% at week 24 in 286 United Kingdom patients; Akiyama et al[12] found 58.7% at week 24 across three JAK inhibitors in Japanese patients; Shirouzu et al[13] described 63.0% at week 12 in a Japanese multicentre study; and the ENEIDA registry in Spain found 58.7% steroid-free remission at 12 months[14]. Overall, FILGUITO’s outcomes fall squarely within the 58%-63% remission range, affirming filgotinib’s reproducible effectiveness across diverse patient populations and practice settings.
Our findings confirm the inverse relationship between prior advanced therapy exposure and filgotinib effectiveness observed in other real-world cohorts. Besides, while differences were not statistically significant, patients previously exposed to vedolizumab appeared to have somewhat higher remission compared to those failing ustekinumab or tofacitinib, even though all groups had a high median number of failed therapies[3,4]. Young et al[11] also found that prior exposure to three or four groups of advanced therapies was significantly associated with non-response at post-induction review (OR = 0.19, 95%CI: 0.07-0.52, P = 0.01). Akiyama et al[12] demonstrated that the number of previous advanced therapies was inversely associated with clinical remission for filgotinib (OR = 0.788, 95%CI: 0.607-1.009, P = 0.065) and tofacitinib (OR = 0.552, 95%CI: 0.348-0.847, P = 0.008). Nogami et al[10] observed comparable effectiveness between biologic-naïve and biologic-experienced patients for both filgotinib and upadacitinib. These patterns suggest that while filgotinib retains effectiveness in heavily pre-treated populations, incrementally higher treatment line exposure may modestly attenuate response rates, a phenomenon consistent with other JAK inhibitors and biologic agents[15]. Endoscopic assessment in FILGUITO showed a trend toward improvement, with mean Mayo endoscopic subscore decreasing from 2.17 at baseline to 1.71 at 12 months, though this did not reach statistical significance. The limited statistical power reflects real-world clinical practice constraints, where systematic endoscopic follow-up may be resource-intensive, uncomfortable for patients, and often deferred in favour of validated disease activity scores and biomarkers as pragmatic surrogates for mucosal healing. In contrast, Young et al[11] reported endoscopic improvement in 64.3% of patients at their most recent follow-up, demonstrating that endoscopic response is achievable with filgotinib when systematically assessed. Regarding extraintestinal manifestations, 30.8% of FILGUITO patients presented with these at baseline. Varying rates of subjective improvement were reported during follow-up, particularly notable early in the treatment course (64.3% at 8 weeks, and 37.5% at 12 months). Filgotinib, as a JAK inhibitor, is approved for the treatment of rheumatoid arthritis, and thus has demonstrated efficacy in this extraintestinal manifestation. However, specific evidence regarding its effect on other manifestations such as skin lesions remains limited and requires further investigation[4]. The significant and progressive reduction in bowel urgency scores observed over the treatment period highlights filgotinib's effectiveness in alleviating one of the most distressing symptoms of UC. Bowel urgency is a distinct and clinically relevant PRO that directly reflects the patient's experience of disease activity and impacts quality of life. Our findings, showing median urgency decreasing from 6 at baseline to 0 at 24 weeks and 52 weeks, indicate marked symptomatic improvement aligned with mucosal healing and clinical remission. To our knowledge, this is the first published real-world study to systematically assess bowel urgency using the urgency as a PRO in UC patients treated with filgotinib. Its inclusion emphasizes the importance of patient-centered measures in assessing treatment response and supports filgotinib's role in improving both objective and subjective disease outcomes in routine clinical practice. In terms of safety, we observed an excellent safety profile over 12 months, with treatment discontinuations in only 2.8% of patients due to isolated cases of fever, headache, autoimmune thrombocytopenia. These findings align with other real-world studies: Young et al[11] reported adverse events in 10.5% of patients with only 2.8% requiring discontinuation, while Gros et al[7] documented adverse events in 16.5% with serious events in just 2.2%. This favourable safety profile in FILGUITO is consistent with comparative analyses showing that filgotinib has fewer adverse events than other JAK inhibitors, with Nogami et al[10] reporting significantly lower adverse event rates (24.5% vs 45.7%, P = 0.005) and discontinuations (6.1% vs 20.0%, P = 0.008) compared to upadacitinib. Several limitations warrant consideration when interpreting these findings. The ambispective observational design inherently introduces potential selection bias and confounding, as treatment decisions were made according to clinical judgment rather than randomization. Channeling bias and physician selection bias may have influenced treatment allocation, potentially favoring filgotinib use in patients perceived as more likely to respond. The relatively modest sample size reduced statistical power for subgroup analyses, limiting our ability to detect significant differences in effectiveness by treatment line or specific prior advanced therapy failure. Additionally, treatment persistence bias may have influenced outcomes, as patients with multiple prior treatment failures represent a highly refractory population often maintained on available therapies despite suboptimal efficacy. Limited endoscopic follow-up data restricted comprehensive assessment of mucosal healing, a key therapeutic target in UC management. Assessment of extraintestinal manifestations relied on subjective patient-reported improvements rather than objective validated instruments, which may overestimate perceived clinical benefit in this domain. The absence of a control group precludes direct comparative effectiveness assessments against other JAK inhibitors or biologic agents. Future research should prioritize head-to-head comparative studies between JAK inhibitors and biologics, longer-term safety surveillance particularly for cardiovascular and malignancy outcomes, identification of predictive biomarkers for treatment response, and optimal sequencing strategies. An important methodological consideration for future research is the standardization of effectiveness analysis such as a modified NRI across real-world studies. The FILGUITO registry continues to generate long-term evidence on durability of response and safety, which will further inform therapeutic positioning in clinical practice.
The FILGUITO registry demonstrates that filgotinib achieves robust and sustained clinical remission rates in real-world practice, with excellent safety profiles. Effectiveness is optimized when filgotinib is positioned at earlier treatment lines, with patients exposed to fewer prior advanced therapies achieving higher remission rates, although the drug retains meaningful effectiveness even in heavily pre-treated populations with multiple biologic failures. The JAK1-preference mechanism appears to confer favorable tolerability while maintaining anti-inflammatory effectiveness, supporting filgotinib's role in the evolving UC treatment landscape.
| 1. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2710] [Article Influence: 301.1] [Reference Citation Analysis (2)] |
| 2. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1580] [Cited by in RCA: 1369] [Article Influence: 152.1] [Reference Citation Analysis (0)] |
| 3. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W; Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 644] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 4. | Caballero-Mateos AM, Cañadas-de la Fuente GA. Game changer: How Janus kinase inhibitors are reshaping the landscape of ulcerative colitis management. World J Gastroenterol. 2024;30:3942-3953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Feagan BG, Danese S, Loftus EV Jr, Vermeire S, Schreiber S, Ritter T, Fogel R, Mehta R, Nijhawan S, Kempiński R, Filip R, Hospodarskyy I, Seidler U, Seibold F, Beales ILP, Kim HJ, McNally J, Yun C, Zhao S, Liu X, Hsueh CH, Tasset C, Besuyen R, Watanabe M, Sandborn WJ, Rogler G, Hibi T, Peyrin-Biroulet L. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397:2372-2384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 323] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 6. | Feagan BG, Matsuoka K, Rogler G, Laharie D, Vermeire S, Danese S, Loftus EV Jr, Beales I, Schreiber S, Kim HJ, Faes M, de Haas A, Masior T, Rudolph C, Peyrin-Biroulet L. Long-term safety and efficacy of filgotinib for the treatment of moderately to severely active ulcerative colitis: Interim analysis from up to 4 years of follow-up in the SELECTION open-label long-term extension study. Aliment Pharmacol Ther. 2024;60:563-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Gros B, Goodall M, Plevris N, Constantine-Cooke N, Elford AT, O'Hare C, Noble C, Jones GR, Arnott ID, Lees CW. Real-world Cohort Study on the Effectiveness and Safety of Filgotinib Use in Ulcerative Colitis. J Crohns Colitis. 2025;19:jjad187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2443] [Article Influence: 122.2] [Reference Citation Analysis (2)] |
| 9. | Dubinsky MC, Irving PM, Panaccione R, Naegeli AN, Potts-Bleakman A, Arora V, Shan M, Travis S. Incorporating patient experience into drug development for ulcerative colitis: development of the Urgency Numeric Rating Scale, a patient-reported outcome measure to assess bowel urgency in adults. J Patient Rep Outcomes. 2022;6:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Nogami A, Asonuma K, Okabayashi S, Ikenouchi M, Matsuda T, Shinzaki S, Fukata M, Kobayashi T. Real-World Comparative Effectiveness and Safety of Filgotinib and Upadacitinib for Ulcerative Colitis: A Multicentre Cohort Study. United European Gastroenterol J. 2024;12:1357-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Young D, Rahmany S, Taylor D, Davis E, Colwill M, Kalyanji Mehta S, Campbell R, Hazel K, Sethi-Arora K, Ritchie S, Heinson AI, Moyses H, Bodger K, Johnston E, Hicks L, Dhar A, Limdi J, Cooney R, Seenan JP, Patel K, Walsh A, Cummings F. Real-world assessment of effectiveness and safety of filgotinib in 286 patients with ulcerative colitis in 9 UK centres. Drugs Context. 2025;14:2024-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Akiyama S, Shimizu H, Tamura A, Yokoyama K, Sakurai T, Kobayashi M, Eizuka M, Yanai S, Nomura K, Shibuya T, Takahara M, Hiraoka S, Sako M, Yoshida A, Tsuruta K, Yoshioka S, Koroku M, Omori T, Saruta M, Matsumoto T, Okamoto R, Tsuchiya K, Fujii T. Comparative Efficacy and Safety of Three Janus Kinase Inhibitors in Ulcerative Colitis: A Real-World Multicentre Study in Japan. Aliment Pharmacol Ther. 2025;61:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 13. | Shirouzu Y, Ishibashi H, Kage M, Mihara Y, Sakakibara Y, Nagata K, Suzuki A, Ohmiya T, Irie T, Araki Y, Mitsuyama K, Takedatsu H, Noake T. Efficacy of Filgotinib in Moderate to Severe Ulcerative Colitis: A Prospective Study Using Partial Mayo Score, Ulcerative Colitis Endoscopic Index of Severity, and Geboes Histopathology Score. Crohns Colitis 360. 2025;7:otaf030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Rodríguez-Lago I, Cudero L, Martínez Pascual C, Mínguez A, Ferreiro-Iglesias R, Arranz L, Royo AM, Martín-Rodríguez D, Mejías MDLÁ, Calafat M, Calvo M, Gargallo-Puyuelo CJ, Granja A, Santos-Fernández J, Ucha P, Ceballos D, Martín-Arranz MD, Ferrer I, Ramos L, Almela P, Fernández-Clotet A, García-Bosch O, Huguet JM, Lozano ML, Riestra S, Sese E, Torres G, Trapero AM, Varela P, Domènech E, Barreiro-de Acosta M. P0849 Real-world efficacy and safety of filgotinib in ulcerative colitis: results from the ENEIDA registry. J Crohns Colitis. 2025;19:i1619-i1620. [DOI] [Full Text] |
| 15. | Ikenouchi M, Fukui H, Yagi S, Nogami A, Kaku K, Sato T, Kawai M, Kamikozuru K, Yokoyama Y, Takagawa T, Tomita T, Kobayashi T, Shinzaki S. Propensity score-matched real-world comparative treatment outcomes of Janus kinase inhibitors for ulcerative colitis in patients with and without prior exposure to anti-tumor necrosis factor α antibody. Intest Res. 2025;23:464-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/