Published online Feb 28, 2025. doi: 10.3748/wjg.v31.i8.99036
Revised: October 9, 2024
Accepted: November 5, 2024
Published online: February 28, 2025
Processing time: 195 Days and 3 Hours
The peritumoral region possesses attributes that promote cancer growth and progression. However, the potential prognostic biomarkers in this region remain relatively underexplored in radiomics.
To investigate the prognostic value and importance of peritumoral radiomics in locally advanced rectal cancer (LARC).
This retrospective study included 409 patients with biopsy-confirmed LARC treated with neoadjuvant chemoradiotherapy and surgically. Patients were divided into training (n = 273) and validation (n = 136) sets. Based on intratumoral and peritumoral radiomic features extracted from pretreatment axial high-resolution small-field-of-view T2-weighted images, multivariate Cox models for progression-free survival (PFS) prediction were developed with or without clinicoradiological features and evaluated with Harrell’s concordance index (C-index), calibration curve, and decision curve analyses. Risk stratification, Kaplan-Meier analysis, and permutation feature importance analysis were performed.
The comprehensive integrated clinical-radiological-omics model (ModelICRO) integrating seven peritumoral, three intratumoral, and four clinicoradiological features achieved the highest C-indices (0.836 and 0.801 in the training and validation sets, respectively). This model showed robust calibration and better clinical net benefits, effectively distinguished high-risk from low-risk patients (PFS: 97.2% vs 67.6% and 95.4% vs 64.8% in the training and validation sets, respectively; both P < 0.001). Three most influential predictors in the comprehensive ModelICRO were, in order, a peritumoral, an intratumoral, and a clinicoradiological feature. Notably, the peritumoral model outperformed the intratumoral model (C-index: 0.754 vs 0.670; P = 0.015); peritumoral features significantly enhanced the performance of models based on clinicoradiological or intratumoral features or their combinations.
Peritumoral radiomics holds greater prognostic value than intratumoral radiomics for predicting PFS in LARC. The comprehensive model may serve as a reliable tool for better stratification and management postoperatively.
Core Tip: This study highlights the superior prognostic value of peritumoral radiomics over intratumoral radiomics in predicting progression-free survival in patients with locally advanced rectal cancer. By integrating peritumoral, intratumoral, and clinicopathological features, our comprehensive model achieved high predictive accuracy, significantly improving risk stratification for disease progression. Notably, peritumoral features emerged as the most influential predictors of pro
- Citation: Liang ZY, Yu ML, Yang H, Li HJ, Xie H, Cui CY, Zhang WJ, Luo C, Cai PQ, Lin XF, Liu KF, Xiong L, Liu LZ, Chen BY. Beyond the tumor region: Peritumoral radiomics enhances prognostic accuracy in locally advanced rectal cancer. World J Gastroenterol 2025; 31(8): 99036
- URL: https://www.wjgnet.com/1007-9327/full/v31/i8/99036.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i8.99036
Rectal cancer presents a major global health concern, with GLOBOCAN 2020 data indicating approximately 0.7 million new diagnoses and 0.34 million deaths worldwide[1]. Approximately 70% of these new cases are classified as locally advanced rectal cancer (LARC); (T3-T4 Nany or Tany N1-N2)[2]. The primary treatment for LARC involves neoadjuvant chemoradiotherapy (NCRT) followed by total mesorectal excision (TME)[3-5], often supplemented with adjuvant chemotherapy to reduce the risk of distant metastasis[6]. These treatment advancements have reduced local recurrence and distant metastasis to approximately 5%-10%[7-9] and 25%-40%[10,11], respectively; however, achieving long-term disease control remains challenging. Progression-free survival (PFS) is a comprehensive indicator that considers multiple disease events-including recurrence, distant metastasis, and death-to evaluate a patient’s condition after the initial treatment, and accurate PFS prediction is crucial for enabling more personalized and risk-adjusted postoperative treatments to improve prognosis.
Pelvic magnetic resonance imaging (MRI) is routinely used in clinical practice and is the preferred modality for dia
The peritumoral region, also known as the tumor microenvironment, has distinctive physical and immune attributes that promote cancer growth and progression[16,17]. For example, transforming growth factor beta within the peritumoral region can affect the tumor growth and metastatic potential[18] by modulating the activity and infiltration of immune cells, inflammatory cells, and cancer-associated fibroblasts. In rectal cancer, the peritumoral adipose tissue promotes cancer progression and metastasis[19-21]. In fact, previous studies have demonstrated that mesorectal fat contains important prognostic information[22-24] and radiomic features that can predict local and distant recurrence in patients with LARC[23]. However, the potential prognostic biomarkers in the peritumoral region have remained relatively un
Therefore, the present study aimed to examine the value of peritumoral radiomics from baseline MRI data, both independently and in combination with intratumoral and clinicoradiological features, in predicting PFS in patients with LARC. We also constructed a comprehensive nomogram with risk stratification to facilitate postoperative treatment decisions.
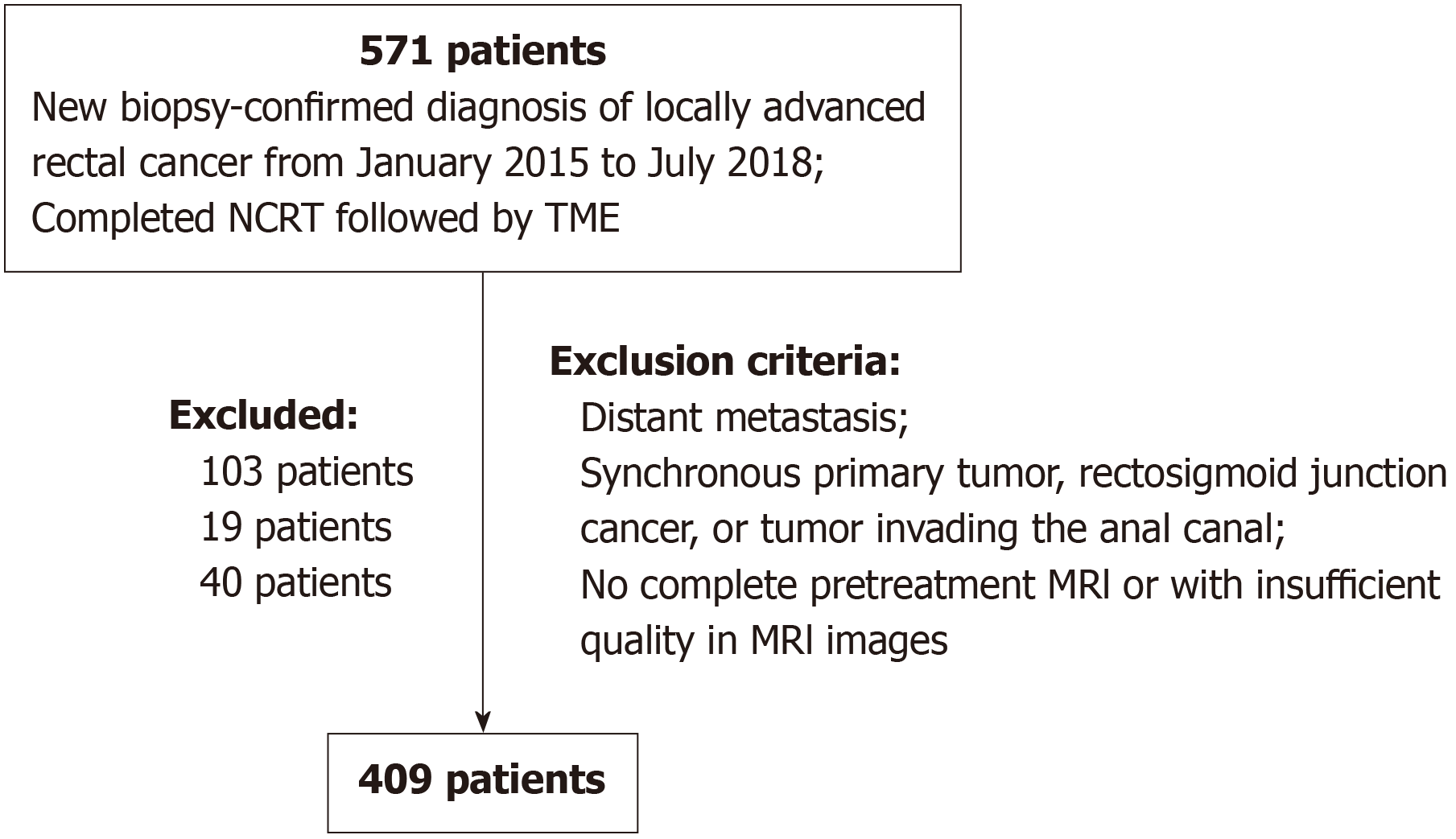
This retrospective study was conducted at a tertiary academic hospital in China. The Institutional Ethics Committee approved this study and waived the requirement for informed consent. To establish the study database, we searched the electronic medical record system for patients with a new biopsy-confirmed diagnosis of LARC who underwent NCRT and TME between January 2015 and July 2018, which yielded 571 patients. Patients were excluded based on the following criteria: Distant metastasis (103 patients); Synchronous primary tumor, rectosigmoid junction cancer, or tumor invading the anal canal (19 patients); And incomplete pretreatment MRI or insufficient image quality (40 patients). The final set included 409 patients who were randomly divided into training and validation sets at a ratio of 2:1. Figure 1 shows the flowchart of patient inclusion and exclusion criteria.
Patient characteristics were extracted from the electronic medical records, encompassing age, sex, body mass index (BMI), NCRT regimen, adjuvant chemotherapy, clinical T stage, clinical N stage, clinical overall stage, along with hematological and biochemical parameters [lymphocyte count, lymphocyte percentage, carcinoembryonic antigen (CEA) level, and carbohydrate antigen-199 level]. Pathological evaluations performed by experienced gastrointestinal cancer pathologists were also documented, including immune response in hematoxylin-eosin stained biopsy sections at diagnosis (available for 178 patients) and postoperative pathological T (ypT) stage, pathological N (ypN) stage, and tumor regression grade (TRG)[32] from surgical pathology specimens after NCRT.
All patients included in this study underwent NCRT followed by TME. Based on the oncologist’s judgement, those with high-risk pathological factors also received postoperative adjuvant chemotherapy. The NCRT regimen is detailed in the Supplementary Information.
Patients were followed up every 3 months in the first 3 years, every 6 months in the subsequent 2 years, and once a year thereafter. Follow-up visits included clinical examination (especially digital rectal examination), hematologic examination with CEA level measurement, abdominal sonography, pelvic MRI, and colonoscopy when necessary.
The endpoint of this study was PFS, which was calculated from the date of treatment initiation to the date of any local recurrence, distant metastasis, or death from any cause.
Patients underwent pelvic MRI within 2 weeks before NCRT with either 1.5-T (Siemens Aera, United States) or 3.0-T MRI scanners (Trio Tim, Siemens Healthcare; Achieva 781-278, Philips Healthcare; Discovery 750/750 W/SIGNA Pioneer, GE Healthcare). Conventional MRI examinations for rectal cancer included axial, coronal, and sagittal T2-weighted imaging (T2WI) sequences followed by diffusion-weighted and contrast-enhanced sequences. The detailed MRI protocols are listed in Supplementary Table 1.
Two radiologists (radiologists 1 and 2) with 15 and 7 years of experience in rectal cancer imaging, respectively-who were blinded to histopathological results retrospectively independently reviewed the MRI data. They mainly evaluated the following: T and N stages (according to the 8th edition of the American Joint Committee on Cancer staging manual)[33], circumferential tumor extent (proportion of the circumferential diameter occupied by the tumor invading the maximal extent of the rectal wall), tumor location (distance from the tumor to the anorectal junction), mesorectal fascia (MRF) status (tumor-MRF distance < 1 mm was defined as positive), and extramural venous invasion (EMVI) according to EMVI scoring system by Smith et al[34]. To evaluate interobserver agreement for radiologist-assessed imaging features, we calculated the intra-class correlation coefficient (ICC) for continuous variables and Cohen’s kappa for categorical variables in 40 randomly selected patients.
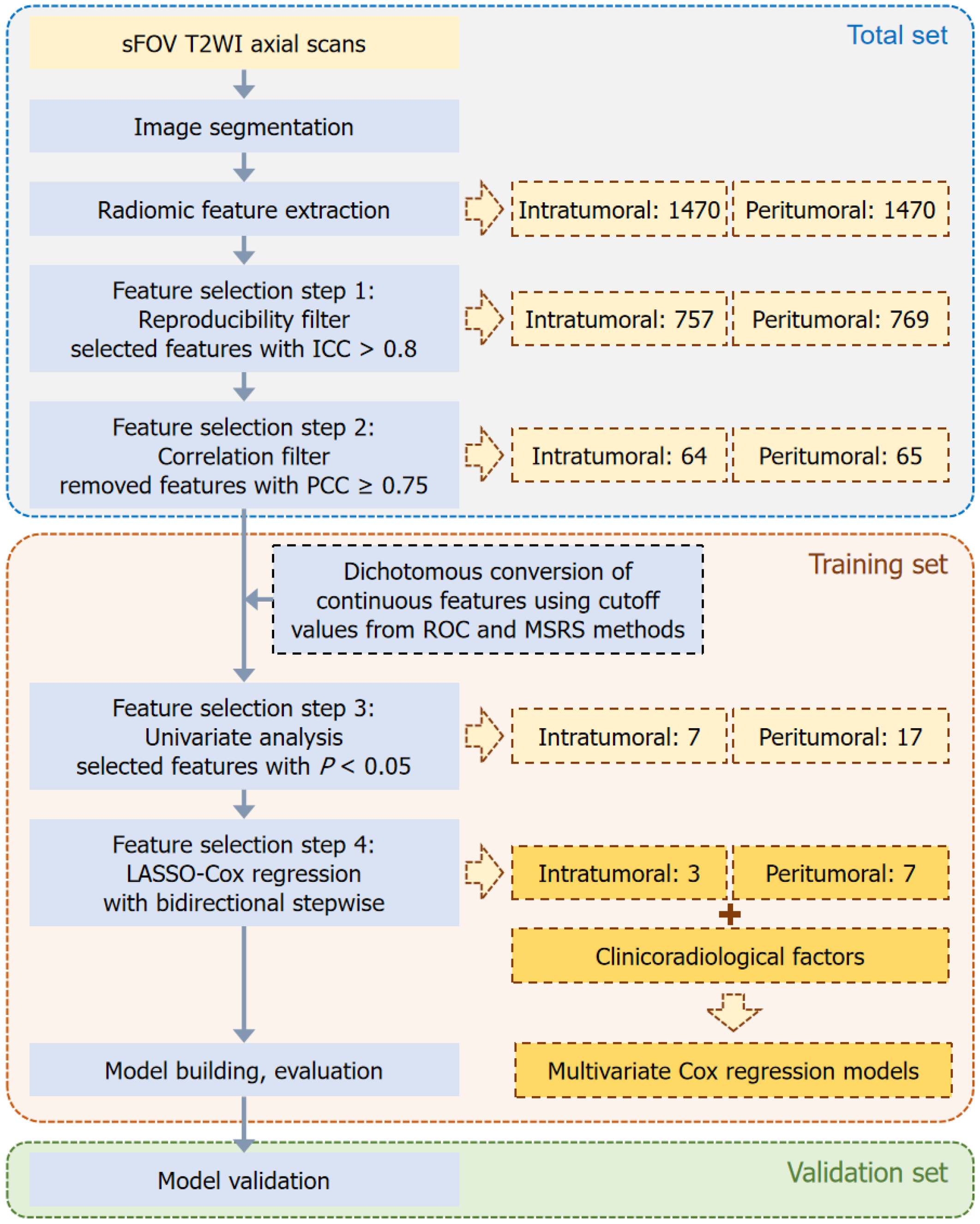
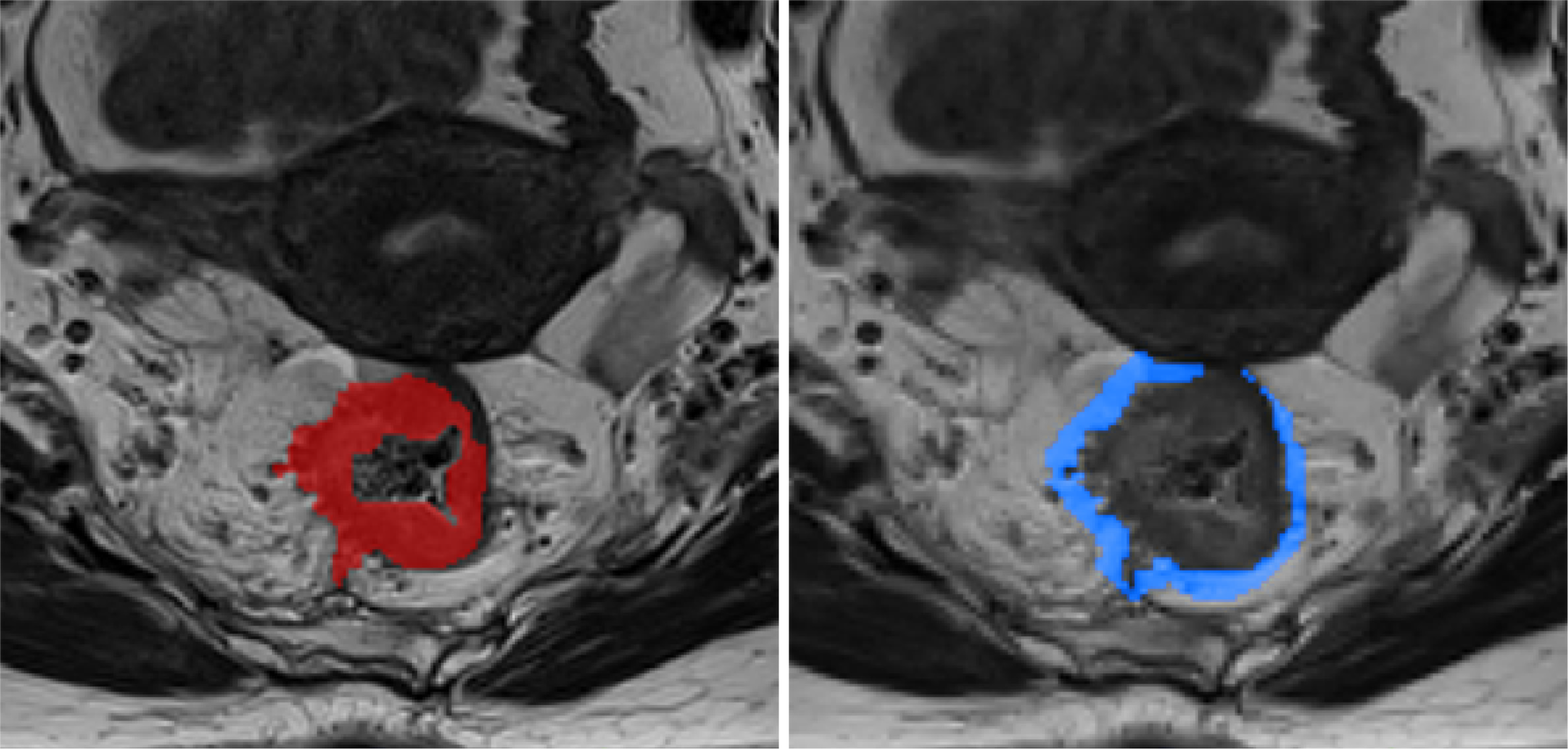
Figure 2 shows our radiomics workflow. Using the open-source software ITK-SNAP (version 3.8.0, http://www.itksnap.org), two radiologists (radiologists 3 and 4) with 4 and 3 years of experience in rectal cancer imaging-manually delineated all regions of interests (ROIs) on axial high-resolution small-field-of-view T2WI slices for three-dimensional segmentation (Figure 3). On each MRI slice, the intratumoral ROI was meticulously delineated to cover the entire rectal tumor, whereas the tube ROI was drawn to encompass both the rectal tube area and tumor. Subsequently, the peritumoral ROI was automatically generated as an overlapping region resulting from a 5-mm dilation of both the intratumoral and tube ROIs. For a subset of 57 randomly selected patients, these ROIs were independently outlined by both radiologists and the dice similarity coefficient was used to assess the accuracy and agreement of their delineations. The remaining 352 patients were randomly divided for individual ROI delineations. Any disagreements in delineation were resolved through con
Following image segmentation, we implemented image normalization by scaling intensity values between 0 and 100. This step was crucial in minimizing variations in image intensity that could arise from the use of different scanners and scanning parameters. To ensure uniformity across all images, they were resampled to a consistent resolution of 1 mm × 1 mm × 1 mm. Subsequently, a total of 1470 radiomic features for each ROI were automatically extracted using the PyRadiomics package in Python[35], including 14 shape, 18 first-order intensity statistics, 73 texture, 91 gradient, 91 exponential, 91 logarithmic, 91 square, 91 square root, 182 log, and 728 wavelet features.
We employed a multistep process to select radiomic features associated with PFS in LARC. Initially, we randomly selected 59 cases to filter features with good interobserver reproducibility (ICC ≥ 0.80). Next, we removed highly correlated features (Pearson’s correlation coefficient ≥ 0.75) and conducted a univariate Cox analysis and calculated the P value to identify features significantly associated with PFS (P < 0.05) in the training set. Notably, only a few intertumoral and peritumoral features were retained. We converted the remaining continuous features into dichotomous variables using Pearson’s correlation coefficient. This conversion was based on the optimal cutoff values determined using receiver operating characteristic curve and maximum selected rank statistic methods. Subsequently, we re-evaluated these dichotomous features using univariate analysis. Finally, we applied the least absolute shrinkage and selection operator (LASSO) with 10-fold cross-validation followed by bidirectional stepwise analysis to refine the feature selection, using the minimal λ as the optimal penalty in LASSO to automatically determine the final feature set.
We built a series of multivariate Cox models for PFS in the training set, each incorporating the following different types of features: Only clinicoradiological, only radiomic (intratumoral or peritumoral), and combined models. Significant clinicoradiological factors affecting PFS were identified using univariate and multivariate analyses.
The model performance was evaluated in terms of discrimination [Harrell’s concordance index (C-index)], calibration, and net clinical benefit. The C-index, which we considered good at approximately 0.7, was used to measure the agreement between the predicted and actual PFS rates. Similarly, calibration curves were plotted to compare the predicted and actual survival, utilizing 1000 times of iterative resampling. Furthermore, decision curve analysis (DCA) evaluated the clinical utility of different models by calculating the net benefits at different threshold probabilities. Additionally, permutation feature importance analysis was used to identify the most influential predictors in the model with the highest C-index. To further investigate the clinical relevance of the identified critical radiomic feature, we evaluated the difference in immune response across groups stratified by this feature within a subset of 178 patients with immune response assessments. Additionally, we analyzed distribution differences in lymphocyte count and percentage both within this subset and across the entire cohort of 409 patients. Finally, patients were assigned risk scores based on prognostic models and stratified into high- or low-risk groups using a cutoff score of zero, with scores above zero indicating high risk and below zero indicating low risk.
We analyzed survival differences between the high- and low-risk groups using Kaplan-Meier analysis and log-rank tests. Additionally, the χ2 and Mann-Whitney U tests were used to compare distribution differences between the training and validation sets and between groups of the most important predictor. All statistical analyses were performed using R software (version 3.2.5), with the caret package for Pearson’s correlation analysis, the survival and survminer packages for Kaplan-Meier analysis, and the glmnet package for LASSO. A two-tailed P value < 0.05 indicated statistical significance.
The final study sample included a total of 409 patients (276 men and 133 women; median age: 56 years; range: 24-81 years; interquartile range: 49-64 years). The clinicoradiological characteristics of the training (n = 273) and validation (n = 136) sets are summarized in Table 1. The Kappa values for T stage, N stage, circumferential tumor extent, MRF status, and EMVI were 0.778, 0.706, 0.760, 0.714, and 0.783, respectively, while the ICC for tumor location was 0.920. All coefficients indicated good interobserver consistency, and there were no significant differences in clinicoradiological variables between the training and validation sets (all P > 0.05), except for in the ypN stage (P = 0.027). During the follow-up period, 46 (16.8%) and 23 (16.9%) patients in the training and validation sets, respectively, experienced disease progression.
| Variable | Total (n = 409) | Training (n = 273) | Validation (n = 136) | 1P value |
| Age (years), median (IQR) | 56 (49-64) | 58 (49-64) | 56 (47-64) | 0.219 |
| Sex | > 0.999 | |||
| Male | 276 (67.5) | 184 (67.4) | 92 (67.6) | |
| Female | 133 (32.5) | 89 (32.6) | 44 (32.4) | |
| BMI | 0.351 | |||
| < 18.5 | 45 (11.0) | 31 (11.4) | 14 (10.3) | |
| 18.5-24 | 178 (43.5) | 112 (41.0) | 66 (48.5) | |
| > 24 | 186 (45.5) | 130 (47.6) | 56 (41.2) | |
| CEA level (ng/mL) | 0.625 | |||
| < 5 | 222 (54.3) | 151 (55.3) | 71 (52.2) | |
| ≥ 5 | 187 (45.7) | 122 (44.7) | 65 (47.8) | |
| CA199 level (U/mL) | 0.695 | |||
| < 35 | 282 (68.9) | 186 (68.1) | 96 (70.6) | |
| ≥ 35 | 127 (31.1) | 87 (31.9) | 40 (29.4) | |
| NCRT regimen | 0.952 | |||
| Capecitabine + RT | 173 (42.3) | 114 (41.8) | 59 (43.4) | |
| CapeOx + RT | 230 (56.2) | 155 (56.8) | 75 (55.1) | |
| Other | 6 (1.5) | 4 (1.5) | 2 (1.5) | |
| Adjuvant chemotherapy | > 0.999 | |||
| No | 104 (25.4) | 69 (25.3) | 35 (25.7) | |
| Yes | 305 (74.6) | 204 (74.7) | 101 (74.3) | |
| Clinical T stage | 0.914 | |||
| cT2 | 29 (7.1) | 20 (7.3) | 9 (6.6) | |
| cT3 | 253 (61.9) | 167 (61.2) | 86 (63.2) | |
| cT4 | 127 (31.1) | 86 (31.5) | 41 (30.1) | |
| Clinical N stage | 0.446 | |||
| cN0 | 65 (15.9) | 45 (16.5) | 20 (14.7) | |
| cN1 | 192 (46.9) | 125 (45.8) | 67 (49.3) | |
| cN2 | 99 (24.2) | 63 (23.1) | 36 (26.5) | |
| cNx | 53 (13.0) | 40 (14.7) | 13 (9.6) | |
| Overall clinical stage | 0.643 | |||
| II | 65 (15.9) | 45 (16.5) | 20 (14.7) | |
| III | 344 (84.1) | 228 (83.5) | 116 (85.3) | |
| Tumor location (cm) | 0.950 | |||
| ≤ 5 | 166 (40.6) | 111 (40.7) | 55 (40.4) | |
| 5.1-10 | 221 (54.0) | 148 (54.2) | 73 (53.7) | |
| > 10 | 22 (5.4) | 14 (5.1) | 8 (5.9) | |
| Circumferential tumor extent | 0.062 | |||
| 1/4-2/4 | 45 (11.0) | 37 (13.6) | 8 (5.9) | |
| 3/4 | 103 (25.2) | 68 (24.9) | 35 (25.7) | |
| 4/4 | 261 (63.8) | 168 (61.5) | 93 (68.4) | |
| MRF status | 0.781 | |||
| Negative | 187 (45.7) | 123 (45.1) | 64 (47.1) | |
| Positive | 222 (54.3) | 150 (54.9) | 72 (52.9) | |
| EMVI | 0.070 | |||
| Negative | 274 (67.0) | 191 (70.0) | 83 (61.0) | |
| Positive | 135 (33.0) | 82 (30.0) | 53 (39.0) | |
| ypT stage | 0.644 | |||
| ypT 0-2 | 228 (55.7) | 150 (54.9) | 78 (57.4) | |
| ypT 3-4 | 181 (44.3) | 123 (45.1) | 58 (42.6) | |
| ypN stage | 0.027a | |||
| ypN0 | 331 (80.9) | 231 (84.6) | 100 (73.5) | |
| ypN1 | 67 (16.4) | 36 (13.2) | 31 (22.8) | |
| ypN2 | 11 (2.7) | 6 (2.2) | 5 (3.7) | |
| TRG | 0.246 | |||
| 1-2 | 218 (53.3) | 140 (51.3) | 78 (57.4) | |
| 3-5 | 191 (46.7) | 133 (48.7) | 58 (42.6) |
Table 2 summarizes the results of univariate and multivariate analyses. In the training set, BMI, MRF status, ypT stage, ypN stage, and TRG were significantly associated with PFS (all P < 0.05). Additionally, a BMI of 18.5-24 or > 24 was a significant independent predictor of PFS compared with a BMI < 18.5 [both hazard ratio (HR) < 0.50, P < 0.05]. Similarly, compared with ypN stage 0, ypN stage of 1 or 2 was a significant negative independent predictor of PFS (both HR > 2; P < 0.05).
| Variable | Training | Univariate analysis | Multivariate analysis | ||
| 3-year PFS (%) | 1P value | HR (95%CI)2 | 2P value | ||
| Age (years) | 273 | 0.518 | |||
| Sex | 0.424 | ||||
| Male | 184 | 86.23 | |||
| Female | 89 | 83.09 | |||
| BMI | 0.013a | ||||
| < 18.5 | 31 | 67.59 | 1 (Ref.) | ||
| 18.5-24 | 112 | 86.39 | 0.44 (0.20-0.98) | 0.045a | |
| > 24 | 130 | 88.40 | 0.40 (0.18-0.91) | 0.029a | |
| CEA level (ng/mL) | 0.312 | ||||
| < 5 | 151 | 87.38 | |||
| ≥ 5 | 122 | 82.51 | |||
| CA199 level (U/mL) | 0.112 | ||||
| < 35 | 186 | 87.54 | |||
| ≥ 35 | 87 | 80.19 | |||
| NCRT regimen | |||||
| Capecitabine + RT | 114 | 83.32 | 0.667 | ||
| CapeOx + RT | 155 | 86.89 | |||
| Other | 4 | 75.00 | |||
| Adjuvant chemotherapy | 0.936 | ||||
| No | 69 | 85.31 | |||
| Yes | 204 | 85.19 | |||
| Clinical T stage | 0.698 | ||||
| cT2 | 20 | 90.00 | |||
| cT3 | 167 | 83.10 | |||
| cT4 | 86 | 88.24 | |||
| Clinical N stage | 0.776 | ||||
| cN0 | 45 | 88.72 | |||
| cN1 | 125 | 84.66 | |||
| cN2 | 63 | 84.04 | |||
| cNx | 40 | 84.71 | |||
| Overall clinical stage | 0.323 | ||||
| II | 45 | 88.72 | |||
| III | 228 | 84.52 | |||
| Tumor location (cm) | 0.535 | ||||
| ≤ 5 | 111 | 85.40 | |||
| 5.1-10 | 148 | 84.36 | |||
| > 10 | 14 | 92.86 | |||
| Circumferential tumor extent | 0.810 | ||||
| 1/4-2/4 | 37 | 86.15 | |||
| 3/4 | 68 | 86.74 | |||
| 4/4 | 168 | 84.37 | |||
| MRF status | 0.011a | ||||
| Negative | 123 | 90.12 | 1 (Ref.) | ||
| Positive | 150 | 81.19 | 1.71 (0.86-3.42) | 0.129a | |
| EMVI | 0.667 | ||||
| Negative | 191 | 85.75 | |||
| Positive | 82 | 84.00 | |||
| ypT stage | 0.005a | ||||
| ypT0-2 | 150 | 89.88 | 1 (Ref.) | ||
| ypT3-4 | 123 | 79.53 | 1.25 (0.59-2.63) | 0.561 | |
| ypN stage | < 0.001a | ||||
| ypN0 | 231 | 88.62 | 1 (Ref.) | ||
| ypN1 | 36 | 69.44 | 2.45 (1.18-5.07) | 0.016a | |
| ypN2 | 6 | 50.00 | 3.88 (1.28-11.79) | 0.017a | |
| TRG | 0.006a | ||||
| 1-2 | 140 | 89.87 | 1 (Ref.) | ||
| 3-5 | 133 | 80.30 | 1.71 (0.83–3.53) | 0.145 | |
We constructed two clinicoradiological models as base models for considering the use of postoperative adjuvant chemotherapy in clinical practice: Model based on the ypT stage and ypN stage (ModelypTN), and model based on BMI, ypN stage, TRG, and MRF status (Modelclin). Notably, Modelclin exhibited higher C-indices than ModelypTN in both the training (0.718 vs 0.670; P = 0.003) and validation sets (0.733 vs 0.670; P = 0.009), indicating better predictive accuracy for PFS (Table 3 and Supplementary Table 2).
| Model | Training set | Validation set | ||||
| C-index (95%CI) | 1P value | 2P value | C-index (95%CI) | 1P value | 2P value | |
| Clinicoradiological model | ||||||
| YpTN | 0.670 (0.593-0.748) | Ref. | 0.003 | 0.670 (0.558-0.781) | Ref. | 0.009 |
| Clin | 0.718 (0.639-0.797) | 0.003 | Ref. | 0.733 (0.634-0.833) | 0.009 | Ref. |
| Radiomics model | ||||||
| Intra | 0.670 (0.603-0.736) | Ref. | 0.297 | 0.669 (0.574-0.764) | Ref. | 0.310 |
| Peri | 0.754 (0.680-0.827) | 0.015 | 0.194 | 0.703 (0.597-0.809) | 0.316 | 0.839 |
| Omics | 0.797 (0.741-0.853) | < 0.001 | < 0.001 | 0.758 (0.670-0.847) | < 0.001 | 0.101 |
| Clinicoradiological-omics model | ||||||
| Clin + intra | 0.764 (0.697-0.832) | Ref. | < 0.001 | 0.765 (0.677-0.853) | Ref. | 0.030 |
| Clin + peri | 0.804 (0.740-0.868) | 0.044 | < 0.001 | 0.778 (0.685-0.871) | 0.432 | 0.010 |
| ICRO | 0.836 (0.787-0.886) | < 0.001 | < 0.001 | 0.801 (0.722-0.879) | 0.001 | < 0.001 |
We found that dice similarity coefficient values for the tumor and tube ROIs were 0.74 and 0.83, respectively, showing good agreement between the two radiologists regarding their delineations. Figure 2 shows the process of the selection of radiomic features. Of the 2940 radiomic features extracted per patient, 1526 features (757 intratumoral and 769 peritumoral) showed high reproducibility (ICC ≥ 0.80) (Supplementary Figure 1). After removing features with Pearson’s correlation coefficient ≥ 0.75, 64 intratumoral and 65 peritumoral features remained. Following univariate analysis and stepwise LASSO-Cox regression (Supplementary Figure 2), three intratumoral and seven peritumoral features were ultimately selected to construct the radiomics models: Intratumoral (Modelintra), peritumoral (Modelperi), and combined intraperitumoral (Modelomics). Notably, Modelperi outperformed Modelintra in predicting PFS (C-index: 0.754 vs 0.670 in the training set, P = 0.015; and 0.703 vs 0.669 in the validation set, P = 0.316). Furthermore, Modelomics, which combines both intratumoral and peritumoral radiomic features, demonstrated the highest discriminative ability for predicting PFS, si
To further validate the added value of peritumoral radiomic features in predicting PFS, we incorporated these features into Modelclin and Modelclin + intra, constructing the new models Modelclin + peri and ModelICRO (integrated clinical-radiological-omics model), respectively. Modelclin + peri significantly improved the C-index, increasing from 0.718 to 0.804 in the training cohort (P < 0.001) and from 0.733 to 0.778 in the validation cohort (P = 0.01). Additionally, ModelICRO demonstrated the highest discriminative capacity, significantly outperforming Modelclin + intra in the training cohort (C-index: 0.836 vs 0.764; P < 0.001) and in the validation cohort (C-index: 0.801 vs 0.765; P = 0.001) (Table 3 and Supplementary Table 2).
Table 3 summarizes C-indices for all models predicting PFS, whereas Supplementary Table 2 shows comparisons of C-indices between models, providing a detailed overview of models’ discriminative performances. The variable parameters of all models are detailed in Table 4 and Supplementary Table 3. Notably, the Modelclin, Modelomics, and ModelICRO achieved the highest C-indices within their respective groups.
| Variable | β | HR (95%CI) | P value | Relative importance weight 1 | Relative importance weight 2 |
| Intratumoral radiomic score1 | 0.936 | 2.55 (1.43-4.54) | 0.001 | 0.3510 | |
| Log.sigma50.mm.3D GLDM DependenceVariance | 1.267 | 0.28 (0.10-0.79) | 0.016 | 0.6047 | |
| Wavelet.HHL_firstorder_RootMeanSquared | 0.621 | 1.86 (0.95-3.64) | 0.069 | 0.0388 | |
| Wavelet.LHL_GLSZM_LargeAreaLowGrayLevelEmphasis | 1.024 | 0.36 (0.12-1.04) | 0.059 | 0.9806 | |
| Peritumoral radiomic score1 | 0.948 | 2.58 (1.90-3.51) | < 0.001 | 1.0000 | |
| Wavelet.LLH_GLCM_ClusterShade | 0.007 | 1.01 (1.00-1.01) | 0.008 | 0.5858 | |
| Wavelet.LLL_GLSZM_LargeAreaLowGrayLevelEmphasis | 0.016 | 1.02 (1.01-1.03) | 0.002 | 0.1473 | |
| Log.sigma30.mm.3D_firstorder_90Percentile | 0.654 | 1.92 (0.93-3.99) | 0.080 | 0.5038 | |
| Wavelet.LHL_GLCM_Correlation | 0.878 | 0.42 (0.22-0.78) | 0.007 | 0.0226 | |
| Wavelet.HLH_firstorder_Kurtosis | 0.889 | 0.41 (0.22-0.77) | 0.005 | 0.3817 | |
| Wavelet.HHH_NGTDM_Contrast | 0.774 | 2.17 (1.02-4.62) | 0.045 | 0.0430 | |
| Square_GLDM_DependenceVariance | 0.865 | 2.37 (1.21-4.65) | 0.012 | 1.0000 | |
| BMI | 0.0943 | 0.5510 | |||
| < 18.5 | 1 (Ref.) | ||||
| 18.5-24 | 0.337 | 0.71 (0.32-1.62) | 0.418 | ||
| > 24 | 0.673 | 0.51 (0.22-1.17) | 0.111 | ||
| MRF status | -0.0867 | -0.3670 | |||
| Negative | 1 (Ref.) | ||||
| Positive | 0.332 | 1.39 (0.69-2.82) | 0.355 | ||
| ypN stage | -0.0510 | -0.0208 | |||
| ypN0 | 1 (Ref.) | ||||
| ypN1 | 0.857 | 2.36 (1.14-4.87) | 0.021 | ||
| ypN2 | 0.958 | 2.61 (0.85-7.97) | 0.093 | ||
| TRG | 0.1019 | 0.8614 | |||
| 1-2 | 1 (Ref.) | ||||
| 3-5 | 0.691 | 2.00 (1.03-3.87) | 0.040 |
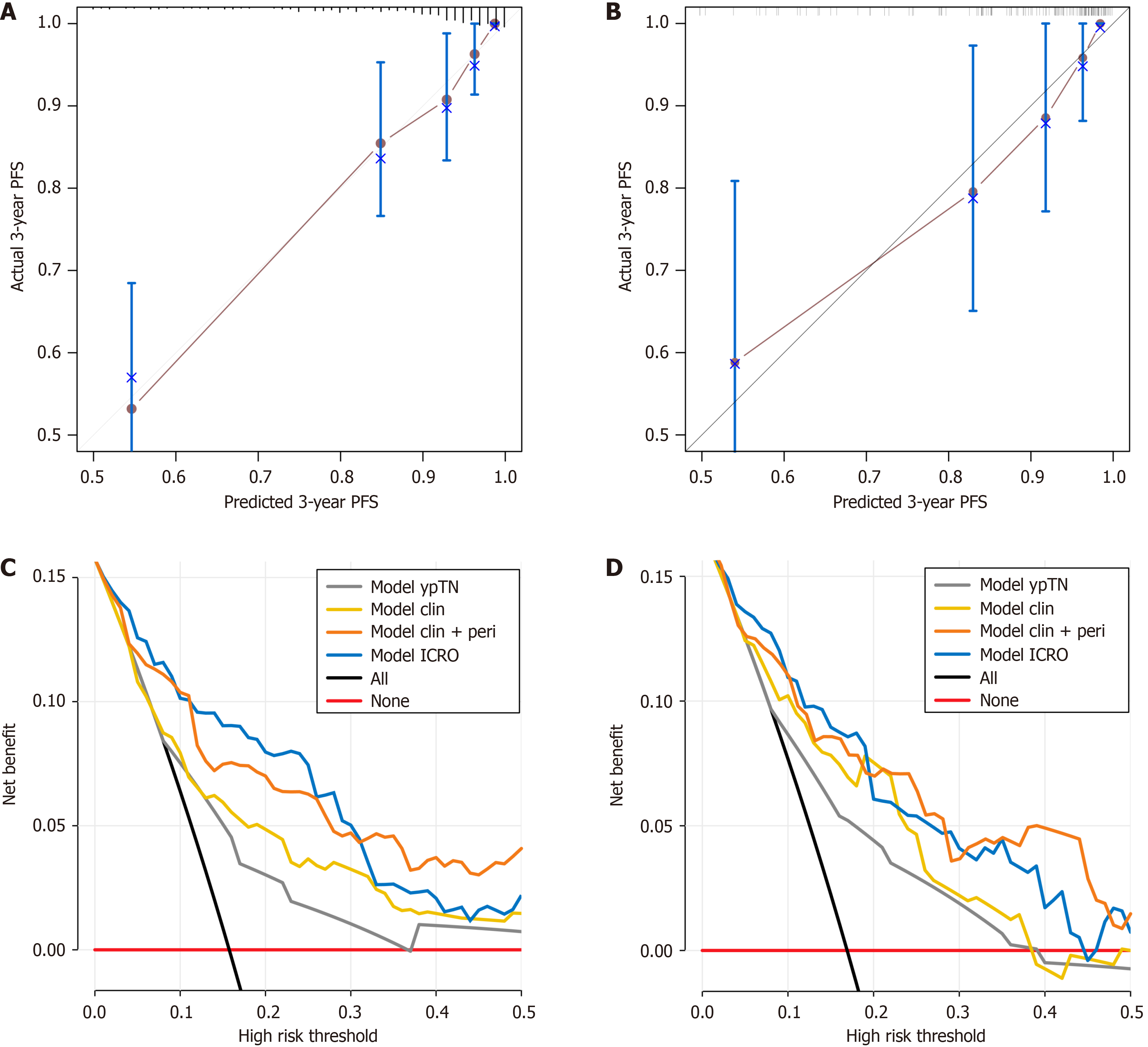
Furthermore, the calibration curves indicated that the ModelICRO more accurately predicted the 3-year PFS than the clinicoradiological (Modelclin) and intraperitumoral radiomics (Modelomics) models, as evidenced by better agreement with the observed PFS in both the training and validation sets (Figure 4A and B, Supplementary Figure 3).
DCA demonstrated that the inclusion of peritumoral radiomic features improved net benefit for Modelclin + peri, Modelomics, and ModelICRO compared to Modelclin, Modelintra, and Modelclin + intra (Figure 4C and D, Supplementary Figure 4). Both Modelclin + peri and ModelICRO showed greater clinical utility than the conventional pathological staging model (ModelypTN) and the enhanced clinicoradiological model (Modelclin). ModelICRO provided the highest net benefit up to a risk threshold of 0.31 in the training set and 0.20 in the validation set. However, since the majority of patients had risks below these thresholds-even those with high-risk factors, such as positive MRF status, had a progression risk of less than 0.2 (Table 2) ModelICRO remained the optimal predictive model for most clinical scenarios.
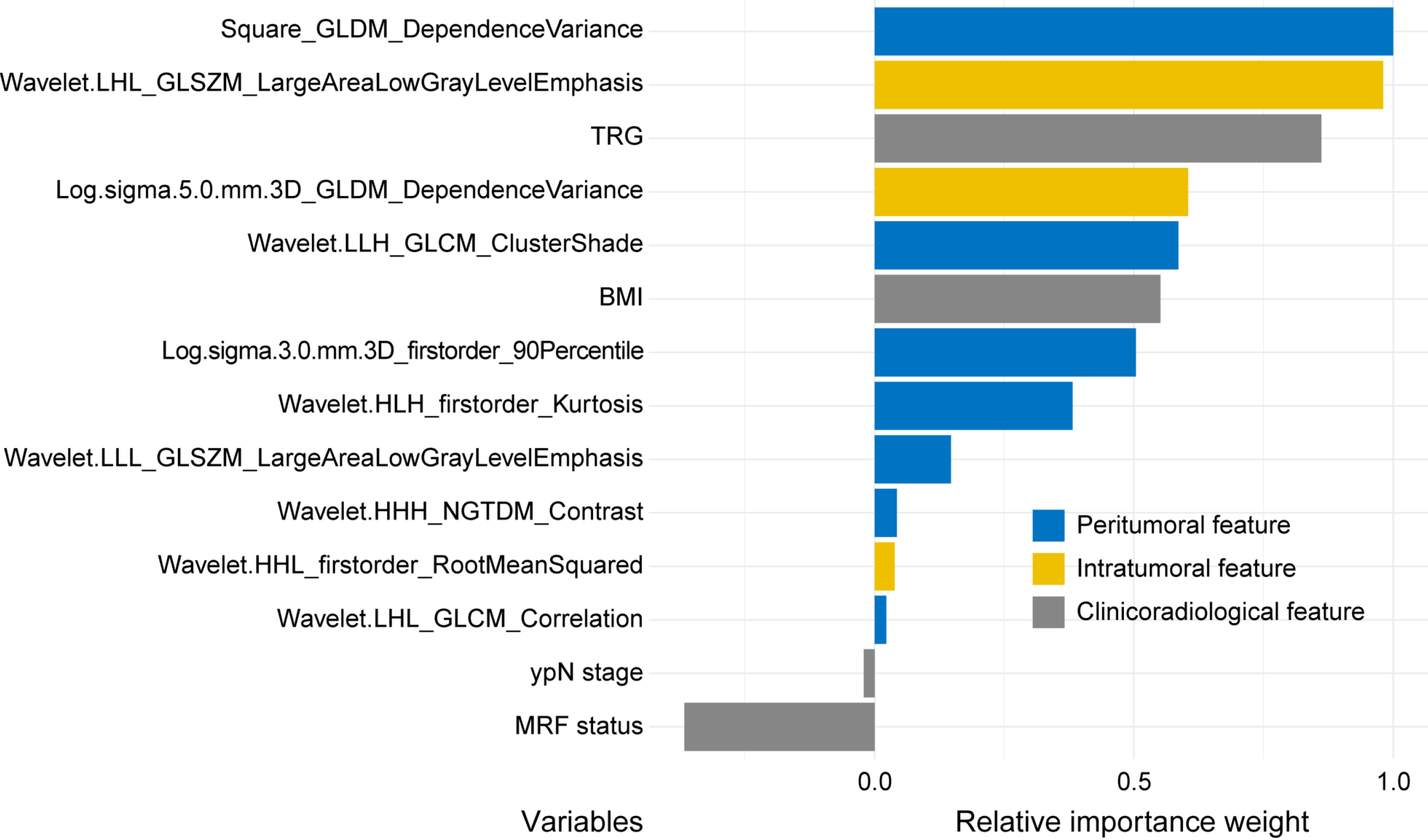
We used permutation feature importance analysis within the clinicoradiological-omics model (ModelICRO) to quantify the relative importance weight of each variable in predicting PFS (Table 4). Remarkably, the most influential predictors were a peritumoral feature [square-grey level dependence matrix (GLDM)-dependence variance], followed by intratu
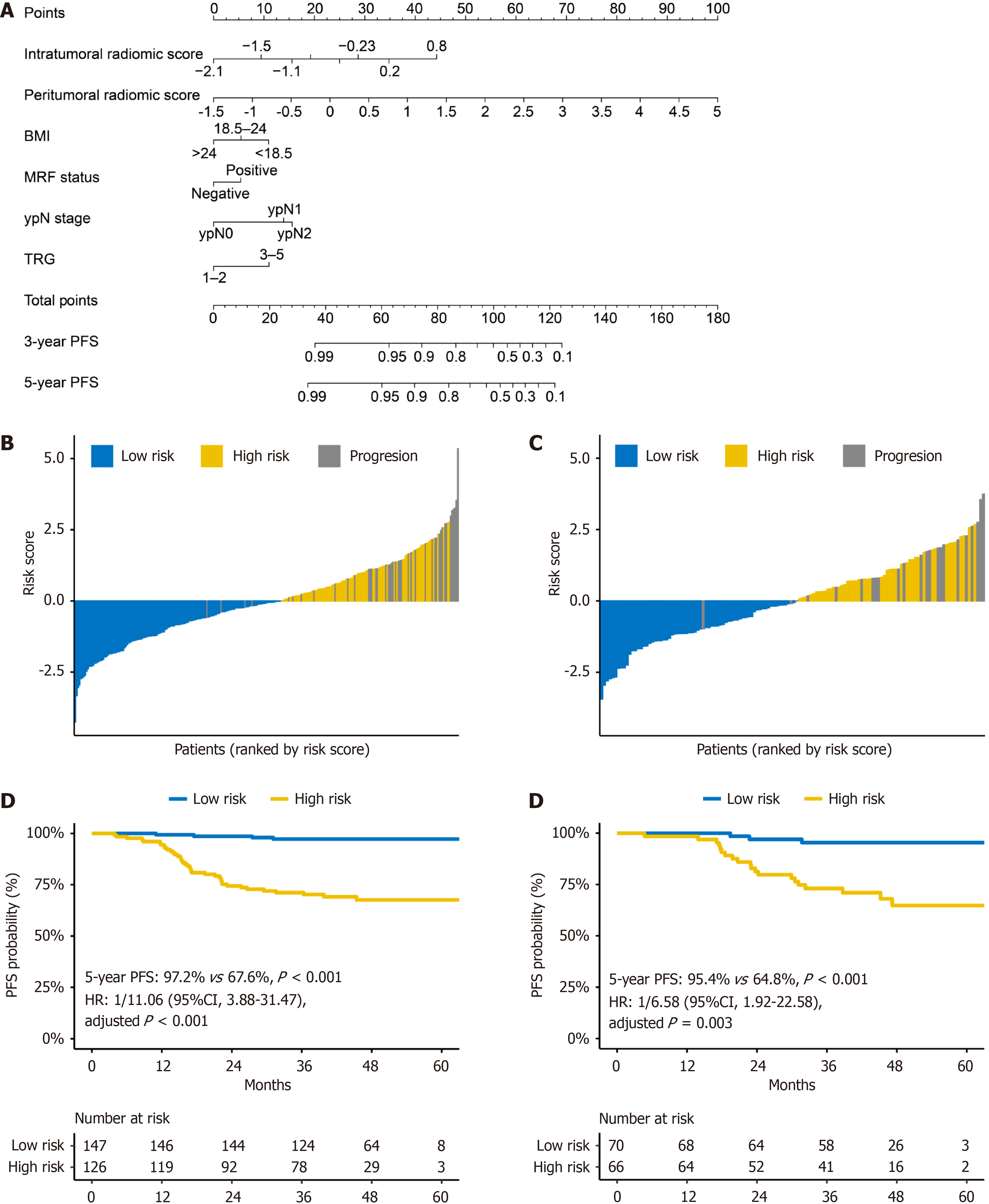
Patients were stratified into high- or low-risk groups using different models, with a cutoff risk score of zero. For more convenient use in clinical settings, we plotted a nomogram for the clinicoradiological omics model with the highest performance (ModelICRO) (Figure 6A). In the nomogram, the peritumoral radiomic score exhibits a much larger point range compared to other important prognostic factors, indicating its additional contribution to the predictive accuracy of the ModelICRO for PFS. As shown in Figure 6B and C, most patients with disease progression within 3 years were in the high-risk group. Notably, Kaplan-Meier analysis (Figure 6D and E) showed that the high-risk group had a significantly worse 3-year PFS than the low-risk group in both the training [3-year PFS: 67.6% vs 97.2%; HR = 11.06; 95% confidence interval (CI): 3.88-31.47; P < 0.001] and validation (3-year PFS: 64.8% vs 95.4%; HR = 6.58; 95%CI: 1.92-22.58; P = 0.003) sets.
The Modelclin and Modelomics models also effectively stratified patients by risk (Supplementary Figure 5), demonstrating survival outcomes similar to those observed in ModelICRO. Survival analysis (Supplementary Figure 6) corroborated the efficacy of risk-based patient stratification based on these two models. In contrast, the pathological staging model (ModelypTN) was less effective in properly stratifying patients into high- and low-risk groups in both the training and validation sets (both P > 0.05) (Supplementary Figure 7).
In this study, we successfully developed and validated a comprehensive model (ModelICRO) to estimate PFS in patients with LARC by incorporating clinicoradiological, intratumoral, and peritumoral radiomic features. This model demon
Accurate prognostic prediction based on available medical data, including clinical, pathological, and imaging data is critical when personalizing medicine for postoperative patients with LARC. Previous research has demonstrated the role of intratumoral radiomics in prognostic prediction[36] and the ability of intratumoral features to reflect biological characteristics within the tumor, such as the degree of differentiation, degree of malignancy, and pathological type. In addition, the peritumoral region also referred to as the tumor microenvironment contains significant prognostic information[16,37,38] and is often undetectable through conventional visual MRI assessments by radiologists. Radiomics can effectively analyze these subtle peritumoral differences, extract a comprehensive set of features for a more thorough evaluation, and reveal important information regarding rectal cancer prognoses. In fact, previous studies have shown that MRI-based peritumoral radiomics is effective in predicting tumor deposits[39], lymphovascular invasion[40], nodal metastasis[41], and treatment response[42] in rectal cancer. However, to the best of our knowledge, the potential value of peritumoral radiomics in predicting PFS has not been comprehensively investigated.
In our study, which included a substantial sample of 409 patients, the peritumoral radiomics model demonstrated superior discriminative performance compared to the intratumoral radiomics model in predicting PFS for patients with LARC. In the training set, the C-indices were 0.754 for the peritumoral model and 0.670 for the intratumoral model (P = 0.015). In the validation set, although the C-index of 0.703 for the peritumoral model did not significantly outperform the intratumoral model’s C-index of 0.669 (P = 0.316), the validation results indicate that the peritumoral model is at least not inferior to the intratumoral model. These results suggest that peritumoral radiomic features hold potentially more significant prognostic value than intratumoral features, reinforcing the importance of incorporating peritumoral radiomic features in prognostic assessments for rectal cancer. This finding aligns with that of other studies in which peritumoral radiomics models surpassed intratumoral models in predicting various prognoses, including recurrence-free survival in a cohort of 346 patients (C-index of peritumoral vs intratumoral radiomics models in the validation set: 0.68 vs 0.64)[26], disease-free survival (DFS) in 209 patients (0.790 vs 0.779)[43], and distance metastasis-free survival in 230 patients (0.703 vs 0.687)[25].
To compare the importance of intratumoral, peritumoral radiomic features, and clinicoradiological features within the model, we conducted a permutation feature importance analysis. The results further emphasized the significance of peritumoral features, which are related to the tumor microenvironment, in predicting PFS in LARC. When ranking the variable importance of peritumoral radiomic score, intratumoral radiomic score, and clinicoradiological features within the ModelICRO, the peritumoral radiomic score emerged as more crucial than the intratumoral score and clinicoradiological features. Notably, further exploration of each variable's importance within this model highlighted that a peritumoral radiomics feature was the most significant predictor, followed by an intratumoral feature, both surpassing the TRG. This underscores the critical importance of integrating peritumoral and intratumoral radiomics characteristics. The top predictor was square GLDM dependence variance, a peritumoral feature that assesses the variation in grey levels and dependency sizes within the peritumoral ROI, with a higher value indicating increased differences in dependencies and a more heterogeneous texture[35]. Clinically, this may suggest greater heterogeneity in the peritumoral region, which could be associated with more aggressive tumor behavior and poorer prognosis, emphasizing the need for careful monitoring for patients exhibiting this characteristic.
Significant distribution differences, as observed simultaneously in biopsy immune response and peripheral lym
In our study, combining peritumoral with intratumoral radiomic features, rather than using intratumoral features alone, significantly enhanced the predictive performance for PFS in LARC. This improvement can be attributed to the comprehensive reflection of heterogeneity within and around the tumor provided by these combined radiomic features. Our model, which utilized only a single T2WI sequence, achieved a C-index of 0.758 for PFS in the validation set. These findings suggest that even with a single-sequence T2WI-based model, incorporating both peritumoral and intratumoral features can achieve high predictive accuracy. In a related study, Cui et al[44] employed a multiparameter MRI-based intratumoral radiomics approach, including axial T2WI, dynamic contrast-enhanced T1 images, and apparent diffusion coefficient maps, to predict DFS. Their T2WI-based radiomics model achieved a C-index of 0.659, and their overall multiparameter model reached a C-index of 0.752 for predicting 3-year DFS. This indicates that multiparameter MRI can enhance the performance of prognostic models. While our single-sequence high-resolution small-field-of-view T2WI model with combined peritumoral and intratumoral features shows promising results, it raises the question of whether integrating multiparameter MRI radiomics, including both peritumoral and intratumoral features, could further improve predictive performance beyond what is achievable with high-resolution T2WI alone. Further research is needed to explore the potential benefits of such an approach for improving prognostic models in rectal cancer.
This study has few limitations. First, it was a retrospective study conducted at a single center and lacked external validation. Therefore, future studies involving multiple centers are required to enhance the effectiveness and relevance of this model. Second, although a peritumoral thickness of 5 mm was reasonable, we did not compare different peritumoral thickness in this study. Third, the manual delineation of intratumoral ROIs was a laborious process subject to variability among different radiologists, even though peritumoral ROI segmentation was automatically created. Hence, to improve the clinical utility of radiomics, future studies should explore the development of an automated ROI-segmentation tool. Fourth, gene-related biomarkers were not included in this study. Incorporating them in future research and exploring their relationship with radiomic features could enhance the interpretability and clinical utility of the models. Integrating multimodal data with intratumoral and peritumoral radiomics may provide deeper insights into cancer progression and outcomes.
This study demonstrated that peritumoral radiomics have greater prognostic value than intratumoral radiomics in predicting PFS in patients with LARC who underwent treatment with NCRT and TME. Therefore, a comprehensive model integrating the radiomic features of peri- and intratumoral regions and clinicoradiological factors may serve as a reliable tool for the better stratification and management of postoperative patients based on their risk of disease progression. Additionally, further research on important peritumoral radiomic features is recommended.
We would like to express our gratitude to Professor Xie CB of the Department of Cancer Prevention at Sun Yat-sen University Cancer Center for conducting the biostatistics review for our study.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68596] [Article Influence: 13719.2] [Reference Citation Analysis (201)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11970] [Article Influence: 2992.5] [Reference Citation Analysis (9)] |
| 3. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1310] [Article Influence: 145.6] [Reference Citation Analysis (0)] |
| 4. | Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1139-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 510] [Article Influence: 127.5] [Reference Citation Analysis (1)] |
| 5. | Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C, Hui B, Liu R, Ma H, Ren J. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int J Biol Sci. 2016;12:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 6. | Tamburini E, Tassinari D, Ramundo M, De Stefano A, Viola MG, Romano C, Elia MT, Zanaletti N, Rudnas B, Casadei-Gardini A, Delrio P, Toma I, Granata V, Petrucelli L, Avallone A. Adjuvant chemotherapy after neoadjuvant chemo-radiotherapy and surgery in locally advanced rectal cancer. A systematic review of literature with a meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2022;172:103627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 7. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, Bessell E, Griffiths G, Thompson LC, Parmar M. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1131] [Article Influence: 66.5] [Reference Citation Analysis (6)] |
| 8. | Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW, van de Velde CJ; Dutch Colorectal Cancer Group. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 888] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 9. | Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, Graeven U, Arnold D, Lang-Welzenbach M, Raab HR, Sülberg H, Wittekind C, Potapov S, Staib L, Hess C, Weigang-Köhler K, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R; German Rectal Cancer Study Group. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 514] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 10. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1387] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 11. | Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, Becker H, Ghadimi M, Mrak K, Merkel S, Raab HR, Sauer R, Wittekind C, Rödel C. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 284] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 12. | Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 710] [Cited by in RCA: 650] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 13. | Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics. 2019;39:367-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 340] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 14. | Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 4188] [Article Influence: 299.1] [Reference Citation Analysis (4)] |
| 15. | Liu Z, Wang S, Dong D, Wei J, Fang C, Zhou X, Sun K, Li L, Li B, Wang M, Tian J. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics. 2019;9:1303-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 676] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 16. | Zhang S, Regan K, Najera J, Grinstaff MW, Datta M, Nia HT. The peritumor microenvironment: physics and immunity. Trends Cancer. 2023;9:609-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 17. | Lee H, Sha D, Foster NR, Shi Q, Alberts SR, Smyrk TC, Sinicrope FA. Analysis of tumor microenvironmental features to refine prognosis by T, N risk group in patients with stage III colon cancer (NCCTG N0147) (Alliance). Ann Oncol. 2020;31:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 812] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 19. | Conti G, Calderan L, Quintero Sierra LA, Conti A, Ossanna R, Boschi F, Marzola P, Ferrarini F, Governa M, Lievens PM, Sbarbati A. Tumor and peritumoral adipose tissue crosstalk: De-differentiated adipocytes influence spread of colon carcinoma cells. Tissue Cell. 2023;80:101990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 20. | Wen YA, Xing X, Harris JW, Zaytseva YY, Mitov MI, Napier DL, Weiss HL, Mark Evers B, Gao T. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 2017;8:e2593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 221] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 21. | Mukherjee A, Bilecz AJ, Lengyel E. The adipocyte microenvironment and cancer. Cancer Metastasis Rev. 2022;41:575-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 22. | Liu J, Yu X, Huang X, Lai Q, Chen J. Associations of muscle and adipose tissue parameters with long-term outcomes in middle and low rectal cancer: a retrospective cohort study. Cancer Imaging. 2023;23:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Jayaprakasam VS, Paroder V, Gibbs P, Bajwa R, Gangai N, Sosa RE, Petkovska I, Golia Pernicka JS, Fuqua JL 3rd, Bates DDB, Weiser MR, Cercek A, Gollub MJ. MRI radiomics features of mesorectal fat can predict response to neoadjuvant chemoradiation therapy and tumor recurrence in patients with locally advanced rectal cancer. Eur Radiol. 2022;32:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 24. | Yoon J, Chung YE, Lim JS, Kim MJ. Quantitative assessment of mesorectal fat: new prognostic biomarker in patients with mid-to-lower rectal cancer. Eur Radiol. 2019;29:1240-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Zhao R, Wan L, Chen S, Peng W, Liu X, Wang S, Li L, Zhang H. MRI-based Multiregional Radiomics for Pretreatment Prediction of Distant Metastasis After Neoadjuvant Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer. Acad Radiol. 2024;31:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Li H, Chen XL, Liu H, Lu T, Li ZL. MRI-based multiregional radiomics for predicting lymph nodes status and prognosis in patients with resectable rectal cancer. Front Oncol. 2022;12:1087882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 27. | Delli Pizzi A, Chiarelli AM, Chiacchiaretta P, d'Annibale M, Croce P, Rosa C, Mastrodicasa D, Trebeschi S, Lambregts DMJ, Caposiena D, Serafini FL, Basilico R, Cocco G, Di Sebastiano P, Cinalli S, Ferretti A, Wise RG, Genovesi D, Beets-Tan RGH, Caulo M. MRI-based clinical-radiomics model predicts tumor response before treatment in locally advanced rectal cancer. Sci Rep. 2021;11:5379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Davey MS, Davey MG, Ryan ÉJ, Hogan AM, Kerin MJ, Joyce M. The use of radiomic analysis of magnetic resonance imaging in predicting distant metastases of rectal carcinoma following surgical resection: A systematic review and meta-analysis. Colorectal Dis. 2021;23:3065-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Stanzione A, Verde F, Romeo V, Boccadifuoco F, Mainenti PP, Maurea S. Radiomics and machine learning applications in rectal cancer: Current update and future perspectives. World J Gastroenterol. 2021;27:5306-5321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (4)] |
| 30. | Feng L, Liu Z, Li C, Li Z, Lou X, Shao L, Wang Y, Huang Y, Chen H, Pang X, Liu S, He F, Zheng J, Meng X, Xie P, Yang G, Ding Y, Wei M, Yun J, Hung MC, Zhou W, Wahl DR, Lan P, Tian J, Wan X. Development and validation of a radiopathomics model to predict pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a multicentre observational study. Lancet Digit Health. 2022;4:e8-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 31. | Xue K, Liu L, Liu Y, Guo Y, Zhu Y, Zhang M. Radiomics model based on multi-sequence MR images for predicting preoperative immunoscore in rectal cancer. Radiol Med. 2022;127:702-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 33. | Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. 8th ed. Springer, New York; 2017: 1024. |
| 34. | Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 337] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 35. | van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts HJWL. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017;77:e104-e107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 4351] [Article Influence: 483.4] [Reference Citation Analysis (0)] |
| 36. | Tibermacine H, Rouanet P, Sbarra M, Forghani R, Reinhold C, Nougaret S; GRECCAR Study Group. Radiomics modelling in rectal cancer to predict disease-free survival: evaluation of different approaches. Br J Surg. 2021;108:1243-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Schneider S, Park DJ, Yang D, El-Khoueiry A, Sherrod A, Groshen S, Streeter O, Iqbal S, Danenberg KD, Lenz HJ. Gene expression in tumor-adjacent normal tissue is associated with recurrence in patients with rectal cancer treated with adjuvant chemoradiation. Pharmacogenet Genomics. 2006;16:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Yang D, Schneider S, Azuma M, Iqbal S, El-Khoueiry A, Groshen S, Agafitei D, Danenberg KD, Danenberg PV, Ladner RD, Lenz HJ. Gene expression levels of epidermal growth factor receptor, survivin, and vascular endothelial growth factor as molecular markers of lymph node involvement in patients with locally advanced rectal cancer. Clin Colorectal Cancer. 2006;6:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Li H, Chen XL, Liu H, Liu YS, Li ZL, Pang MH, Pu H. MRI-based multiregional radiomics for preoperative prediction of tumor deposit and prognosis in resectable rectal cancer: a bicenter study. Eur Radiol. 2023;33:7561-7572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 40. | Zhang Y, He K, Guo Y, Liu X, Yang Q, Zhang C, Xie Y, Mu S, Guo Y, Fu Y, Zhang H. A Novel Multimodal Radiomics Model for Preoperative Prediction of Lymphovascular Invasion in Rectal Cancer. Front Oncol. 2020;10:457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Ma S, Lu H, Jing G, Li Z, Zhang Q, Ma X, Chen F, Shao C, Lu Y, Wang H, Shen F. Deep learning-based clinical-radiomics nomogram for preoperative prediction of lymph node metastasis in patients with rectal cancer: a two-center study. Front Med (Lausanne). 2023;10:1276672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 42. | Chen BY, Xie H, Li Y, Jiang XH, Xiong L, Tang XF, Lin XF, Li L, Cai PQ. MRI-Based Radiomics Features to Predict Treatment Response to Neoadjuvant Chemotherapy in Locally Advanced Rectal Cancer: A Single Center, Prospective Study. Front Oncol. 2022;12:801743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 43. | Qin S, Lu S, Liu K, Zhou Y, Wang Q, Chen Y, Zhang E, Wang H, Lang N. Radiomics from Mesorectal Blood Vessels and Lymph Nodes: A Novel Prognostic Predictor for Rectal Cancer with Neoadjuvant Therapy. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 44. | Cui Y, Wang G, Ren J, Hou L, Li D, Wen Q, Xi Y, Yang X. Radiomics Features at Multiparametric MRI Predict Disease-Free Survival in Patients With Locally Advanced Rectal Cancer. Acad Radiol. 2022;29:e128-e138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/