Published online Feb 28, 2025. doi: 10.3748/wjg.v31.i8.102629
Revised: December 5, 2024
Accepted: January 10, 2025
Published online: February 28, 2025
Processing time: 91 Days and 17.9 Hours
Intraoperative determination of resection margin and adequate residual liver parenchyma are the key points of hepatectomy for the treatment of liver tumors. Intraoperative ultrasound and indocyanine green fluorescence navigation are the most commonly used methods at present, but the technical barriers limit their promotion.
To evaluate the value of the three-dimensional location approach with silk thread (3D-LAST) in precise resection of liver tumors.
From September 2020 to January 2022, 8 patients with liver tumors including hepatocellular carcinoma, intrahepatic cholangiocarcinoma, hilar cholangiocarcinoma, and gastric cancer liver metastasis were included in this study. All patients underwent 3D-LAST in precise resection of liver tumors.
All patients (8/8, 100%) underwent the operation successfully without any complications. During the mean follow-up of 8.7 months, all patients survived without tumor recurrence.
In conclusion, the 3D-LAST is a safe and effective new method for liver intraoperative navigation, which is practical and easy to promote.
Core Tip: The aim of this study is to evaluate the value of the three-dimensional location approach with silk thread (3D-LAST) in precise resection of liver tumors. Eight patients with liver tumors including hepatocellular carcinoma, intrahepatic cholangiocarcinoma, hilar cholangiocarcinoma, and gastric cancer liver metastasis underwent the operation successfully without any complications. During the mean follow-up of 8.7 months, all patients survived without tumor recurrence. In conclusion, the 3D-LAST is a safe and effective new method for liver intraoperative navigation, which is practical and easy to promote.
- Citation: Zhang ZH, Feng QB, Jiang C, Huang JW, Li JX. Three-dimensional location approach with silk thread guided hepatectomy for liver tumor. World J Gastroenterol 2025; 31(8): 102629
- URL: https://www.wjgnet.com/1007-9327/full/v31/i8/102629.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i8.102629
Hepatectomy is widely used for the treatment of liver tumors. In recent decades, the concept and practice of hepatectomy have developed from irregular, regular and anatomical to the current precise resection. Necessary assistive technologies have enabled these advances. Intraoperative ultrasound (IOUS) localization and indocyanine green (ICG) fluorescence imaging guidance are two frequently-used approaches for laparoscopic hepatectomy[1,2]. IOUS is an invaluable auxiliary means widely accepted in surgery for real-time diagnostic information to determine resection range and navigate the surgical path[3]. However, the major limitation of IOUS is the time cost during the procedure for paging the sono
Three-dimensional (3D) visualization involves extracting features and producing volumetric images based on computed tomography (CT) through a computer postprocessing technique. This tool offers a reasonable approach to the clinical decision for the potential to display the complex internal anatomy in an intuitive and stereoscopic manner[7]. In the past few decades, applying 3D simulation software for liver volume calculation, virtual simulation surgery, portal hypertension monitor, and surgical navigation has proven to be safe and effective[8].
Therefore, we propose a new method to find obvious anatomical markers and calculate the resection range according to 3D positioning before operation. During the operation, the scope of resection was delineated with silk thread, and resection was performed. This is a new practical approach, which we named as 3D location approach with silk thread (3D-LAST).
From September 2020 to January 2022, 8 patients underwent 3D-LAST guided hepatectomy. The study was approved by the review committee of West China Hospital of Sichuan University.
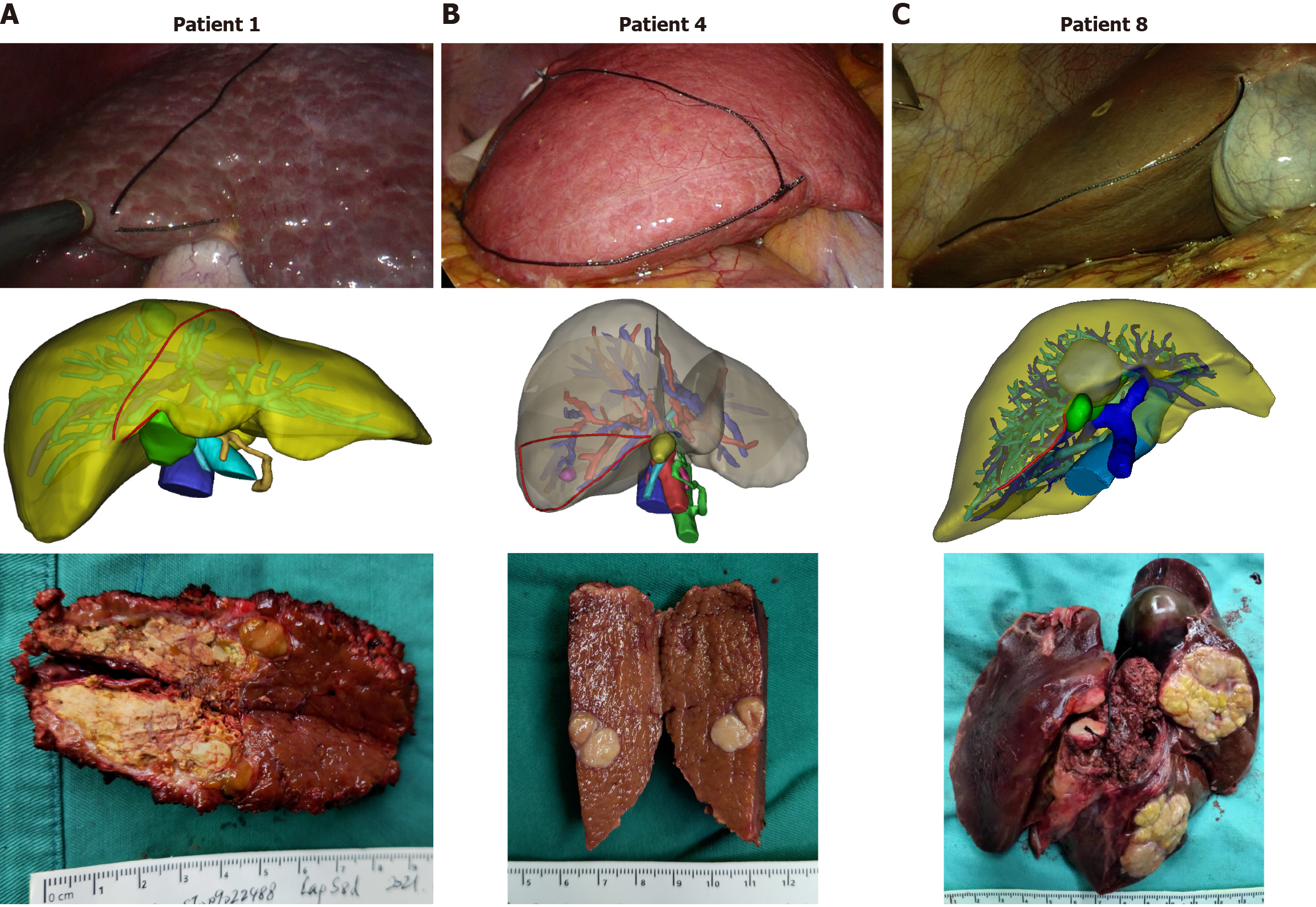
(1) 3D reconstruction of the intrahepatic vessels and tumors; (2) Small virtual sticks were used to demarcate the key points of the preoperatively planned cutting edge; (3) The lines were used to connect with the key points on the surface of the virtual liver model, and the length of each line was measured; (4) Prepare silk thread with the same length and place it at the anatomical position of the liver surface; and (5) Cut the liver alone the shape of the silk thread.
During the insertion of silk, the surgeon will repeatedly compare the preoperative 3D reconstruction model. In addition, the internal anatomy of the liver was deconstructed and compared with the 3D reconstruction model to assist in adjusting the position of the silk thread during the operation.
The inclusion criteria included: No metastasis to other organs except liver lesions; liver function was Child-Pugh grade A; the remaining liver volume was sufficient; no contraindications to laparoscopic surgery, or anesthesia.
Exclusion criteria: Child-Pugh grade B or C; Model of End-stage Liver Disease score > 15; insufficient residual liver volume; the presence of distant metastasis; pregnancy or lactation; hyperthyroidism.
Anatomical hepatectomy was performed according to tumor location, size, adjacent structures, liver function, residual liver volume and other factors. The principle is a margin of at least 1 cm.
During the study period from September 2020 to January 2022, 5 patients with hepatocellular carcinoma, 1 patient with intrahepatic cholangiocarcinoma, 1 patient with hilar cholangiocarcinoma, and 1 patient with gastric cancer liver metastasis were assessed for liver resection. There were 5 males and 3 females. The mean age of these patients was 54.3 ± 10.2 years (34-66 years). Preoperative 3D positioning was conducted and the scope of resection was delineated with a surgical suture successfully performed in all 8 patients without complications. The treatment results of these 8 patients are shown in Table 1. The 90-day operative mortality was zero. Complications worse than Dindo-Clavien IIIa was not observed at a mean follow-up time of 8.7 months (4-16 months), there was no evidence of tumor recurrence or extrahepatic metastasis. At the time of reporting, the patients are all alive and lead normal lives.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
| Age, years | 46 | 57 | 66 | 55 | 54 | 58 | 64 | 34 |
| Sex | F | F | M | F | M | M | M | M |
| HBV | + | - | - | + | + | - | + | + |
| Diagnosis | HCC | ICC | HCCA | HCC | HCC | GLM | HCC | HCC |
| Liver cirrhosis | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
| Child-Pugh | A | A | A | A | A | A | A | A |
| Tumor size (cm) | 3.2 × 2.8 | 4.3 × 2.7 | 2.6 × 1.5 | 1.2 × 1.0 | 2.3 × 2.2 | 2.7 × 1.6 | 7.1 × 6.3 | 4.4 × 3.6 |
| ASA score | I | I | II | I | I | I | II | I |
| Operation method | Lap | Open | Lap | Lap | Lap | Lap | Open | Lap |
| Resected liver segment | S8 | S5 + S6 + S7 + S8 | S1 + S2 + S3 + S4 | S6 | S8 | S5 | S5 | S2 + S3 + S4 |
| Operative time (minute) | 160 | 245 | 370 | 130 | 170 | 125 | 110 | 175 |
| Blood loss (mL) | 80 | 230 | 400 | 70 | 50 | 80 | 50 | 150 |
We take one patient as an example, 58-year-old male, who found a liver lesion 10 months after gastric cancer surgery. Enhanced CT showed that the lesion was located in the liver S5, about 1.5 cm in diameter, and considered metastatic lesions. We performed 3D-LAST guided hepatectomy on this patient (Figure 1).
Other representative 3D-LAST surgical procedures are shown in Figure 2.
Laparoscopic liver resection (LLR) has been increasingly ubiquitous and the indications are nearly as broad as open surgery attributed to technical innovations and experience accumulation[4,9-11]. In contrast to laparotomy, LLR has prominent advantages involving reduced pain with fewer perioperative complications, and faster recovery[12,13]. Nevertheless, lack of tactile and depth perception, narrowed operating space, and limited visual field caused an inherent challenge for the expanded application of laparoscopy[14,15]. IOUS and ICG fluorescence imaging are mainly applied as real-time navigation tools to address the issue in recent years[2,4]. IOUS is performed directly on the surface of the liver without blind spots and dead corners, thus the accuracy of lesion detection and localization can be enhanced[16]. The Southampton Consensus guidelines 2018 released by the European Guidelines Meeting strongly recommend the use of IOUS in every case of laparoscopic liver surgery because it is significantly associated with intraoperative diagnosis and resection plan[3]. However, most of the IOUS is operated by sonographers. In most cases, surgeons had to call sonographers intraoperatively and pause the operation, which prolonged operative time with a strong reliance on ultrasound equipment and techniques[17]. Besides, IOUS may overestimate the disease by confusing regenerative nodules with tumors in cirrhotic livers[18]. ICG is a harmless water-soluble near-infra-red fluorescent, by uses specifically tailored scopes to detect the fluorescence, which can be of great help in the intraoperative visualization of anatomical structures identification[19]. It is nowadays widely accepted as a useful tool in numerous liver surgery fields for its high sensitivity and clear contrast in surgical navigation[20]. However, 10mm is considered to be the maximum tissue penetration range for near-infra-red light, which limited the application of ICG for lesions deep in the liver[5]. In addition, an excessive dose of ICG may cause pseudo training, and the success rate of tumor staining is associated with blood supply, liver cirrhosis, and necrosis[6]. Hence, an innovative convenient, time-saving, and accurate localization method for deep liver tumors is of great clinical value.
In this study, preoperative 3D positioning was conducted to identify obvious anatomical markers and calculate the resection range in patients with a single liver tumor. During the operation, margin lines with the corresponding length calculated by the preoperative 3D software were arranged and the edges were delineated with silk thread. This approach represents a time-saving and accurate optimal cutting plane navigation option. The operation time of IOUS and ICG-guided hepatectomy was significantly prolonged. The reported operation time was 400 (345-450) minutes[21], while the operation time of our study was only 165 (110-370) minutes. This approach not only overcome the dependence on IOUS, thus saving the operation time, and reducing the technical and condition requirements, but can be applied to a variety of navigating conditions for the liver tumors, regardless of the size and depth. Precise assessment of the number of lesions and whether there exist metastasize are especially crucial to the success of this surgical procedure. It should be pointed out that the sample size of this study is small, and further prospective studies with large samples are still needed in the future. The learning curve will also be explained. Designing a simple and widely used 3D reconstruction program is also a future goal.
Collectively, in selected patients with liver tumors, this novel resection can be used as an alternative surgical method. Given the requirement for accurate preoperative diagnosis and 3D localization, this operation is suggested to carry out in conditional hospitals. Furthermore, more cases are needed to validate and popularize our procedure.
| 1. | Pace C, Nardone V, Roma S, Chegai F, Toti L, Manzia TM, Tisone G, Orlacchio A. Evaluation of Contrast-Enhanced Intraoperative Ultrasound in the Detection and Management of Liver Lesions in Patients with Hepatocellular Carcinoma. J Oncol. 2019;2019:6089340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Inoue Y, Arita J, Sakamoto T, Ono Y, Takahashi M, Takahashi Y, Kokudo N, Saiura A. Anatomical Liver Resections Guided by 3-Dimensional Parenchymal Staining Using Fusion Indocyanine Green Fluorescence Imaging. Ann Surg. 2015;262:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, Aroori S, Belli G, Besselink M, Briceno J, Gayet B, D'Hondt M, Lesurtel M, Menon K, Lodge P, Rotellar F, Santoyo J, Scatton O, Soubrane O, Sutcliffe R, Van Dam R, White S, Halls MC, Cipriani F, Van der Poel M, Ciria R, Barkhatov L, Gomez-Luque Y, Ocana-Garcia S, Cook A, Buell J, Clavien PA, Dervenis C, Fusai G, Geller D, Lang H, Primrose J, Taylor M, Van Gulik T, Wakabayashi G, Asbun H, Cherqui D. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 523] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 4. | Zhang P, Luo H, Zhu W, Yang J, Zeng N, Fan Y, Wen S, Xiang N, Jia F, Fang C. Real-time navigation for laparoscopic hepatectomy using image fusion of preoperative 3D surgical plan and intraoperative indocyanine green fluorescence imaging. Surg Endosc. 2020;34:3449-3459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Baiocchi GL, Diana M, Boni L. Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: State of the art and future directions. World J Gastroenterol. 2018;24:2921-2930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (2)] |
| 6. | Li J, Li X, Zhang X, Wang H, Li K, He Y, Liu Z, Zhang Z, Yuan Y. Indocyanine green fluorescence imaging-guided laparoscopic right posterior hepatectomy. Surg Endosc. 2022;36:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Caton MT Jr, Wiggins WF, Nunez D. Three-Dimensional Cinematic Rendering to Optimize Visualization of Cerebrovascular Anatomy and Disease in CT Angiography. J Neuroimaging. 2020;30:286-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Fang C, An J, Bruno A, Cai X, Fan J, Fujimoto J, Golfieri R, Hao X, Jiang H, Jiao LR, Kulkarni AV, Lang H, Lesmana CRA, Li Q, Liu L, Liu Y, Lau W, Lu Q, Man K, Maruyama H, Mosconi C, Örmeci N, Pavlides M, Rezende G, Sohn JH, Treeprasertsuk S, Vilgrain V, Wen H, Wen S, Quan X, Ximenes R, Yang Y, Zhang B, Zhang W, Zhang P, Zhang S, Qi X. Consensus recommendations of three-dimensional visualization for diagnosis and management of liver diseases. Hepatol Int. 2020;14:437-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 9. | Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schön MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 415] [Reference Citation Analysis (0)] |
| 10. | Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg. 2016;263:761-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 605] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 11. | Cipriani F, Shelat VG, Rawashdeh M, Francone E, Aldrighetti L, Takhar A, Armstrong T, Pearce NW, Abu Hilal M. Laparoscopic Parenchymal-Sparing Resections for Nonperipheral Liver Lesions, the Diamond Technique: Technical Aspects, Clinical Outcomes, and Oncologic Efficiency. J Am Coll Surg. 2015;221:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, Hausken J, Tønnessen TI, Abildgaard A, Barkhatov L, Yaqub S, Røsok BI, Bjørnbeth BA, Andersen MH, Flatmark K, Aas E, Edwin B. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg. 2018;267:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 510] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 13. | Zhang Z, Ji J, Qiu G, Hou Z, Mi S, Jin Z, Dai Y, Xie Q, Zeng Y, Huang J. Surgical indications for solid hepatic benign tumors: An updated literature review. Biosci Trends. 2023;17:325-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 14. | Moris D, Vernadakis S. Laparoscopic Hepatectomy for Hepatocellular Carcinoma: The Opportunities, the Challenges, and the Limitations. Ann Surg. 2018;268:e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Wottawa CR, Cohen JR, Fan RE, Bisley JW, Culjat MO, Grundfest WS, Dutson EP. The role of tactile feedback in grip force during laparoscopic training tasks. Surg Endosc. 2013;27:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Short Version). Ultraschall Med. 2017;38:377-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Xu YW, Fu H. Application of intraoperative ultrasound in liver surgery. Hepatobiliary Pancreat Dis Int. 2021;20:501-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Takigawa Y, Sugawara Y, Yamamoto J, Shimada K, Yamasaki S, Kosuge T, Makuuchi M. New lesions detected by intraoperative ultrasound during liver resection for hepatocellular carcinoma. Ultrasound Med Biol. 2001;27:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Berardi G, Colasanti M, Meniconi RL, Ferretti S, Guglielmo N, Mariano G, Burocchi M, Campanelli A, Scotti A, Pecoraro A, Angrisani M, Ferrari P, Minervini A, Gasparoli C, Wakabayashi G, Ettorre GM. The Applications of 3D Imaging and Indocyanine Green Dye Fluorescence in Laparoscopic Liver Surgery. Diagnostics (Basel). 2021;11:2169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Wang X, Teh CSC, Ishizawa T, Aoki T, Cavallucci D, Lee SY, Panganiban KM, Perini MV, Shah SR, Wang H, Xu Y, Suh KS, Kokudo N. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann Surg. 2021;274:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 21. | Pu C, Wu T, Wang Q, Yang Y, Zhang K. Feasibility of novel intraoperative navigation for anatomical liver resection using real-time virtual sonography combined with indocyanine green fluorescent imaging technology. Biosci Trends. 2024;17:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |