Published online Dec 7, 2025. doi: 10.3748/wjg.v31.i45.113690
Revised: September 27, 2025
Accepted: October 22, 2025
Published online: December 7, 2025
Processing time: 93 Days and 20.4 Hours
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract, predominantly arising in the stomach (approximately 60%) and small intestine (approximately 30%), and accounting for 1%-3% of all gastrointestinal malignancies. In most cases, GISTs originate within the gastrointestinal tract; However, in rare instances, they may develop in extra-GISTs (EGISTs). Among these, gallbladder-derived EGISTs are exceedingly uncommon, with only nine cases reported to date.
We present the case of a 66-year-old woman who presented with recurrent right upper quadrant abdominal pain and subsequently underwent cholecystectomy. Histopathological examination with immunohistochemistry revealed CD117(+), DOG-1(+), and CD34(+), with no evidence of a primary gastrointestinal lesion, thereby confirming the diagnosis of primary gallbladder EGIST. According to the 2017 Chinese consensus on GISTs, based on the modified NIH 2008 criteria, the tumor was classified as very low risk. Consequently, the patient did not receive adjuvant targeted therapy such as imatinib, and the patient remained disease-free during a 6-month follow up.
Primary gallbladder EGISTs are exceedingly rare, with insidious onset and nonspecific clinical manifestations. Histopathological examination combined with immunohistochemistry remains the cornerstone of definitive diagnosis.
Core Tip: Primary extra-gastrointestinal stromal tumor (EGIST) of the gallbladder is exceptionally rare, with only a handful of cases reported worldwide. We describe a 66-year-old female patient diagnosed with gallbladder EGIST, confirmed by immunohistochemical staining for CD117(+), DOG-1(+), and CD34(+). The tumor was classified as very low risk based on the modified NIH criteria and Chinese consensus guidelines, and the patient remained recurrence-free at follow-up. This case underscores the diagnostic challenges and clinical significance of gallbladder EGISTs, highlighting the importance of integrating imaging, pathology, and risk stratification for optimal management.
- Citation: Wang P, Zhu CR, Yao J, Xu P, Zhang K, Zhu J, Ge XY, Chen Y, Wang WZ. Primary extra-gastrointestinal stromal tumor of the gallbladder: A case report. World J Gastroenterol 2025; 31(45): 113690
- URL: https://www.wjgnet.com/1007-9327/full/v31/i45/113690.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i45.113690
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the digestive tract, typically composed of spindle cells, epithelioid cells, or a mixture of both. They may arise at any site along the gastrointestinal tract from the esophagus to the rectum. In terms of distribution, 50%-60% occur in the stomach, 20%-30% in the small intestine, 10% in the colon, and approximately 5% in the esophagus. GISTs are believed to originate from interstitial cells of Cajal (ICCs), which are located within the muscularis propria and the myenteric plexus[1]. The majority of GISTs are associated with activating mutations in the KIT (CD117) or PDGFRA genes, leading to constitutive activation of tyrosine kinase receptors[2]. CD117(+) is a hallmark immunohistochemical feature, commonly accompanied by CD34(+), while DOG-1 expression is observed in a subset of cases.
In addition to the gastrointestinal tract, GISTs may also develop in extraintestinal sites such as the mesentery, omentum, pancreas, prostate, vagina, and pleura. Tumors arising outside the digestive tract are termed extra-GISTs (EGISTs)[3]. The pathological characteristics and molecular expression profiles of EGISTs are similar to those of conventional GISTs[4]. Both GISTs and EGISTs tend to have an insidious onset. GISTs arise within the gastrointestinal tract, where the confined space predisposes tumors to compress adjacent abdominal organs or obstruct the lumen during growth. This often results in abdominal distension, abdominal pain, nausea, vomiting, and gastrointestinal bleeding. Although these symptoms are nonspecific, they typically lead to earlier clinical presentation and facilitate earlier diagnosis compared with EGISTs. In contrast, EGISTs develop outside the gastrointestinal wall, without the spatial constraints of the digestive tract[5]. As a result, patients are often asymptomatic or present with nonspecific manifestations in the early stages, and tumors are usually detected only after reaching a substantial size or causing compression or invasion of adjacent organs and tissues. Although gallbladder GISTs are exceedingly rare, in March 2025, Northern Jiangsu People's Hospital Affiliated to Yangzhou University admitted a patient diagnosed with primary gallbladder EGIST. This report provides a detailed description of the case, aiming to explore the clinical presentation, differential diagnosis, pathological features, and therapeutic strategies of primary gallbladder EGISTs.
A 66-year-old woman presented in March 2025 to the Affiliated Northern Jiangsu People’s Hospital of Yangzhou University with recurrent right upper quadrant abdominal pain. The patient had experienced episodes of right upper quadrant pain for 2 years without obvious precipitating factors, each lasting approximately 30 minutes and resolving spontaneously.
Two weeks prior to admission, the pain had worsened and was accompanied by nausea and vomiting, without chills or fever.
The patient had no prior history of GISTs. Her past medical history was notable for thyroid nodules, for which she underwent subtotal thyroidectomy (right lobe and isthmus); postoperative pathology confirmed subacute thyroiditis.
No significant family history was reported.
On physical examination, there was tenderness in the right upper quadrant, with a positive Murphy’s sign. No palpable abdominal mass was detected. The skin and sclera were not icteric, and neither the liver nor the spleen was palpable below the costal margin.
Laboratory investigations revealed no significant abnormalities in complete blood count or liver function tests. Tumor markers including carbohydrate CA50, CA19-9, a-fetoprotein, and carcinoembryonic antigen were all within normal limits.
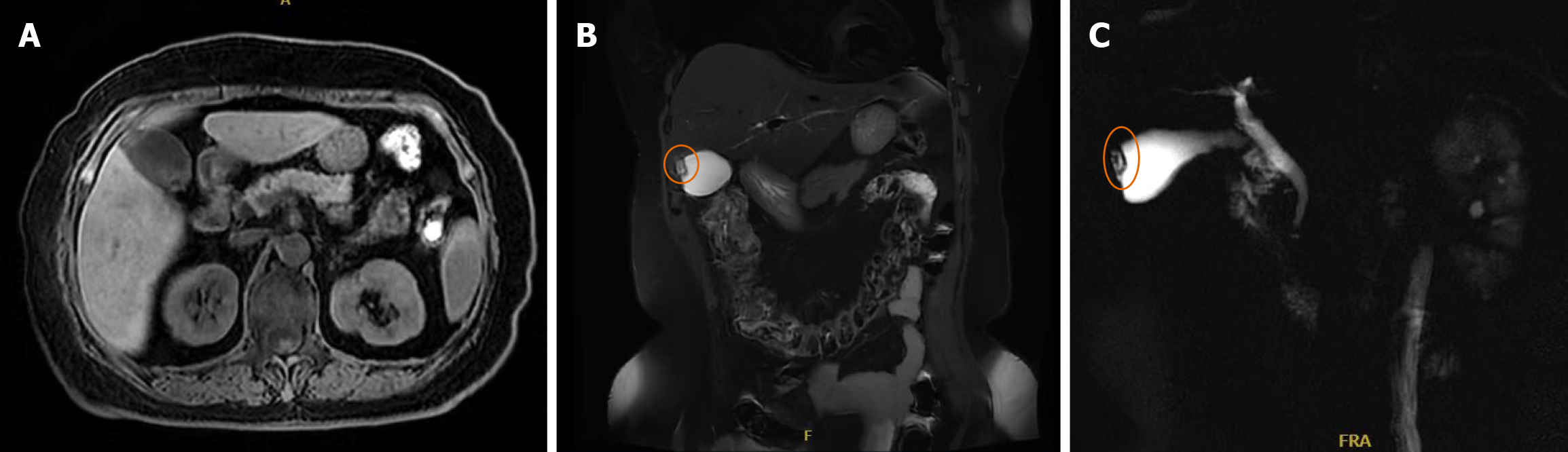
Abdominal ultrasonography suggested gallbladder calculi with possible adenomyomatosis. Magnetic resonance cholangiopancreatography also supported a possible diagnosis of gallbladder adenomyomatosis (Figure 1). Based on these findings, laparoscopic cholecystectomy was performed. Intraoperatively, mild adhesions were noted between the gallbladder and omentum, which were carefully dissected. The gallbladder measured approximately 9 cm × 4 cm. The liver, duodenum, common bile duct, colon, and small intestine showed no obvious abnormalities. The postoperative course was uneventful, and the patient was discharged on postoperative day three.
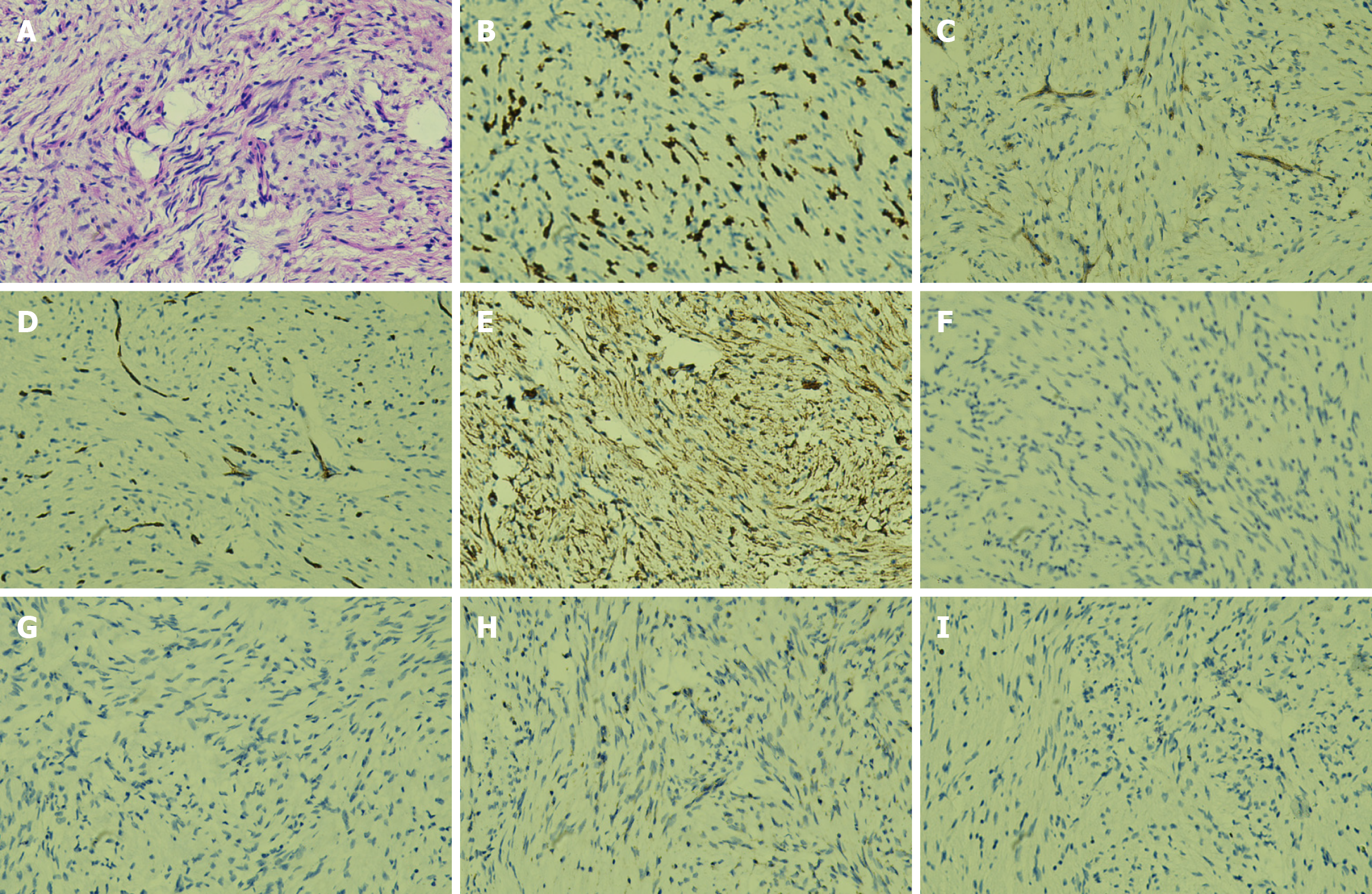
Pathological examination Gross pathological examination revealed a gallbladder measuring 9 cm × 3.5 cm × 2.5 cm with a roughened mucosal surface and wall thickness of 0.2 cm. One gallstone was identified, measuring 0.3-0.4 cm in diameter. Microscopic hematoxylin and eosin staining demonstrated proliferative spindle-shaped cells (Figure 2A). Immunohistochemical staining showed CD117(+; Figure 2B), DOG-1(+; Figure 2C), CD34(+; Figure 2D), and smooth muscle actin (SMA; +; Figure 2E); desmin(-; Figure 2F), S-100(-; Figure 2G), and succinate dehydrogenase subunit B (SDHB; -; Figure 2H); and a Ki-67 proliferation index of approximately 1% (Figure 2I). Based on histopathological and immunohistochemical findings, the lesion was diagnosed as EGIST of spindle cell type, with a maximum tumor diameter of approximately 0.4 cm. No significant mitotic figures, cytological atypia, or necrosis were observed. According to risk stratification criteria, the tumor was classified as very low risk. Therefore, the patient did not receive adjuvant therapy with imatinib or other chemotherapeutic agents. The patient has been closely followed postoperatively, and at the time of manuscript preparation remains alive and free of disease recurrence.
The cholecystectomy specimen revealed primary EGIST of the gallbladder. Microscopic examination revealed a spindle cell neoplasm with fascicular and whorled arrangement, maximum diameter approximately 0.4 cm, located within a localized thickened mucosal area (1.8 cm × 1.5 cm × 1.0 cm). No significant cytological atypia, necrosis, or mitotic figures were observed (0/50 HPFs). Immunohistochemistry showed CD117(+), DOG-1(+), SMA(+), while desmin, S-100, and SDHB were negative. The Ki-67 proliferation index, assessed in the most proliferative region, was approximately 1%. According to the 2017 Chinese consensus, based on the modified NIH 2008 criteria, the tumor was classified as very low risk. Based on morphological and immunophenotypic features, the final diagnosis was primary gallbladder EGIST, spindle cell type, very low-risk category.
After admission, the patient underwent laparoscopic cholecystectomy. Postoperative pathology combined with immunohistochemistry confirmed the diagnosis of EGIST. The postoperative course was uneventful, and the patient was discharged on day 3 after surgery. According to the Chinese consensus on GISTs, based on the modified NIH 2008 criteria, the tumor was classified as very low risk; therefore, the patient did not receive adjuvant targeted therapy such as imatinib.
The patient has been closely followed postoperatively, and at the time of manuscript preparation, she remains alive with no evidence of disease recurrence.
This study reports a case of primary gallbladder EGIST in a 66-year-old female patient, with the diagnosis confirmed by immunohistochemical staining for CD117, DOG-1, and CD34. Malignant mesenchymal tumors of the gallbladder are exceedingly rare, and to date, only nine cases of benign or malignant gallbladder EGISTs with an ICC phenotype have been reported (Table 1)[6-14]. In the present case, according to the Chinese Consensus 2017 (based on the modified NIH 2008 criteria), the tumor was classified as very low risk GIST. Because EGISTs originate outside the gastrointestinal wall, they often have an insidious onset, with no specific symptoms or signs in the early stages. Unlike conventional GISTs, patients with EGISTs typically lack classic gastrointestinal symptoms such as bleeding, obstruction, or perforation.
| Case | Ref. | Age (year)/sex | Clinical presentation | Tumor size (cm) | Pathology & IHC | Molecular detection | Treatment | Outcome |
| 1 | OrtizHidalgo et al[6], 2000 | 69/F | RUQ pain | 2 × 2 | CD117 (+), CD34 (+) | N/A | Laparoscopic cholecystectomy | N/A |
| 2 | MendozaMarin et al[7], 2002 | 34/F | RUQ pain | 1.5 | CD117 (+), VIM (+), CD34 (-), SMA (-) | N/A | Cholecystectomy | N/A |
| 3 | Park et al[8], 2004 | 72/F | RUQ pain | 6 × 3 | CD117 (+), VIM (+), CD34 (-), Desmin (-), SMA (-) | Mutations of the c-kit proto-oncogene were not found | Cholecystectomy | Liver metastasis 7 months after surgery, died after 9 months |
| 4 | Peerlinck et al[9], 2004 | 79/F | RUQ pain with fever | 4 | CD117 (+), Desmin (+), SMA (+) | N/A | Simple cholecystectomy | Progressive intra-abdominal disease 13 months after surgery, died after 15 months |
| 5 | Furihata et al[10], 2005 | 68/F | RUQ pain | 6 × 5 × 4 | CD117 (+), VIM (+), SMA (+), CD34 (-), Desmin (-) | Exon 9 of the c-kit gene (+) | Extended right hepatectomy with hepaticojejunostomy after portal vein embolization | No sign of metastasis or recurrence > 12 months postoperatively |
| 6 | Al-Daraji et al[11], 2009 | 48/F | Pain with fever | 2 × 1 | CD117 (+), CD34 (+) | N/A | Cholecystectomy | Died 5 days after surgery |
| 7 | Petrou et al[12], 2011 | 72/F | RUQ pain | 7.5 × 3.5 | CD117 (-), PDGFR (+) | Exon 18 of PDGFRA gene (+) | Cholecystectomy | N/A |
| 8 | Bolanaki et al[13], 2012 | 77/F | RUQ pain | 7 | CD117 (+), SMA (+), CD34 (-) | Failed to detect c-kit gene mutations in exons 9, 11, 13, and 17 | Exploratory laparotomy | Died 10 days postoperatively |
| 9 | Kostov and Kobakov[14], 2012 | 41/F | N/A | N/A | CD117 (+), CD34 (-) | N/A | Cholecystectomy | N/A |
Instead, EGISTs are more frequently discovered as abdominal masses, abdominal distension, vague abdominal pain, or incidentally during imaging examinations. Consequently, most patients present for medical attention due to the detection of an abdominal mass, often at an advanced stage. The lack of disease-specific clinical features renders the early diagnosis and management of EGIST particularly challenging[15]. EGISTs lack specific biomarkers for early detection, making preoperative diagnosis difficult. Previous studies have demonstrated that GIST risk stratification directly influences surgical planning and serves as an essential basis for evaluating postoperative recurrence risk. Pathological parameters such as mitotic index are commonly used for risk assessment[16].
Magnetic resonance imaging (MRI), as a noninvasive diagnostic modality, plays a critical role in determining risk stratification. In particular, MRI with apparent diffusion coefficient (ADC) map analysis shows value in differentiating benign from malignant tumors. GISTs of different risk categories often demonstrate distinct features on MRI. Owing to its superior soft-tissue resolution, MRI provides detailed visualization of tumor morphology, including size, shape, and margins, which are closely correlated with the biological behavior of GISTs, thereby aiding in accurate risk assessment. Additionally, MRI offers unique advantages in characterizing intratumoral features. Multiparametric and multiplanar imaging techniques enable comprehensive evaluation of tumor-tissue relationships, internal architecture, and functional status[17]. Among these, diffusion-weighted imaging and quantitative ADC value analysis can assess the restriction of intratumoral water diffusion, thereby reflecting biological behavior and prognosis, which is particularly advantageous in risk stratification[18]. Combined application of MRI and ADC map analysis provides valuable predictive information on GIST risk categories and has clinical importance in disease assessment and treatment planning[19].
From a diagnostic perspective, the combined use of morphological assessment and immunohistochemistry, particularly diffuse and strong positivity for DOG-1 and CD117, allows for a definitive diagnosis in most cases of GIST. It is recommended to perform mutational testing for KIT and PDGFRA genes, at minimum including KIT exons 9, 11, 13, and 17, as well as PDGFRA exons 12 and 18[20]. In the present case, KIT and PDGFRA mutational testing was not performed. We fully recognize that mutational analysis plays an important role in the diagnosis, prognostication, and therapeutic decision-making of EGISTs. Previous studies have demonstrated that mutations in KIT exons 9, 11, 13, and 17 as well as PDGFRA exons 12 and 18 are closely associated with clinical behavior and sensitivity to tyrosine kinase inhibitors (TKIs). Therefore, molecular subtyping has been recommended as a necessary component by international guidelines. In this case, due to the small tumor size (maximum diameter 0.4 cm), spindle cell type morphology, and classification as very low risk, mutational analysis was not routinely performed at the time of diagnosis. Nevertheless, we acknowledge this as a limitation of our study. In the future, systematic molecular testing should be incorporated into the diagnostic and therapeutic process of EGISTs to guide precision treatment and accumulate further evidence-based data.
The primary treatment for localized GIST is complete surgical resection with negative margins (R0 resection). However, R0 resection alone does not guarantee cure for all primary GISTs[21]. For patients with primary GIST at high risk of recurrence, adjuvant therapy with imatinib is warranted. Postoperative recurrence risk stratification remains the most important criterion for determining the indication for adjuvant treatment[22]. According to the Chinese Expert Consensus on the Diagnosis and Treatment of Gastrointestinal Stromal Tumors (2017 edition, based on the modified NIH 2008 criteria), patients with an intermediate or high risk of recurrence are considered appropriate candidates for adjuvant therapy. At present, the internationally recognized standards for risk assessment of GIST including the NIH risk classification system (2008), the World Health Organization criteria (2013), and the Armed Forces Institute of Pathology criteria were all established based on the clinicopathological characteristics of GISTs arising within the gastrointestinal tract. Whether these systems are equally applicable to tumors arising outside the gastrointestinal tract, such as EGISTs, remains uncertain. Current research has not yet clarified whether GISTs and EGISTs share a common origin and represent the same biological entity. If they are biologically distinct, separate diagnostic and therapeutic standards or clinical guidelines specifically for EGISTs will be required[23]. The clinical significance of EGISTs lies in their considerable diagnostic complexity, potential for highly aggressive behavior, and treatment strategies that are dependent on molecular subtyping, making their management uniquely challenging. Preoperative diagnosis relies heavily on advanced imaging interpretation and clinical expertise, while definitive diagnosis is primarily achieved through histopathological exa
Although EGISTs represent a rare subtype of GIST, they pose unique diagnostic and therapeutic challenges. Their insidious onset, wide anatomical distribution, diverse molecular features, and generally unfavorable prognosis necessitate a high degree of clinical vigilance. With ongoing advances in molecular biology, the oncogenic mechanisms and therapeutic targets of EGISTs are increasingly elucidated. In the future, precision stratification and tailored targeted therapy hold promise for improving clinical outcomes and survival in this rare and challenging disease entity.
| 1. | Park CH, Kim GH, Lee BE, Song GA, Park DY, Choi KU, Kim DH, Jeon TY. Two staging systems for gastrointestinal stromal tumors in the stomach: which is better? BMC Gastroenterol. 2017;17:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1743] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 3. | Usama F, Rasikh R, Hassam K, Rahman M, Khalil Ur Rehman F, Khan IW, Lau DT. An update on gastrointestinal stromal tumors (GISTs) with a focus on extragastrointestinal stromal tumors (EGISTs). Gastroenterol Rep (Oxf). 2025;13:goaf068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Costa Almeida C, Caroço TV, Albano M, Carvalho L. Extragastrointestinal stromal tumour (EGIST) presented as a mesenteric and retroperitoneal mass. BMJ Case Rep. 2019;12:e232481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Nguyen Thanh T, Nguyen TTN, Le TB, Le DD, Nguyen VM, Le DK. Extragastrointestinal stromal tumor presenting as an exophytic prostatic mass. Radiol Case Rep. 2020;15:1142-1148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Ortiz-Hidalgo C, de Leon Bojorge B, Albores-Saavedra J. Stromal tumor of the gallbladder with phenotype of interstitial cells of Cajal: a previously unrecognized neoplasm. Am J Surg Pathol. 2000;24:1420-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Mendoza-Marin M, Hoang MP, Albores-Saavedra J. Malignant stromal tumor of the gallbladder with interstitial cells of Cajal phenotype. Arch Pathol Lab Med. 2002;126:481-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Park JK, Choi SH, Lee S, Min KO, Yun SS, Jeon HM. Malignant gastrointestinal stromal tumor of the gallbladder. J Korean Med Sci. 2004;19:763-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Peerlinck ID, Irvin TT, Sarsfield PT, Harington JM. GIST (gastro-intestinal stromal tumour) of the gallbladder: a case report. Acta Chir Belg. 2004;104:107-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Furihata M, Fujimori T, Imura J, Ono Y, Furihata T, Shimoda M, Kato M, Kita J, Ohkura Y, Kubota K. Malignant stromal tumor, so called "gastrointestinal stromal tumor", with rhabdomyomatous differentiation occurring in the gallbladder. Pathol Res Pract. 2005;201:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Al-Daraji WI, Prescott RJ, Al-Mahmoud RM, Husain EA, Haider SA. Cytological findings in a primary GIST of the gallbladder. Cytopathology. 2009;20:332-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Petrou A, Alexandrou P, Papalambros A, Saetta A, Fragkou P, Kontos M, Brennan N, Manzelli A, Bramis K, Felekouras E. A Malignant Gastrointestinal Stromal Tumor of the Gallbladder Immunoreactive for PDGFRA and Negative for CD 117 Antigen (c-KIT). HPB Surg. 2011;2011:327192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Bolanaki H, Delladetsima I, Argyropoulou P, Kapranou A, Kakolyris S, Simopoulos C, Karayiannakis AJ. Primary Malignant Gastrointestinal Stromal Tumor (GIST) of the Gallbladder: Report of a Case. J Gastrointest Cancer. 2012;43 Suppl 1:S151-S155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Kostov DV, Kobakov GL. Gastrointestinal stromal tumour of the gallbladder. HPB (Oxford). 2012;14:150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Elagami MM, Khalid A, Kumar V, Singhal M, Grossman MA. Perirectal Extragastrointestinal Stromal Tumor: An Unusual Presentation. Cureus. 2021;13:e15529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Joensuu H, Eriksson M, Hall KS, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE, Schlemmer M, Bauer S, Wardelmann E, Sarlomo-Rikala M, Nilsson B, Sihto H, Ballman KV, Leinonen M, DeMatteo RP, Reichardt P. Risk factors for gastrointestinal stromal tumor recurrence in patients treated with adjuvant imatinib. Cancer. 2014;120:2325-2333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Zhu X, He Y, Wang M, Shu Y, Lai X, Gan C, Liu L. Intratumoral and Peritumoral Multiparametric MRI-Based Radiomics Signature for Preoperative Prediction of Ki-67 Proliferation Status in Glioblastoma: A Two-Center Study. Acad Radiol. 2024;31:1560-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Inoue A, Ota S, Yamasaki M, Batsaikhan B, Furukawa A, Watanabe Y. Gastrointestinal stromal tumors: a comprehensive radiological review. Jpn J Radiol. 2022;40:1105-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (2)] |
| 19. | Mao H, Zhang B, Zou M, Huang Y, Yang L, Wang C, Pang P, Zhao Z. MRI-Based Radiomics Models for Predicting Risk Classification of Gastrointestinal Stromal Tumors. Front Oncol. 2021;11:631927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Oppelt PJ, Hirbe AC, Van Tine BA. Gastrointestinal stromal tumors (GISTs): point mutations matter in management, a review. J Gastrointest Oncol. 2017;8:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Rutkowski P, Skoczylas J, Wisniewski P. Is the Surgical Margin in Gastrointestinal Stromal Tumors Different? Visc Med. 2018;34:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Lu PW, Raut CP. Adjuvant therapy for primary GIST: is longer really better? A narrative review. Gastrointest Stromal Tumor. 2021;4:4. [DOI] [Full Text] |
| 23. | Arellano-Gutiérrez G, Martínez-Aldrete LF, Pérez-Fabián A, Maldonado-García EL. Primary extra-gastrointestinal stromal tumor (EGIST) of the mesentery: Case report and review of literature. Ann Med Surg (Lond). 2020;60:480-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Yao M, Zhou X, Tan Y. The first case of primary extragastrointestinal stromal tumor of the kidney: a case description. Quant Imaging Med Surg. 2025;15:3723-3728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Renberg S, Zhang Y, Karlsson F, Bränström R, Åhlen J, Jalmsell L, Linder-Stragliotto C, Haglund de Flon F, Papakonstantinou A. The role of neoadjuvant imatinib in gastrointestinal stromal tumor patients: 20 years of experience from a tertial referral center. Int J Cancer. 2022;151:906-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Kelly CM, Gutierrez Sainz L, Chi P. The management of metastatic GIST: current standard and investigational therapeutics. J Hematol Oncol. 2021;14:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 177] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/