Published online Oct 28, 2025. doi: 10.3748/wjg.v31.i40.112408
Revised: August 14, 2025
Accepted: September 11, 2025
Published online: October 28, 2025
Processing time: 93 Days and 18.1 Hours
The recent editorial by Parente et al provides a balanced overview of machine perfusion (MP) in liver transplantation. While its potential to improve graft pre
Core Tip: Machine perfusion (MP) may enhance graft preservation and modulate immune responses, but high costs, logistical barriers, and lack of standardized viability criteria limit its routine use. Long-term outcome data and head-to-head comparisons between perfusion strategies remain scarce, and uptake is low in Korean liver transplant centers. Robust multicenter trials integrating immune profiling and biomarker-guided immunosuppression are needed before MP can be endorsed as standard practice.
- Citation: Kim SH. Machine perfusion in liver transplantation: A step forward, but still on the runway. World J Gastroenterol 2025; 31(40): 112408
- URL: https://www.wjgnet.com/1007-9327/full/v31/i40/112408.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i40.112408
I read with great interest the article by Parente et al[1] published in the World Journal of Gastroenterology. The authors provide a timely and thoughtful overview of current machine perfusion (MP) strategies, underscoring their potential to transform donor organ preservation and improve graft outcomes. As a liver transplant specialist, I applaud the balanced discussion and welcome the spotlight on this rapidly evolving field.
While the editorial makes a compelling argument for the clinical relevance of MP in liver transplantation by syn
First, while the clinical relevance of MP is increasingly acknowledged, its routine implementation in high-volume transplant programs faces significant barriers. Chief among these are the high costs associated with perfusion devices and consumables, logistical complexity, and the need for specialized personnel and infrastructure. These limitations are particularly acute in resource-constrained settings and smaller transplant centers with limited access to donor organs. Moreover, real-world data on the cost-effectiveness of MP especially in comparison to static cold storage for marginal grafts such as those from donation after circulatory death and extended criteria donors remain insufficient, further hindering widespread adoption.
Second, despite growing interest in viability assessment during normothermic MP, there remains no consensus on standardized criteria. Current clinical trials employ a heterogeneous array of parameters, including lactate clearance, bile production, potential of hydrogen, and glucose metabolism to evaluate graft suitability. However, these thresholds vary widely across centers and protocols, resulting in inconsistencies in decision-making and clinical outcomes. While the authors acknowledge viability assessment as a future objective, this aspect merits stronger emphasis. Emerging data, particularly from viability-guided trials, suggest that dynamic functional markers, such as lactate clearance, bile production, and perfusate metabolomics during normothermic MP may offer predictive insights beyond traditional histological and enzymatic parameters[2]. This is further supported by clinical reviews summarizing multiple trials in which bile output and lactate dynamics were used to guide graft utilization decisions[3]. Moreover, the integration of omics-based technologies into perfusion platforms holds promise for enhancing precision in graft selection and immunological profiling.
Third, while short-term benefits such as reduced ischemia-reperfusion injury are well established, long-term outcome data remain scarce. There is a pressing need for well-designed multicenter trials that extend beyond short-term endpoints and rigorously assess long-term outcomes, including graft and patient survival, the incidence and severity of biliary complications, and the rate of re-transplantation beyond the first postoperative year. Such studies are essential to move beyond anecdotal experience and early-phase evidence, and to establish robust, generalizable data that can inform clinical guidelines, support health policy decisions, and justify broader implementation of MP technologies in diverse healthcare settings.
Fourth, a key limitation in the current literature is the lack of direct comparative evidence between different MP strategies, namely, hypothermic and normothermic MP and their relative advantages in distinct clinical contexts, such as extended criteria donors, donation after circulatory death, and ABO-incompatible liver transplantation. Without head-to-head comparisons, it remains unclear which approach is optimal for specific risk profiles, thereby limiting the development of tailored perfusion protocols and evidence-based decision-making. Table 1 summarizes the comparative features of hypothermic MP and normothermic MP, highlighting differences in metabolic activity, viability assessment, immunomodulatory potential, and logistical considerations[3,4].
| Parameter | Hypothermic MP (HOPE/D-HOPE) | Normothermic MP |
| Metabolic activity | Suppressed; reduces oxygen demand and cellular stress | Maintained; mimics physiological conditions |
| Viability assessment | Limited; lacks dynamic functional readouts | Real-time assessment via lactate clearance, bile output, etc. |
| Biliary protection | Strong evidence for reduced ischemic biliary injury | Mixed results; less consistent protection |
| Immunomodulatory potential | Underexplored; minimal data on immune modulation | Emerging evidence of Treg induction, cytokine shifts |
| Logistical complexity | Relatively simple; lower equipment and staffing requirements | More complex; requires specialized devices and trained personnel |
| Cost | Lower; fewer consumables and infrastructure needs | Higher; expensive devices and perfusate components |
| Clinical integration | Increasing use, especially in Europe and for marginal grafts | Growing adoption; more common in viability-guided protocols |
| Limitations | No active metabolism; limited therapeutic intervention window | Risk of overinterpretation of viability markers; cost barriers |
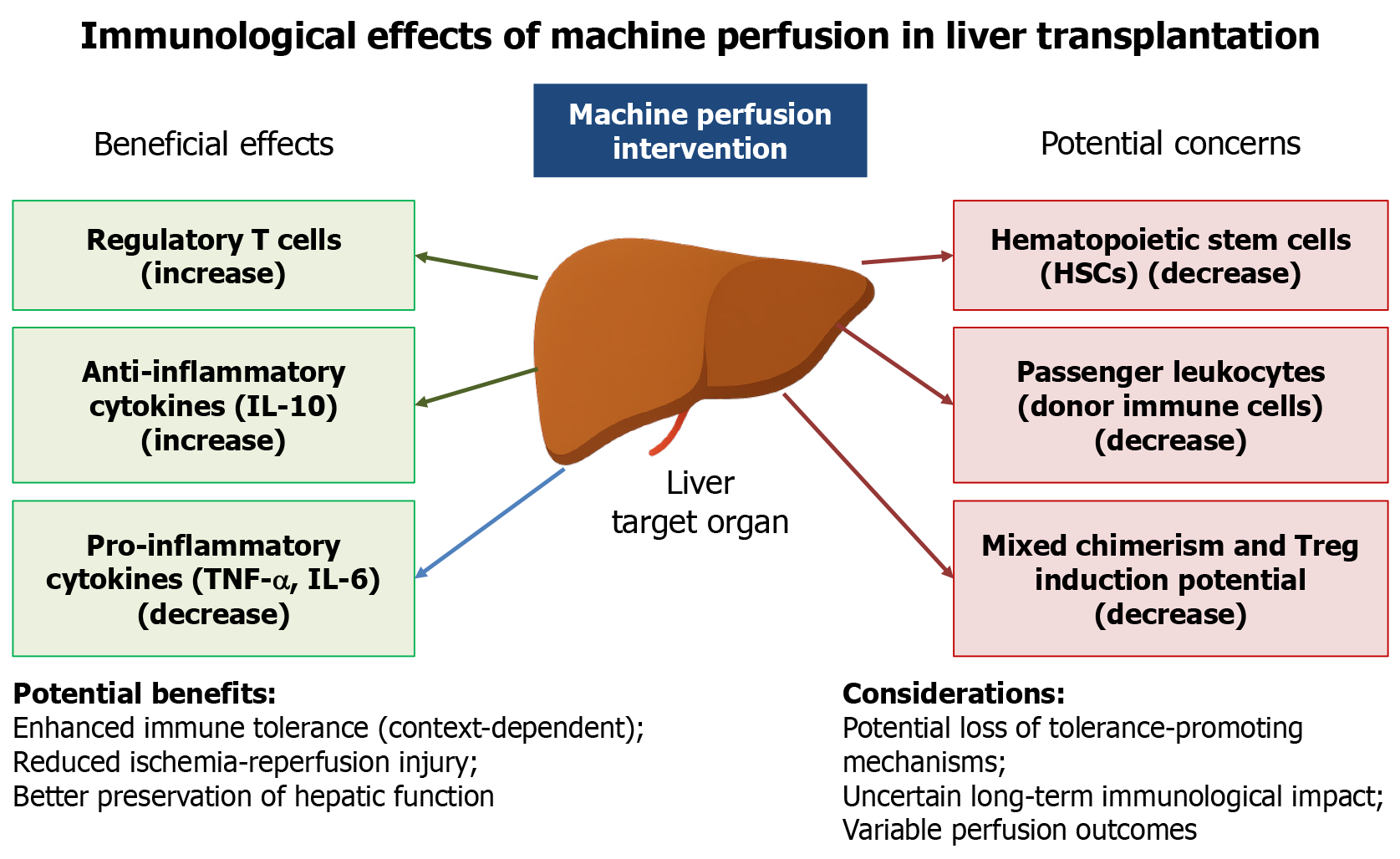
Finally, recent studies suggest that normothermic MP not only preserves graft function but actively reshapes the immune landscape of the liver prior to transplantation[5-7]. Beyond conventional functional assessment, accumulating evidence suggests that MP may contribute to immunological tolerance through multiple mechanisms, including the induction of regulatory T cells (Tregs), enhanced secretion of anti-inflammatory cytokines such as interleukin-10, and downregulation of pro-inflammatory mediators like tumor necrosis factor-alpha and interleukin-6[8,9]. These immunomodulatory effects highlight the potential of MP to influence post-transplant immune responses and graft acceptance. However, these potential benefits remain controversial (Figure 1). Other reports indicate that perfusing organs before transplantation could result in the depletion of beneficial hematopoietic stem cells and passenger leukocytes, which might otherwise contribute to mixed chimerism and peripheral Treg generation[10,11]. Moreover, experimental models have shown conflicting effects on antigen-presenting cell function and alloantibody responses, suggesting that the immune consequences of MP are highly context-dependent[4].
In parallel with these mechanistic insights, recent multicenter experiences have demonstrated tangible clinical benefits of MP. The organ care system liver PROTECT randomized trial showed that normothermic MP significantly reduced early allograft dysfunction and ischemic biliary complications across 20 United States transplant centers[12]. Additionally, the national MP program in Italy has reported successful transplantation of most perfused organs particularly in donation after circulatory death liver transplants thereby expanding the donor pool and improving graft utilization[13]. These examples underscore the growing clinical momentum behind MP and reinforce its translational potential in routine transplant practice.
These divergent findings and emerging clinical data highlight the need for mechanistic studies integrating perfusate immune profiling, graft-infiltrating leukocyte characterization, and longitudinal immune monitoring in recipients. Clinically, modulation of the hepatic immune environment via MP may attenuate alloimmune activation, lowering early rejection risk and potentially improving long-term graft survival. This immunologic “reset” could reduce the need for high-dose immunosuppression, thereby decreasing drug-related complications such as infection, nephrotoxicity, and metabolic syndrome, while alleviating the financial burden of prolonged use of costly immunosuppressants. These hypotheses require validation through well-designed multicenter trials correlating MP-induced immune signatures with safe and effective immunosuppression minimization strategies. Such data would be essential to determine whether MP can reliably induce tolerance or improve long-term immunological outcomes across diverse transplant scenarios.
Based on my institutional experience, I have performed more than 900 Liver transplants without the use of MP, as I have not yet identified a clear clinical need for such equipment in our practice. If MP were unequivocally proven to deliver consistent and substantial benefits, its adoption would be justified regardless of cost. Our outcomes have remained satisfactory without MP and to my knowledge, most liver transplant centers in Korea have not implemented MP because it likely reflects the absence of compelling evidence supporting its routine use. Although numerous studies on MP have emerged in recent years, conclusive proof of its necessity remains lacking. In my view, MP is still in the investigational stage, and widespread adoption should be deferred until robust, high-quality preferably region-specific data demonstrate clear advantages over current standard practice.
The potential of MP to influence long-term immunological tolerance and mitigate biliary injury particularly in ABO-incompatible or sensitized recipients remains a promising yet underexplored area. As MP technologies advance, future research should aim to integrate perfusion metrics not only with graft viability but also with immunologic modulation and individualized risk stratification. To date, few studies have systematically examined how perfusion affects alloim
In addition to scientific and clinical challenges, broader ethical and policy-related considerations must be addressed to ensure equitable and sustainable adoption of MP technologies. Despite the growing enthusiasm for MP technologies, there is a notable absence of discussion around the ethical, regulatory, and policy-related challenges that may impede their equitable implementation. In low-resource settings, the high cost of perfusion devices and consumables raises concerns about distributive justice and access disparities. Ethical dilemmas may arise when advanced technologies are available only to select institutions or patient populations, potentially exacerbating existing inequities in transplant care. Furthermore, regulatory frameworks governing MP use remain fragmented across regions, with limited guidance on standardization, safety oversight, and reimbursement policies. The integration of novel technologies like MP demands proactive policy development, stakeholder engagement, and ethical governance to ensure that innovation translates into inclusive and sustainable clinical practice. In low-resource settings, challenges such as access disparities, fragmented regulatory oversight, and the lack of reimbursement frameworks may significantly hinder implementation.
Although the current discussion is primarily adult-focused, I believe that MP may theoretically offer significant benefits in pediatric liver transplantation particularly by improving graft viability in split grafts and grafts from donors after circulatory death, expanding the donor pool, and reducing cold ischemia time in small-volume recipients.
Addressing these clinical, scientific, logistical, ethical, and policyrelated challenges will be critical to advancing MP from a promising experimental innovation to an established standard of care in liver transplantation.
A well-deserved commendation goes to the authors for synthesizing the current landscape of MP and advocating its broader clinical adoption. While MP technologies are steadily evolving and hold clear promise, their potential immunomodulatory benefits particularly in high-risk transplant settings such as donation after circulatory death and ABO-incompatible recipients remain underexplored. By bridging functional recovery with immune modulation, normothermic MP may offer a novel avenue to improve long-term graft outcomes and reduce immunologic complications. However, robust multicenter data encompassing both clinical efficacy and mechanistic insights together with ethical and policy considerations, will be essential before its routine use can be fully endorsed.
I would like to express my sincere appreciation to all members of our liver transplantation team for their unwavering support and collaboration.
| 1. | Parente A, Sun K, Dutkowski P, Shapiro AJ, Schlegel A. Routine utilization of machine perfusion in liver transplantation: Ready for prime time? World J Gastroenterol. 2024;30:1488-1493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | van Leeuwen OB, de Vries Y, Fujiyoshi M, Nijsten MWN, Ubbink R, Pelgrim GJ, Werner MJM, Reyntjens KMEM, van den Berg AP, de Boer MT, de Kleine RHJ, Lisman T, de Meijer VE, Porte RJ. Transplantation of High-risk Donor Livers After Ex Situ Resuscitation and Assessment Using Combined Hypo- and Normothermic Machine Perfusion: A Prospective Clinical Trial. Ann Surg. 2019;270:906-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 3. | Mugaanyi J, Dai L, Lu C, Mao S, Huang J, Lu C. A Meta-Analysis and Systematic Review of Normothermic and Hypothermic Machine Perfusion in Liver Transplantation. J Clin Med. 2022;12:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 4. | Hautz T, Brandacher G, Schneeberger S. Editorial: Immunology of machine perfused organs and tissues. Front Immunol. 2022;13:1104268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Parente A, Osei-Bordom DC, Ronca V, Perera MTPR, Mirza D. Organ Restoration With Normothermic Machine Perfusion and Immune Reaction. Front Immunol. 2020;11:565616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Nguyen MC, Zhang C, Chang YH, Li X, Ohara SY, Kumm KR, Cosentino CP, Aqel BA, Lizaola-Mayo BC, Frasco PE, Nunez-Nateras R, Hewitt WR, Harbell JW, Katariya NN, Singer AL, Moss AA, Reddy KS, Jadlowiec C, Mathur AK. Improved Outcomes and Resource Use With Normothermic Machine Perfusion in Liver Transplantation. JAMA Surg. 2025;160:322-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 7. | Moein M, Whittemore C, Lin KM, Hurst E, Essop T, Bahreini A, Saidi RF. Intervention During Normothermic Machine Perfusion of Solid Organs: a New Era in Organ Revitalization. Curr Transpl Rep. 2025;12:3. [DOI] [Full Text] |
| 8. | Jassem W, Xystrakis E, Ghnewa YG, Yuksel M, Pop O, Martinez-Llordella M, Jabri Y, Huang X, Lozano JJ, Quaglia A, Sanchez-Fueyo A, Coussios CC, Rela M, Friend P, Heaton N, Ma Y. Normothermic Machine Perfusion (NMP) Inhibits Proinflammatory Responses in the Liver and Promotes Regeneration. Hepatology. 2019;70:682-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 9. | Lee ACH, Edobor A, Lysandrou M, Mirle V, Sadek A, Johnston L, Piech R, Rose R, Hart J, Amundsen B, Jendrisak M, Millis JM, Donington J, Madariaga ML, Barth RN, di Sabato D, Shanmugarajah K, Fung J. The Effect of Normothermic Machine Perfusion on the Immune Profile of Donor Liver. Front Immunol. 2022;13:788935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Elahimehr R, Scheinok AT, McKay DB. Hematopoietic stem cells and solid organ transplantation. Transplant Rev (Orlando). 2016;30:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Mergental H, Laing RW, Kirkham AJ, Perera MTPR, Boteon YL, Attard J, Barton D, Curbishley S, Wilkhu M, Neil DAH, Hübscher SG, Muiesan P, Isaac JR, Roberts KJ, Abradelo M, Schlegel A, Ferguson J, Cilliers H, Bion J, Adams DH, Morris C, Friend PJ, Yap C, Afford SC, Mirza DF. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. 2020;11:2939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 369] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 12. | Markmann JF, Abouljoud MS, Ghobrial RM, Bhati CS, Pelletier SJ, Lu AD, Ottmann S, Klair T, Eymard C, Roll GR, Magliocca J, Pruett TL, Reyes J, Black SM, Marsh CL, Schnickel G, Kinkhabwala M, Florman SS, Merani S, Demetris AJ, Kimura S, Rizzari M, Saharia A, Levy M, Agarwal A, Cigarroa FG, Eason JD, Syed S, Washburn WK, Parekh J, Moon J, Maskin A, Yeh H, Vagefi PA, MacConmara MP. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022;157:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 315] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 13. | De Carlis R, Lauterio A, Centonze L, Buscemi V, Schlegel A, Muiesan P, De Carlis L; Italian DCD Collaborator Group. Current practice of normothermic regional perfusion and machine perfusion in donation after circulatory death liver transplants in Italy. Updates Surg. 2022;74:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/