Published online Oct 28, 2025. doi: 10.3748/wjg.v31.i40.111727
Revised: July 29, 2025
Accepted: September 23, 2025
Published online: October 28, 2025
Processing time: 111 Days and 19.8 Hours
Chronic liver disease (CLD) causes approximately two million deaths each year, and its clinical diagnosis and management remain challenging. Ultrasound is currently the most widely used technique for disease detection.
To propose a practical cut-off value for identifying patients with hepatocellular carcinoma (HCC) among those with compensated advanced CLD or healthy individuals using the GALAD score, an algorithm based on a formula that incorporates gender, age, serum alpha-fetoprotein (AFP), AFP-L3, and des-gamma-carboxy prothrombin values.
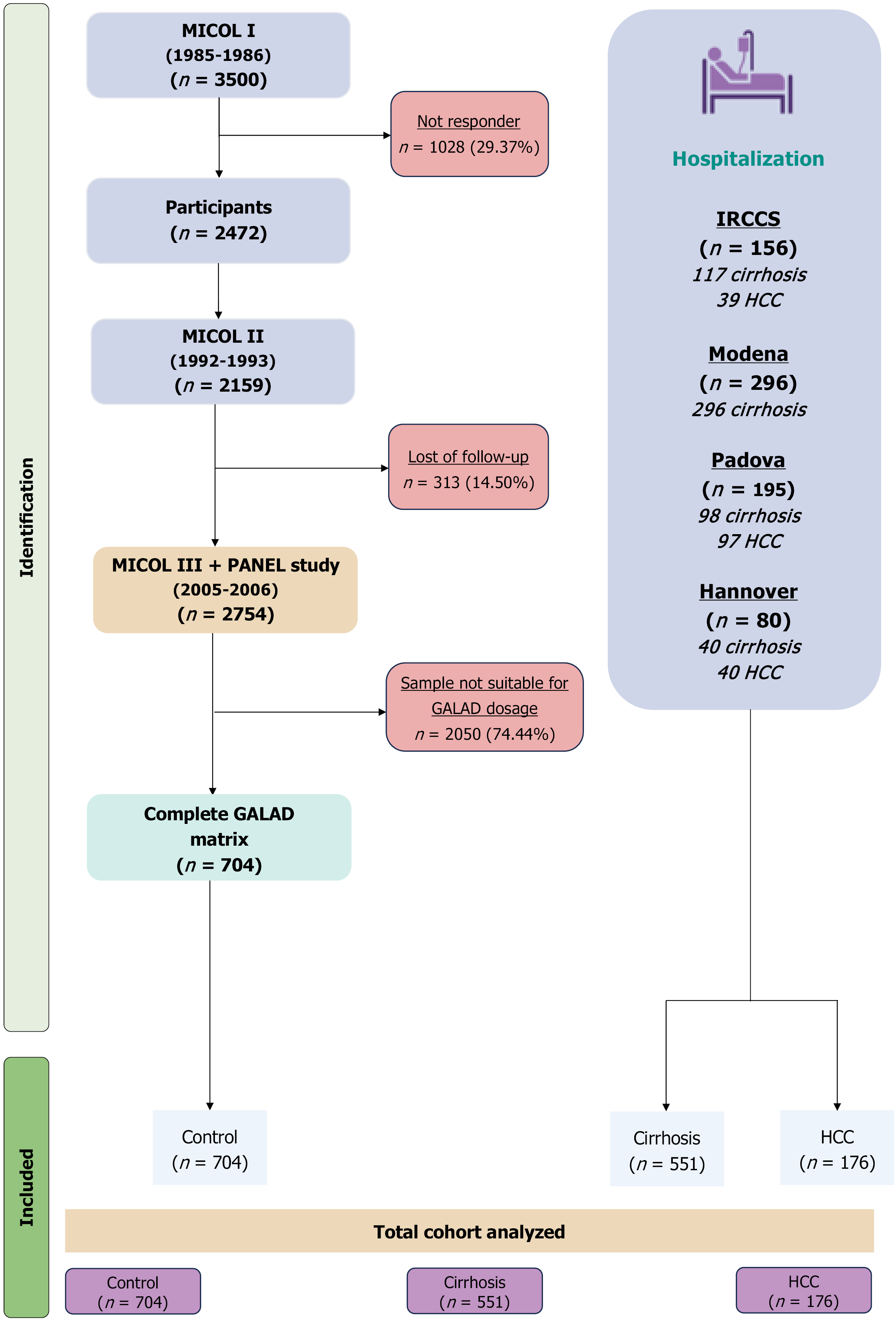
This cross-sectional analysis was conducted using prospectively collected data from five cohorts (n = 1431) comprising healthy individuals, cirrhosis, and HCC patients. These subjects were enrolled from an Italian retrospective cohort, including patients from the IRCCS “Saverio de Bellis”, Department of Gastroenterology, the University of Modena and Reggio Emilia Gastroenterology Department, and the Padua University Hospital and the Department of Gastroenterology, Hepatology, Infectious diseases and Endocrinology, Hannover Medical School.
Using healthy subjects as reference, a GALAD score cut-off of -1.67 identified HCC with a sensitivity of 89.77% and specificity of 97.59%. Individuals with GALAD values > -1.67 exhibited a moderate to very high probability (over 90%) of having HCC. When cirrhotic patients were used as the reference category, a cut-off of -0.77 yielded a sensitivity of 78.17% and a specificity of 89.55%.
We strongly recommend incorporating this GALAD cut-off into clinical guidelines for the screening of patients with a compensated advanced CLD who are at high risk of developing HCC. Given the rapid global rise in metabolic-associated steatotic liver disease (MASLD)-related CLD, future research should prioritize larger MASLD cohorts to establish the most appropriate GALAD cut-off for diagnostic use, compared to healthy controls and to patients with other forms of CLD.
Core Tip: The GALAD score, combining gender, age, alpha-fetoprotein (AFP), AFP-L3, and des-gamma-carboxy prothrombin levels, is a promising tool for identifying hepatocellular carcinoma (HCC). In this multi-center study involving 1431 subjects, we propose a cut-off of -1.67 to distinguish HCC from healthy individuals, and -0.77 to differentiate HCC from cirrhotic patients, achieving high sensitivity and specificity. These thresholds can enhance early HCC detection and are recommended for integration into screening protocols. Future studies should validate these findings, particularly in metabolic-associated steatotic liver disease-related liver disease, which is becoming increasingly prevalent worldwide.
- Citation: Villa E, Donghia R, Coletta S, Bonfiglio C, Critelli RM, Ancona A, Shahini E, Iacovazzi PA, Cozzolongo R, Pavone F, Carella N, Pontisso P, Martini A, Al Aoua S, Bantel H, Giannelli G. Insights into the GALAD score: A new optimal cut-off for hepatocellular carcinoma. World J Gastroenterol 2025; 31(40): 111727
- URL: https://www.wjgnet.com/1007-9327/full/v31/i40/111727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i40.111727
The epidemiology of hepatocellular carcinoma (HCC) has been evolving in recent years, with incidence and mortality rates projected to rise significantly over the next 30 years, particularly in Europe and the United States[1-4]. The incidence of HCC is dramatically increasing because of the marked rise in metabolic-associated steatotic liver disease (MASLD), even though hepatitis B and hepatitis C infections are decreasing[4]. Approximately 70%-95% of HCC cases occur in individuals with chronic liver disease (CLD), however, the risk is especially elevated among patients with cirrhosis, particularly males aged 40 to 60 in Western countries, with an annual chance of progression ranging from 1% to 8%[5]. Interestingly, HCC in people under 20 years has distinct features from HCC in older adults[5,6].
The prognosis and survival of patients with HCC are closely linked to the stage of disease, with early-stage disease offering the greatest opportunity for curative treatment options such as surgical resection, liver transplantation and radiofrequency ablation[7,8]. This is widely endorsed by all the scientific societies, which recommend the surveillance program in patients with cirrhosis as the standard of care. Current international guidelines advocate for abdominal ultrasound with or without alpha-fetoprotein (AFP) testing every six months[2]. Both scientific evidence and real-world clinical experience underscore the advantages of the surveillance programs in terms of overall survival, demonstrating a 37% reduction in HCC-related mortality. Furthermore, earlier recognition of HCC through adaptation of surveillance programs lead to a remarkable improvement of the overall prognosis exceeding three years. However, existing surveillance strategies have notable limitations. Ultrasound performance is highly dependent on operator expertise and equipment quality, while AFP testing suffers from low diagnostic accuracy and limited specificity[9-12]. As a result, the need to improve or complement current surveillance methods is a pressing issue under active debate. The investigation of new biomarkers is still an unmet need, requiring cross-sectional studies and appropriate prospective longitudinal clinical trials, which are difficult to realize[13-15].
Recently, a novel score known as GALAD-which incorporates gender, age, AFP-L3, AFP and des-gamma-carboxy prothrombin (DCP) has been proposed and evaluated in several cross-sectional studies, with limited longitudinal validation[15]. Additionally, the GALAD score has been suggested to correlate with overall survival. In all studies reported to date, GALAD has demonstrated superior diagnostic accuracy compared to AFP or DCP alone, as well as to other scores such as albumin-bilirubin (ALBI) or age-male-ALBI-platelets (aMAP). Despite the advantages of the GALAD score over AFP used in clinical practice, it has not yet been adopted as the standard of care, even though the assays for the three biomarkers included in the score are reliable and reproducible[16-18]. On the other hand, one limitation clinicians may face is the absence of established reference ranges, making it difficult to distinguish between normal and pathological values. Furthermore, the score lacks stratification by age and sex, despite these being components of the algorithm.
The aim of this study is to evaluate the diagnostic accuracy of GALAD score in patients with liver cirrhosis and HCC, and to define cut-offs for pathological values, in a large and multicentric cohort.
The study was designed as a cross-sectional analysis with a prospective component. The subjects evaluated in this study belonged to four different cohorts. The first group (MICOL I-II-III) included healthy individuals as well as a subset of participants with cirrhosis or HCC. Details about the MICOL cohort have been previously published[18,19]. The MICOL study, a prospective cohort study conducted at IRCCS “Saverio de Bellis” Research Hospital (Castellana Grotte, Italy), began in 1985 with a systematic 1-in-5 random sample of patients aged ≥ 30 years. Follow-ups occurred in 1992, 2005-2006, and 2013-2016. To address cohort aging, MICOL III (PANEL study) was introduced in 2005-2006, enrolling participants aged 30-50 using the same sampling method. The second group consisted of patients diagnosed with cirrhosis or HCC from our Gastroenterology Unit. The final three cohorts included patients with cirrhosis or HCC from the Gastroenterology Unit of the University of Modena and Reggio Emilia and the University Hospital of Padova as well as from Hannover Medical School. To be eligible for the study, participants had to be at least 18 years old and provide consent for serum sample storage in the Biobank. Patients with CLD had various etiologies, including viral, alcoholic or autoimmune hepatitis, MASLD, primary biliary cholangitis, primary sclerosing cholangitis, or cryptogenic when no other causes were identified. Patients with CLD were required to be enrolled in regular HCC prevention programs and have preserved liver function, classified as Child-Pugh class A or B, with a model for end-stage liver disease (MELD) score below 15. Additionally, they must have had no detectable liver mass in the previous six months, as confirmed by ultrasound, multiphasic computed tomography (CT) scan, magnetic resonance imaging (MRI) when clinically indicated, or liver biopsy in suspected HCC cases without cirrhosis.
Healthy controls were defined as individuals with no clinical, laboratory, or imaging evidence of liver disease at enrollment and throughout the follow-up period. Exclusion criteria include lack of consent for study participation, significant medical comorbidities with a life expectancy of less than one-year, hepatic decompensation, recent cancer history, prior solid organ transplant, and warfarin therapy, which may elevate DCP levels and lead to false-positive HCC diagnoses.
All studies were conducted in accordance with the ethical standards of the institutional research committee [Human Studies Committee of the IRCCS Oncological Hospital-Giovanni Paolo II, Bari, Italy approval for the Great Age-Aging based on MICOL Study (No. 144, April 09, 2019)]; patients with compensated advanced CLD from the Gastroenterology Unit “IRCCS Saverio de Bellis”: Human Studies Committee of the IRCCS Oncological Hospital-Giovanni Paolo II, Bari, Italy (No. 28, January 25, 2023; ClinicalTrials.gov, No. NCT05781568); patients from Modena (No. 1389, May 20, 2008; ClinicalTrials.gov, No. NCT01657695); patients from Padua (Ethical Committee based on resolution, No. 154, February 28, 2005); patients form Hannover (No. 10129_BO_K_2021) and with the 2024 Helsinki declaration.
All participants provided informed consent following the principles outlined in the Declaration of Helsinki. The baseline information collection encompassed population demographics, medical history, routine liver biochemical tests, and a blood sample stored in a biobank for research use (i.e., biomarker tests). We classified CLD as viral, alcoholic liver disease, MASLD, or other (see above). We assessed liver disease severity using the Child-Pugh classification and MELD score to evaluate liver compensation in clinical practice. At each follow-up, blood samples were collected, and medical history updates were recorded. Based on these assessments, the study population was categorized into three subgroups: (1) Individuals without CLD, who remained free of liver disease at both the initial MICOL study and the second follow-up after 10 years; (2) Patients with compensated liver cirrhosis; and (3) Patients with a confirmed diagnosis of HCC. The diagnosis and surveillance of advanced CLD were based on blood tests and the presence of characteristic radiological features on one or more imaging modalities (ultrasound, MRI, or CT scan), in accordance with national and international guidelines (Figure 1)[2,12].
The GALAD score was determined using the automated Fujifilm TASWakoTM i30 analyzer that quantitatively measures the biomarker (AFP, AFP-L3, and DCP) concentrations by microfluidic electrophoretic separation, as previously described[13,20]. The formula included AFP, AFP-L3, and DCP values, as well as variables such as age and gender (encoding 1 for males and 0 for females), as follows: Z = -10.08 + 1.67 × gender + 0.09 × age + 0.04 × AFP-L3 + 2.34 × log10 (AFP) + 1.33 × log10 (DCP)[13]. The reportable range for AFP concentration is 0.3-1000 ng/mL, while the range for AFP-L3 is 0.5%-99.5%, and the range for DCP concentration is 0.1-950 ng/mL, with expected values typically below 7.5 ng/mL[11]. The clinical database used in this analysis was openly available in FigShare at https://doi.org/10.6084/m9.figshare.28794065.v1. All analyses were performed by an unblinded statistician.
Subjects’ and blood characteristics are reported as mean ± SD or median and interquartile range for continuous variables and as frequency and percentages (%) for categorical variables. The Shapiro-Wilk test was used to assess the normality. The χ2 test was used to evaluate the relationship between groups (i.e., healthy, cirrhosis, and HCC) for categorical variables, and the Kruskal-Wallis equality rank test for more than two independent groups. Multiple pairwise comparisons were made using Dunn’s test with Bonferroni’s adjustment. We utilized a complete matrix for GALAD score and outcome diseases. For other variables, missing data accounted for less than 20%[21], a threshold that permits robust and accurate statistical inference. The probability of diagnosing HCC using the GALAD score was calculated from the z-score of a logistic regression model and classified into five risk classes: Very low (0%-10%), low (10%-25%), moderate (25%-50%), high (50%-75%), and very high (75%-100%). GALAD’s predictive performance was assessed using receiver operating characteristic (ROC) analysis to evaluate the sensitivity-specificity tradeoff and identify a new optimal diagnostic cut point. This cut-off point maximizes Youden’s index while minimizing the distance from (0, 1) in the ROC space, which represents optimal sensitivity and specificity. Due to the challenge of obtaining an external cohort for validation, particularly in ensuring a disease-free control group, a K-fold cross-validation was used to provide a realistic estimate of predictive performance. This approach was preferred over randomly splitting data into two groups[22,23]. A 10-fold cross-validation (K = 10) was selected. And a calibration plot were built to evaluate how well a model predicts the probabilities (Supplementary Figures 1 and 2). Furthermore, a retrospective power analysis was performed to show that our sub-cohort was adequate for calculating the new clinical threshold. The analyses were carried out using StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC., and RStudio (“Chocolate Cosmos” Release).
We divided our final cohort of 1431 subjects into three sub-cohorts: 704 (49.20%) healthy individuals, 511 (35.71%) with compensated cirrhosis, and 176 (12.30%) with HCC. Table 1 shows the patient characteristics. The healthy group was significantly younger (50.84 ± 13.69) compared to the cirrhosis (60.27 ± 11.97) and HCC (67.49 ± 9.79) groups. Diabetes was significantly associated with advanced CLD, with a similar prevalence in HCC patients (30.68%) compared to those with cirrhosis (30.85%). Blood sugar, bilirubin, alanine aminotransferase/glutamic pyruvic transaminase, aspartate aminotransferase/glutamic oxaloacetic transaminase, and gamma-glutamyl transferase were all significantly higher in patients with advanced CLD. A similar significant difference was observed in biomarkers used in the GALAD algorithm, namely AFP, AFP-L3, and DCP (803.28 ± 3418.54, 20.48 ± 25.82, and 130.36 ± 482.28, respectively) in the HCC category.
| Parameters | Total cohort | Healthy (n = 704) | Advanced CLD | P value1 | |
| Cirrhosis (n = 551) | HCC (n = 176) | ||||
| Age (years) | 56.52 ± 13.97 | 50.84 ± 13.69 | 60.27 ± 11.97 | 67.49 ± 9.79 | 0.0001 |
| Gender (male) | 908 (63.45) | 413 (58.66) | 356 (64.61) | 139 (78.98) | < 0.0012 |
| BMI (kg/m2) | 27.57 ± 5.05 | 28.03 ± 4.92 | 27.09 ± 5.15 | 26.87 ± 5.13 | 0.0002 |
| Diabetes (yes) | 261 (18.24) | 37 (5.26) | 170 (30.85) | 54 (30.68) | < 0.0012 |
| Child-Pugh | 0.0022 | ||||
| A | 362 (65.70) | 94 (53.41) | |||
| B | 140 (25.41) | 69 (39.20) | |||
| C | 49 (8.89) | 13 (7.39) | |||
| Etiology | < 0.0012 | ||||
| HBV | 52 (9.52) | 9 (5.26) | |||
| HCV | 164 (30.04) | 74 (43.27) | |||
| MASLD | 100 (18.32) | 16 (9.36) | |||
| Alcohol | 126 (23.08) | 55 (32.16) | |||
| Cryptogenic | 3 (0.55) | 7 (4.09) | |||
| Other | 101 (18.50) | 10 (5.85) | |||
| Blood | |||||
| Blood sugar (mg/dL) | 109.71 ± 32.43 | 105.71 ± 19.48 | 123.71 ± 58.79 | 123.23 ± 41.65 | 0.03 |
| Bilirubin (mg/dL) | 2.22 ± 5.14 | 0.88 ± 0.31 | 3.00 ± 6.20 | 5.64 ± 9.08 | 0.0001 |
| GPT (U/L) | 32.99 ± 52.13 | 14.80 ± 7.51 | 54.28 ± 77.23 | 50.12 ± 42.79 | 0.0001 |
| GOT (U/L) | 33.63 ± 53.05 | 10.89 ± 2.89 | 50.78 ± 55.36 | 77.01 ± 94.40 | 0.0001 |
| GGT (U/L) | 54.68 ± 102.74 | 12.99 ± 6.73 | 103.51 ± 132.82 | 139.30 ± 151.20 | 0.0001 |
| AFP (ng/mL) | 109.31 ± 1236.92 | 2.06 ± 1.19 | 24.66 ± 289.01 | 803.28 ± 3418.54 | 0.0001 |
| AFP-L3 (%) | 4.31 ± 12.53 | 0.44 ± 3.04 | 4.09 ± 9.01 | 20.48 ± 25.82 | 0.0001 |
| DCP (ng/mL) | 20.30 ± 178.29 | 0.81 ± 13.41 | 10.05 ± 62.80 | 130.36 ± 482.28 | 0.0001 |
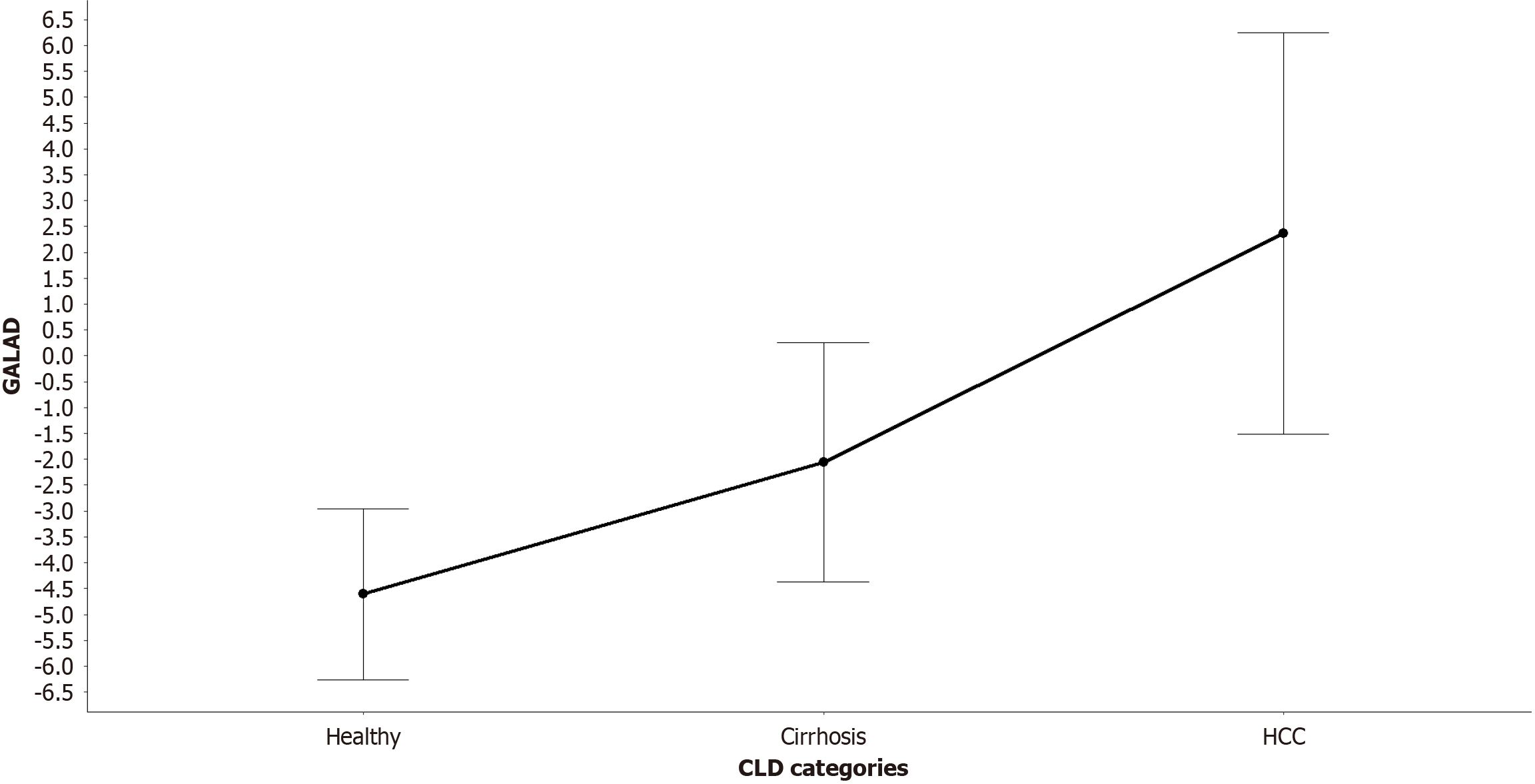
GALAD scores differed significantly across groups (Table 2), with the control group showing the lowest values (-4.61 ± 1.65), followed by progressively increasing values in the cirrhosis (-2.06 ± 2.31) and HCC (2.36 ± 3.88) groups (shown in Figure 2).
The distribution of GALAD by liver condition, as well as by gender and age group (> 50 vs ≤ 50 years) is shown in the Supplementary Figure 3. The area under curve (AUC), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy values to assess GALAD’s performance in all advanced CLD combinations are reported in Table 3. For each combination, K-fold validation was also performed, and the difference between the K-fold estimate and the classical approach was calculated.
| Response variable | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
| Control (Ref.) | ||||||
| HCC | 0.9725; (K-fold = 0.9743); Δ = 0.0018 | 85.23 | 98.86 | 94.94 | 96.40 | 96.14 |
| Cirrhosis (Ref.) | ||||||
| HCC | 0.8609; (K-fold = 0.8657); Δ = 0.0048 | 47.73 | 95.28 | 76.36 | 85.09 | 83.77 |
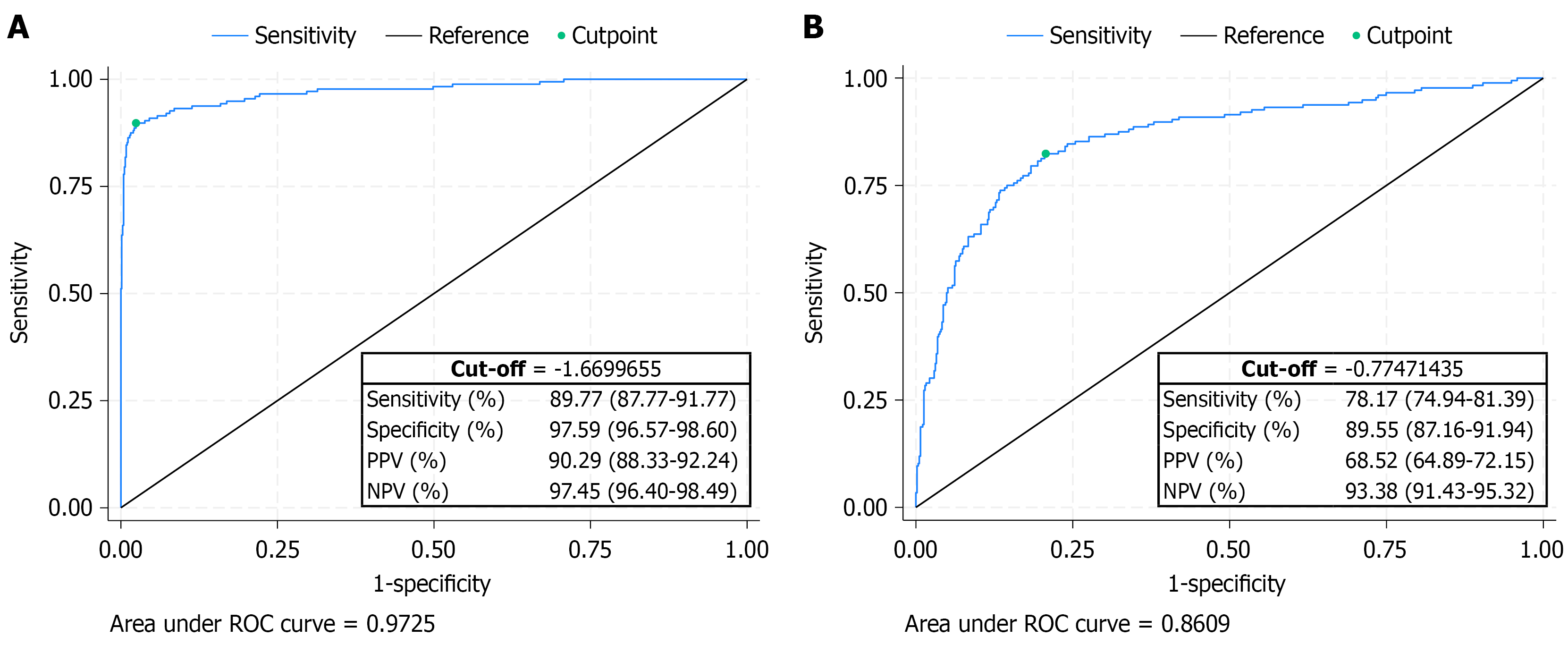
Using healthy subjects as reference, the differentiation from the HCC group demonstrated the highest diagnostic accuracy (AUC = 0.97), which was also reflected in sensitivity, specificity, PPV, NPV, and overall accuracy. When cirrhosis was used as the reference category, GALAD’s performance had an AUC of 0.86 (shown in Figure 3). Calibration plots were built showing an overall good agreement between predicted and observed probabilities of HCC. The model is well-calibrated in the low and high predicted risk ranges (Supplementary Figures 1 and 2).
For the comparison between healthy individuals and patients with HCC, the cut-off point of -1.67 determined by the Youden index maximized both sensitivity and specificity (shown in Figure 3A). At this threshold, the GALAD score demonstrated strong performance in identifying HCC-positive cases (sensitivity: 89.77%) and high reliability in ruling out negatives (specificity: 97.59%). Moreover, the PPV was 90.29%, while the NPV reached 97.45%. Understandably, depending on the type of comparison (e.g., healthy individuals vs HCC) the test was more reliable in excluding HCC than in confirming it, as reflected by the higher NPV compared to the PPV. Patients with a GALAD > -1.67 had about a 90% chance of having a moderate or high risk of having HCC (Table 4).
| GALAD categories | Very low risk (0%-10%) | Low risk (10%-25%) | Moderate risk (25%-50%) | High risk (50%-75%) | Very high risk (75%-100%) |
| HCC vs healthy | |||||
| ≤ -1.6699655 | 953 (100.00) | 88 (51.16) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| > -1.6699655 | 0 (0.00) | 84 (48.84) | 99 (100.00) | 59 (100.00) | 148 (100.00) |
| Cirrhosis vs HCC | |||||
| ≤ -0.77471435 | 953 (100.00) | 172 (100.00) | 41 (100.00) | 0 (0.00) | 0 (0.00) |
| > -0.77471435 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 15 (100.00) | 148 (100.00) |
For the comparison between patients with cirrhosis and those with HCC, a cut-off point of -0.77 determined by the Youden index, maintained a good sensitivity and specificity. The NPV (93.38%) was again very good while the PPV was slightly lower (68.52%) (shown in Figure 3B). Patients with a GALAD > -0.77 had a high to very high risk of having HCC (Table 4).
In this multicenter study, we evaluated the diagnostic performance of the GALAD score in differentiating HCC from both healthy individuals and patients with compensated advanced CLD. Our findings confirm the robustness of GALAD as a diagnostic tool, particularly in distinguishing HCC from healthy individuals, and to a slightly lesser extent, from patients with cirrhosis. The GALAD score demonstrated excellent diagnostic accuracy in distinguishing HCC from healthy controls, with an AUC of 0.97. At the optimal cut-off of -1.67 (determined by Youden’s index), GALAD achieved high sensitivity (85.23%) and specificity (98.86%), with strong predictive values (PPV: 94.94%, NPV: 96.40%). These values support the potential of GALAD to be a reliable, non-invasive biomarker-based tool in screening and early detection programs. Importantly, the test proved more reliable in ruling out HCC than confirming it, as evidenced by the higher NPV compared to PPV a common feature in screening tests where minimizing false negatives is critical. To date, only a few studies have evaluated GALAD performance against healthy controls. Among them, the study by Liu et al[20] reported comparable results, further reinforcing the potential of GALAD as a valuable screening tool, particularly in regions with high HCC prevalence.
When cirrhosis was used as the reference group a scenario more reflective of clinical reality GALAD maintained good discriminative ability (AUC = 0.86), although performance was moderately reduced. At a cut-off of -0.77, sensitivity remained acceptable (47.73%), while specificity remained high (95.28%), confirming GALAD’s utility in risk stratification among patients with cirrhosis. The drop in sensitivity in this context underscores the inherent challenge of distinguishing HCC from advanced liver disease using biomarkers alone, as background liver dysfunction and inflammation may obscure diagnostic signals. Notably, GALAD performance varies depending on HCC stage, with diagnostic accuracy improving from early to advanced stages[21]. In this study, GALAD’s performance in early-stage HCC (Barcelona Clinic Liver Cancer 0-A) was comparable to that in patients with Barcelona Clinic Liver Cancer B, in terms of sensitivity, specificity, and overall accuracy. Similar results were also reported by Vo et al[22], further supporting GALAD’s diagnostic value in early-stage HCC. This consistency is expected, as all HCC cases in our study were detected through surveillance and classified as early-stage disease.
Notably, our analysis revealed that GALAD scores varied significantly by liver condition, sex, and age, with higher values in males and in older individuals. This variability reflects the components of the GALAD algorithm and underlines the importance of interpreting scores in context. However, one current limitation of the GALAD score remains the lack of clearly established normal reference ranges, particularly across different demographic subgroups. This absence complicates integration into routine surveillance pathways and may contribute to its limited clinical adoption despite favorable performance metrics. Compared to existing tools, GALAD consistently outperformed AFP alone and other scoring systems such as aMAP and ALBI, as reported in prior studies[18,23]. Moreover, the automated quantification of AFP, AFP-L3, and DCP enhances reproducibility and minimizes operator dependency, a known limitation of ultrasonography, which remains the cornerstone of current surveillance guidelines.
Furthermore, in the paper by Yang et al[24], which compared the performance of GALAD with ultrasound, they demonstrated that GALAD showed superior performance in diagnosing HCC. Therefore, the authors concluded that the GALAD score also complements ultrasound, proving particularly useful in patients with advanced liver dysfunction or obesity, conditions that can lead to false-negative ultrasound results. For this reason, they introduced the GALADUS score, which combines GALAD with ultrasound results and demonstrated superiority over using either method alone[24].
Cross-validation with a 10-fold approach further confirmed the reliability of our findings, minimizing the risk of overfitting and providing a realistic performance estimate. Retrospective power calculations confirmed that our sample size was adequately powered to detect clinically relevant differences. Despite these strengths, our study has limitations. First, the cross-sectional nature of the analysis limits the assessment of GALAD’s predictive capacity over time. Second, while the multi-center design improves generalizability, variations in patient selection and imaging practices may introduce heterogeneity. Finally, the absence of an external validation cohort limits the broader applicability of the optimal cut-offs identified.
In conclusion, future research should focus on the longitudinal validation of GALAD in real-world surveillance cohorts, ideally integrated with imaging and emerging biomarkers. Standardization of assay procedures and the definition of risk-adapted thresholds potentially stratified by sex, age, and etiology are critical steps toward clinical implementation.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68525] [Article Influence: 13705.0] [Reference Citation Analysis (201)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6413] [Article Influence: 801.6] [Reference Citation Analysis (9)] |
| 3. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3434] [Article Influence: 429.3] [Reference Citation Analysis (3)] |
| 4. | Koshy A. Evolving Global Etiology of Hepatocellular Carcinoma (HCC): Insights and Trends for 2024. J Clin Exp Hepatol. 2025;15:102406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 5. | Trinchet JC, Bourcier V, Chaffaut C, Ait Ahmed M, Allam S, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Goria O, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Buffet C, Bacq Y, Wartelle C, Dao T, Benhamou Y, Pilette C, Silvain C, Christidis C, Capron D, Thiefin G, Hillaire S, Di Martino V, Nahon P, Chevret S; ANRS CO12 CirVir Group. Complications and competing risks of death in compensated viral cirrhosis (ANRS CO12 CirVir prospective cohort). Hepatology. 2015;62:737-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Khanna R, Verma SK. Pediatric hepatocellular carcinoma. World J Gastroenterol. 2018;24:3980-3999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 7. | Wang QB, Li J, Zhang ZJ, Li YK, Liang YB, Chen XM, Luo WL, Lakang Y, Yang ZS, Liu GY, Liu Y, Li SX, Ke Y. The effectiveness and safety of therapies for hepatocellular carcinoma with tumor thrombus in the hepatic vein, inferior vena cave and/or right atrium: a systematic review and single-arm meta-analysis. Expert Rev Anticancer Ther. 2025;25:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 51] [Article Influence: 51.0] [Reference Citation Analysis (1)] |
| 8. | Li YK, Wu S, Wu YS, Zhang WH, Wang Y, Li YH, Kang Q, Huang SQ, Zheng K, Jiang GM, Wang QB, Liang YB, Li J, Lakang Y, Yang C, Li J, Wang JP, Kui X, Ke Y. Portal Venous and Hepatic Arterial Coefficients Predict Post-Hepatectomy Overall and Recurrence-Free Survival in Patients with Hepatocellular Carcinoma: A Retrospective Study. J Hepatocell Carcinoma. 2024;11:1389-1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 82] [Reference Citation Analysis (0)] |
| 9. | Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology. 2021;73:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 255] [Article Influence: 51.0] [Reference Citation Analysis (2)] |
| 10. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 976] [Article Influence: 44.4] [Reference Citation Analysis (1)] |
| 11. | Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008;6:1418-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Tzartzeva K, Singal AG. Testing for AFP in combination with ultrasound improves early liver cancer detection. Expert Rev Gastroenterol Hepatol. 2018;12:947-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4428] [Article Influence: 885.6] [Reference Citation Analysis (4)] |
| 14. | Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 1408] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 15. | Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, Hull D, Patman G, Kagebayashi C, Hussain S, Graham J, Reeves H, Satomura S. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 16. | Singal AG, Tayob N, Mehta A, Marrero JA, El-Serag H, Jin Q, Saenz de Viteri C, Fobar A, Parikh ND. GALAD demonstrates high sensitivity for HCC surveillance in a cohort of patients with cirrhosis. Hepatology. 2022;75:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 17. | Wang X, Zhang Y, Yang N, He H, Tao X, Kou C, Jiang J. Evaluation of the Combined Application of AFP, AFP-L3%, and DCP for Hepatocellular Carcinoma Diagnosis: A Meta-analysis. Biomed Res Int. 2020;2020:5087643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Villa E, Donghia R, Baldaccini V, Tedesco CC, Shahini E, Cozzolongo R, Ascari S, Pesole PL, Coletta S, Critelli RM, Lasagni S, Schepis F, Semellini F, Giannelli G. GALAD outperforms aMAP and ALBI for predicting HCC in patients with compensated advanced chronic liver disease: A 12-year prospective study. Hepatol Commun. 2023;7:e0262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Osella AR, Misciagna G, Guerra VM, Chiloiro M, Cuppone R, Cavallini A, Di Leo A. Hepatitis C virus (HCV) infection and liver-related mortality: a population-based cohort study in southern Italy. The Association for the Study of Liver Disease in Puglia. Int J Epidemiol. 2000;29:922-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Jiang W, Li X, Zhao H, Wang S. The Diagnostic Performance of AFP, AFP-L3, DCP, CA199, and Their Combination for Primary Liver Cancer. J Hepatocell Carcinoma. 2025;12:513-526. [PubMed] [DOI] [Full Text] |
| 21. | Hou J, Berg T, Vogel A, Piratvisuth T, Trojan J, De Toni EN, Kudo M, Malinowsky K, Findeisen P, Hegel JK, Schöning W, Madin K, Kroeniger K, Lik-Yuen Chan H, Sharma A. Comparative evaluation of multimarker algorithms for early-stage HCC detection in multicenter prospective studies. JHEP Rep. 2025;7:101263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Vo TD, Mai SH, Lam HT. Evaluating the GALAD Score for Detection of Hepatocellular Carcinoma in Patients With Cirrhosis. J Clin Gastroenterol. 2025;59:915-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 23. | Cagnin S, Donghia R, Martini A, Pesole PL, Coletta S, Shahini E, Boninsegna G, Biasiolo A, Pontisso P, Giannelli G. Galad Score as a Prognostic Marker for Patients with Hepatocellular Carcinoma. Int J Mol Sci. 2023;24:16485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 24. | Yang JD, Addissie BD, Mara KC, Harmsen WS, Dai J, Zhang N, Wongjarupong N, Ali HM, Ali HA, Hassan FA, Lavu S, Cvinar JL, Giama NH, Moser CD, Miyabe K, Allotey LK, Algeciras-Schimnich A, Theobald JP, Ward MM, Nguyen MH, Befeler AS, Reddy KR, Schwartz M, Harnois DM, Yamada H, Srivastava S, Rinaudo JA, Gores GJ, Feng Z, Marrero JA, Roberts LR. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol Biomarkers Prev. 2019;28:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/