Published online Oct 21, 2025. doi: 10.3748/wjg.v31.i39.111323
Revised: July 29, 2025
Accepted: September 12, 2025
Published online: October 21, 2025
Processing time: 116 Days and 4.9 Hours
Hepatology encompasses various aspects, such as metabolic-associated fatty liver disease, viral hepatitis, alcoholic liver disease, liver cirrhosis, liver failure, liver tumors, and liver transplantation. The global epidemiological situation of liver diseases is grave, posing a substantial threat to human health and quality of life. Characterized by high incidence and mortality rates, liver diseases have emerged as a prominent global public health concern. In recent years, the rapid advan
Core Tip: This minireview highlights artificial intelligence innovations in hepatology. It develops multi-modal data fusion strategies integrating imaging, genomics, lab results, and clinical records via transformer models and graph neural networks to uncover latent associations and build disease knowledge graphs. Convolutional neural networks enable automated, high-accuracy liver lesion detection and quantification in pathology/imaging. Artificial intelligence optimizes personalized antiviral therapy and liver transplant outcomes through drug-response prediction and graft-recipient matching. Natural language processing extracts critical insights from electronic health records. These approaches significantly advance diagnosis, treatment personalization, and translational research in precision hepatology.
- Citation: Zheng Y, Li H, Wang R, Jiang CS, Zhao YT. Multi-model applications and cutting-edge advancements of artificial intelligence in hepatology in the era of precision medicine. World J Gastroenterol 2025; 31(39): 111323
- URL: https://www.wjgnet.com/1007-9327/full/v31/i39/111323.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i39.111323
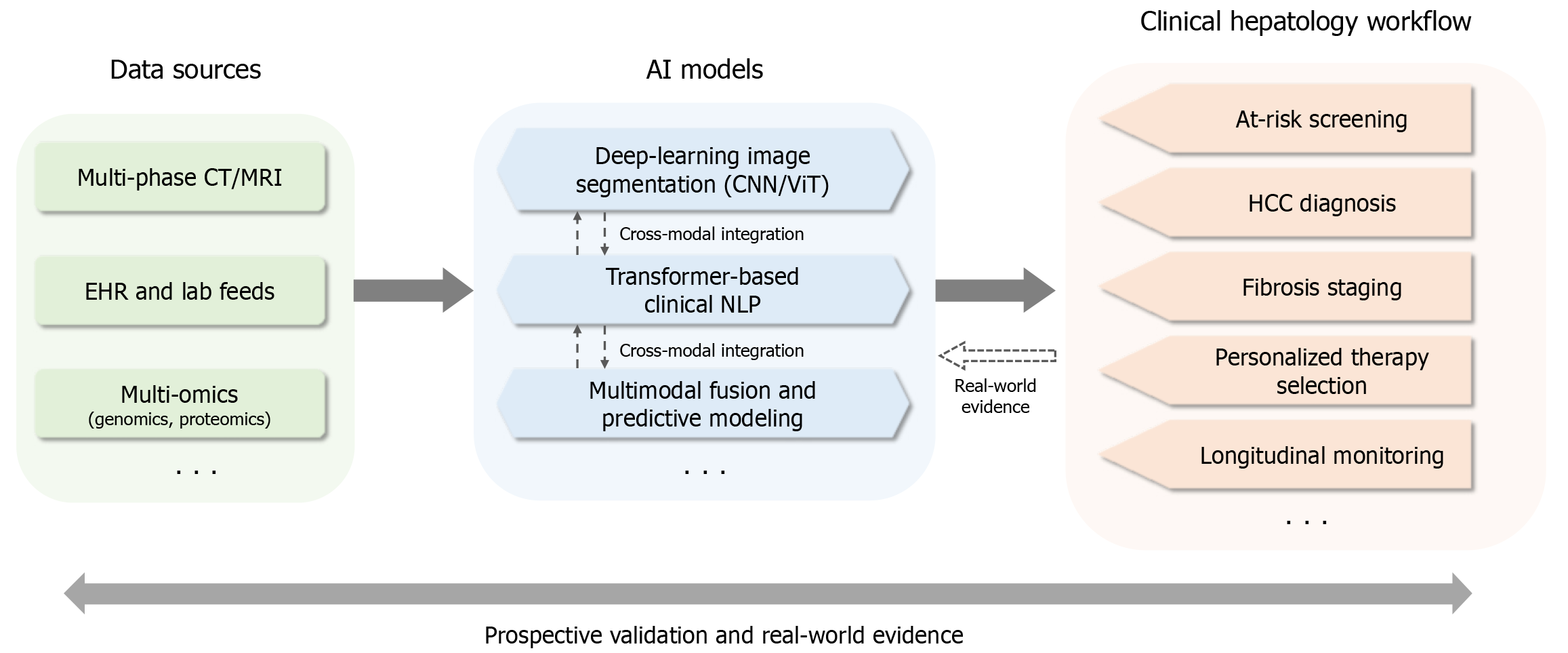
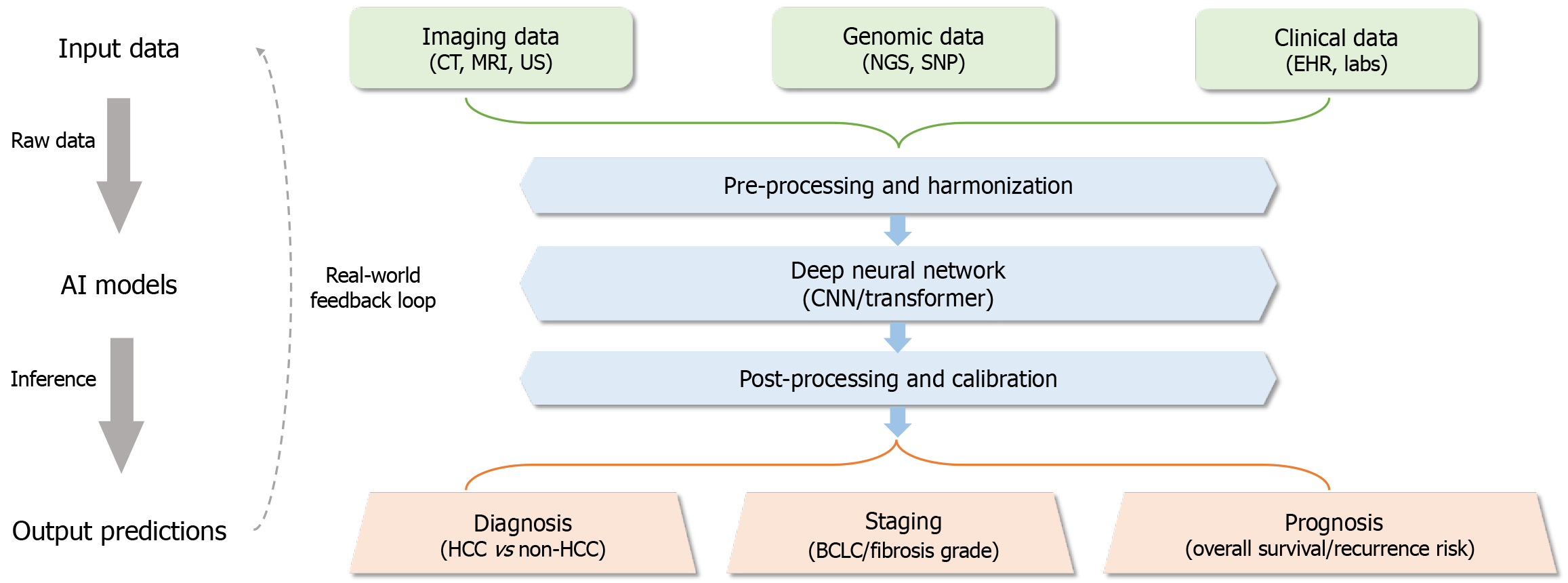
A conceptual framework (Figure 1) showing the interaction between artificial intelligence (AI) models and clinical workflows in hepatology. Figure 2 shows the AI-assisted diagnostic pipeline.
In the field of disease diagnosis and classification, radiomics and deep learning (DL) techniques are widely utilized in liver image analysis to evaluate liver steatosis and cirrhosis staging[1-5]. Radiomics extracts morphological, texture, and advanced features from liver images, combining them with machine learning algorithms to construct classification models. These models distinguish patients with metabolic-associated fatty liver diseases from healthy controls or assess the severity of steatosis[1]. DL, particularly convolutional neural networks (CNNs), excels in image analysis by automatically extracting more representative features, significantly improving diagnostic accuracy and robustness[5].
In terms of predicting disease progression and risk, machine learning models are employed to forecast the risk of non-alcoholic steatohepatitis progressing to fibrosis and the prognosis of patients with acute liver failure[6]. Traditional statistical methods struggle with complex data relationships and high-dimensional data, whereas machine learning algorithms-such as least absolute shrinkage and selection operator regression, random forests, support vector machines (SVMs), gradient-boosting trees (e.g., extreme gradient boosting, light gradient boosting machine), and DL models [e.g., multilayer perceptrons, recurrent neural networks (RNNs)]-effectively handle complex clinical data. These algorithms construct predictive models, identify risk factors associated with disease progression, and predict patient outcomes[7-10].
Additionally, natural language processing (NLP) technology plays a significant role in analyzing electronic health records (EHRs). EHRs contain vast amounts of unstructured text data, such as doctors’ diagnostic records, progress notes, and medication histories. NLP extracts valuable information from this text to aid diagnosis and classification[11,12]. For instance, named entity recognition identifies symptoms reported by patients (e.g., fatigue, jaundice) and laboratory test results (e.g., elevated alanine aminotransferase, hepatitis B virus DNA positivity). Text classification algorithms differentiate between types of viral hepatitis and assess the severity of liver function impairment[13]. Furthermore, NLP integrates text information with structured data to build predictive models, evaluating the risk of progression to cirrhosis or hepatocellular carcinoma and issuing early warnings[14].
AI plays a critical role in patient monitoring by analyzing real-time physiological indicators such as heart rate, blood pressure, and oxygen saturation levels. These AI systems are capable of predicting changes in a patient’s condition and issuing early warnings, which is particularly vital in resource-constrained settings where comprehensive monitoring and timely interventions may be limited. The predictive capabilities of AI systems enable timely interventions by healthcare providers, helping to prevent deterioration of conditions and improve patient outcomes.
In the realm of real-time clinical decision-making, AI systems provide valuable support by rapidly analyzing the latest patient data, including lab results, medication records, and physiological metrics. For instance, AI can predict a patient’s response to a specific treatment or identify potential risks of complications, such as in liver disease patients. This real-time decision support enhances diagnostic and therapeutic efficiency while minimizing the likelihood of human error. In resource-limited environments, where healthcare providers often face dual pressures of time and resource constraints, AI-driven support becomes especially critical.
The integration of AI with EHR systems is a pivotal strategy for improving healthcare efficiency and patient care quality. By seamlessly connecting with EHRs, AI systems can extract and consolidate historical patient data, lab results, and medication records, providing clinicians with a more comprehensive foundation for decision-making. This integra
AI significantly contributes to optimizing antiviral and liver transplant treatment regimens. In antiviral therapy, AI predicts patients’ responses to different antiviral drugs by analyzing clinical characteristics, viral features, and treatment histories using machine learning models such as SVMs, random forests, and DL. By integrating genomic data (e.g., single nucleotide polymorphisms) and proteomics data, researchers identify genetic variations associated with drug meta
In liver transplantation, AI assesses graft quality by analyzing imaging and genomic data. Machine learning models integrate patients’ clinical features, laboratory indices, and graft characteristics to predict the likelihood of postoperative complications and identify genetic risk factors[17,18]. AI also optimizes recipient matching by predicting the success probability of donor-recipient pairs based on clinical features, graft characteristics, and postoperative outcomes. Post-transplantation, AI monitors patients’ recovery in real time, predicts potential risks, and tailors’ treatment plans based on genomic data[17].
Traditional liver imaging and histopathological analysis rely heavily on clinicians’ experience and visual judgment, leading to subjectivity and inefficiency. AI has revolutionized liver image analysis, particularly in lesion detection, classification, and diagnosis. In liver imaging, CNNs are widely used for detecting and classifying hepatocellular carcinoma, hepatic cysts, and vascular tumors. Radiomics technology analyzes computed tomography (CT) or magnetic resonance imaging (MRI) images to extract features associated with fibrosis and cirrhosis, constructing machine learning models to predict fibrosis and cirrhosis staging. Radiomics provides non-invasive, efficient tools for clinical decision-making[19]. In histopathological image analysis, DL techniques, such as CNNs, are used to identify fibrosis, inflammation, and other pathological features. DL models automatically quantify collagen deposition and inflammatory cell infiltration, offering objective evidence for fibrosis diagnosis and staging[5]. Transformer models also demonstrate exceptional performance in histopathological image analysis.
Traditional prediction methods rely on clinical experience and statistical models, such as the Cox proportional hazards model, which face limitations in handling complex, dynamic clinical data. AI provides innovative solutions for liver disease prediction and risk assessment, particularly in analyzing time-series data and constructing personalized predictive models. RNNs are widely applied in predicting the progression of liver diseases. RNNs, capable of processing time-series data, utilize memory mechanisms to capture temporal dependencies. Liver disease patients’ clinical data, such as laboratory results, imaging features, and treatment responses, are often represented as time-series data. RNNs effectively analyze these data to predict future disease progression. For example, RNNs can predict the risk of chronic hepatitis B progressing to cirrhosis by analyzing viral load, liver function indicators, and treatment responses[6]. Additionally, RNNs integrate multimodal clinical data (e.g., laboratory results and gene expression data) to provide comprehensive predictions. RNNs’ advantages include capturing temporal dependencies, constructing personalized models, and fusing multimodal data, significantly improving prediction accuracy[20].
SVMs are widely used in predicting mortality risks in liver diseases. SVMs analyze patients’ clinical data (e.g., age, gender, liver function indicators, and comorbidities) to construct mortality risk prediction models[21]. By mapping data to high-dimensional spaces using kernel functions (e.g., radial basis function kernels), SVMs handle non-linear relationships in clinical data, enhancing model performance. For instance, SVMs can predict mortality risks in cirrhotic patients by integrating laboratory results, imaging features, and treatment responses[15]. SVMs excel in classification tasks, handling non-linear relationships, and synthesizing multifactorial data, though their performance depends on kernel function selection and parameter tuning, and they are sensitive to noisy data.
Basic research in hepatology and translational medicine aim to elucidate disease mechanisms and apply research findings to clinical treatment. Traditional methods, relying on experimental techniques and statistical analyses, face limitations in data processing and complexity. AI provides innovative solutions, particularly in integrating multi-omics data and identifying therapeutic targets. AI integrates genomics, proteomics, and metabolomics data to uncover interconnections across data layers, offering comprehensive insights into disease mechanisms. Genomics focuses on the structure, function, and expression of the entire genome. AI, particularly DL, processes large genomic datasets to identify mutations or expression abnormalities associated with liver diseases[15]. For example, CNNs identify gene mutations linked to fibrosis and hepatocellular carcinoma, while long short-term memory networks analyze time-series gene expression data to reveal dynamic changes in liver diseases[15,22]. Graph neural networks (GNNs) construct gene interaction networks to identify key genes and modules. Proteomics investigates protein expression, function, and interactions, with AI aiding in analyzing proteomic data to discover critical protein nodes and pathways[23]. For instance, GNNs analyze protein interaction networks to identify modules associated with liver diseases, while SVMs classify proteomic data from patients and healthy controls. Metabolomics examines metabolite types, concentrations, and changes, with AI identifying metabolic pathways and biomarkers linked to liver diseases[24,25]. For example, random forests classify metabolomic biomarkers in patients and healthy controls, while GNNs analyze metabolic networks to discover disease-related modules[25].
Machine learning models identify potential therapeutic targets by analyzing multi-omics data. For instance, SVMs classify genes and proteins associated with liver diseases to screen for therapeutic targets. Additionally, machine learning optimizes drug design, enhancing efficacy and safety. For example, generative adversarial networks design novel drug molecules with optimized pharmacological properties[26]. Furthermore, machine learning discovers new therapeutic applications for existing drugs. Random forests analyze drug databases to identify compounds with potential hepatological applications, facilitating drug repurposing[27,28].
The frontier technologies of AI in hepatology primarily encompass DL models, radiomics, NLP, and multi-modal data fusion (Table 1).
| Model | Advantages | Disadvantages/limitations | Primary applications in hepatology |
| CNNs | Efficient feature extraction; rapid processing of large datasets; high accuracy and automation; integrates multimodal data | May require large labeled datasets; limited interpretability | Liver image analysis (magnetic resonance imaging/computed tomography/ultrasonography); lesion detection and classification; fibrosis staging; integration with clinical data for prognosis |
| Transformer | Powerful sequence modeling; multi-task processing; excels at global feature extraction and multiscale analysis | High computational complexity; limited adaptability to small datasets | Multi-modal data fusion (imaging + genomics + clinical records); prediction of survival outcomes; gene-imaging association analysis |
| GNNs | Models complex graph structures; dynamically updates knowledge graphs; enables reasoning across multi-source data | Requires well-structured graph data; computational cost may be high for large networks | Construction of disease knowledge graphs; identification of therapeutic targets; analysis of gene-protein-metabolite interactions |
| Radiomics | Extracts high-dimensional features from images; non-invasive and efficient; improves diagnostic and prognostic performance | Dependent on image quality and standardization; requires validation in multi-center settings | Diagnosis and classification of liver cancer; cirrhosis staging; prediction of survival outcomes |
| NLP | Extracts valuable information from unstructured text; integrates multi-source medical information | Limited by data quality and heterogeneity; may require domain-specific tuning | Analysis of electronic health records; construction of knowledge graphs for clinical decision support |
| Multi-modal fusion | Enhances predictive performance by integrating imaging, genomic, lab, and clinical data | Complexity in aligning and fusing heterogeneous data sources | Precise diagnosis; severity assessment; personalized treatment planning; comprehensive predictive modeling |
CNNs: CNNs play a significant role in liver image analysis. By analyzing MRI, CT, and ultrasound images, CNNs extract morphological and texture features for precise lesion identification[5,29]. Additionally, CNNs can integrate imaging features, such as measuring lesion size and signal intensity, with clinical data to stage liver cancer or liver fibrosis and assess patient prognosis, providing objective diagnostic metrics and evidence for personalized treatment[30]. The ad
Transformer models: Transformer models demonstrate immense potential in multi-modal data fusion. By integrating liver imaging data, laboratory test results, genomics data, and clinical records, Transformer models can uncover latent associations among diverse datasets. For example, these models can combine imaging features with laboratory data to predict the survival outcomes of liver cancer patients or integrate genomics and imaging data to reveal gene-imaging associations[31,32]. Transformer models also demonstrate exceptional performance in histopathological image analysis, leveraging self-attention mechanisms to extract global features and fuse multiscale features for identifying fibrosis distribution patterns and classifying histopathological images. While transformers excel in global feature extraction and multiscale analysis, challenges such as high computational complexity and limited adaptability to small datasets require further optimization. The multi-task processing capabilities and powerful sequence modeling abilities of transformer models make them highly promising for applications in hepatology.
GNNs: GNNs are pivotal in the construction of knowledge graphs for liver diseases. By integrating multi-source data, such as genes, proteins, metabolites, diseases, and drugs, GNNs can build complex knowledge graphs to reveal associations among genes, proteins, and metabolites. These models enable reasoning and predictions based on knowledge graphs, identifying potential therapeutic targets and mechanisms of drug action[23,33,34]. The strengths of GNNs lie in their ability to model complex graph structures and dynamically update knowledge graphs.
Radiomics is a technique that extracts high-dimensional features from medical images, with a focus on quantifying tissue structure and functional characteristics. Radiomics has shown remarkable advantages in the diagnosis and classification of liver cancer, enabling the extraction of tumor morphological and texture features to distinguish liver cancer from benign liver lesions[35,36]. Furthermore, radiomics can be applied to the staging of liver cirrhosis and the prediction of survival outcomes for liver cancer patients[19,37]. The integration of DL techniques has significantly enhanced the performance of radiomics models, making them more precise and efficient in hepatology applications.
NLP technologies provide innovative solutions for extracting valuable information from unstructured medical texts, such as patient records and research papers[11,12]. NLP-based knowledge graph construction techniques can extract liver disease-related knowledge from medical texts and integrate multi-source medical information to build knowledge graphs, thereby supporting clinical decision-making[13,14].
By integrating imaging, laboratory examinations, genomics, and clinical data, multi-modal data fusion significantly enhances model predictive capabilities and provides robust support for the diagnosis and optimization of treatment plans for liver diseases[38]. Multi-modal data fusion methods primarily include feature-level fusion, decision-level fusion, and model-level fusion. For instance, multi-modal fusion models based on transformer can simultaneously process imaging, laboratory, and genomic data to deliver more comprehensive predictive results[39]. Additionally, multi-modal data fusion technology can be utilized for precise diagnosis, severity assessment, and the development of personalized treatment plans for liver diseases.
The application of AI technology in hepatology is primarily evident in the domains of diagnosis, treatment, and basic research. In terms of diagnosis, AI models based on DL are highly efficient in analyzing medical imaging (such as ultrasound, CT, and MRI), significantly improving the accuracy and efficiency of diagnosing liver diseases, including cirrhosis, liver cancer, and fatty liver disease. AI excels particularly in identifying early-stage lesions and detecting small lesions, providing clinicians with reliable auxiliary tools. In the realm of treatment, AI has driven the development of precision medicine by integrating patient omics and clinical data, enabling the optimization of treatment plans and the prediction of patients’ responses to different drugs, thus facilitating personalized therapy. Additionally, in the field of basic research, AI technology has played a crucial role in analyzing complex genomic and proteomic data, uncovering the molecular mechanisms of liver diseases and providing new directions for drug development.
Despite the promising prospects of AI in hepatology, its implementation faces numerous challenges. In terms of data dependency and limitations, the reliance of AI technology on large labeled datasets represents a major bottleneck in hepatology. Liver disease data are often scarce, isolated, and highly heterogeneous, posing significant challenges for the training and validation of AI models. Specifically, issues such as overfitting, inadequate generalization capability, biases in training data, and problems related to data heterogeneity and standardization are particularly prominent. These challenges limit the effectiveness of AI models in real-world clinical scenarios.
Furthermore, the translation of AI prototypes into clinically validated tools remains a significant hurdle. Unlike radiology or dermatology, where several AI-based devices have received Food and Drug Administration clearance, hepatology-specific AI tools are notably scarce in regulatory-approved clinical use. This gap underscores the challenges in validating AI models for diverse patient populations and integrating them into routine clinical workflows, beyond academic proof-of-concept studies. Regarding model interpretability and clinical acceptance, the “black-box” nature of AI models constitutes another major challenge in their clinical application. While DL models perform exceptionally well in diagnosing liver diseases, their complex decision-making processes are difficult for clinicians to understand. This lack of transparency reduces trust in AI technology among healthcare providers, limiting its widespread adoption in clinical practice.
In the domain of ethical and regulatory issues, the application of AI technology in hepatology also raises ethical and regulatory concerns. AI algorithms may produce unfair predictive outcomes due to biases in training data, potentially leading to discrimination against specific patient groups. Furthermore, the clinical application of AI requires clear attribution of responsibility, particularly in cases of erroneous AI diagnoses or flawed treatment recommendations. Determining accountability between physicians and AI systems remains an unresolved issue. Lastly, the preparedness of healthcare systems for AI integration is another critical concern. Many healthcare institutions lack adequate preparation in terms of technical infrastructure, human resources, and financial investment to support the stable operation and widespread use of AI systems. Additionally, the acceptance and skill levels of healthcare professionals regarding AI technology can significantly impact its implementation in clinical practice.
In response to the aforementioned challenges, the following potential solutions are proposed.
Federated learning and domain adaptation: Federated learning can enhance model generalization while safeguarding patient privacy. Domain adaptation techniques can address data heterogeneity, enabling AI models to better accom
Data sharing and standardization: Establishing multi-institutional collaborative platforms for data sharing is crucial for advancing the standardization and interoperability of liver disease data, thereby addressing issues of data heterogeneity and scarcity.
Enhanced model explainability: Developing more transparent AI models, such as explainable AI, is essential for im
Ethical frameworks and regulatory policies: The establishment of robust ethical frameworks and regulatory policies is paramount to ensuring the safe, fair, and transparent application of AI in hepatology. Moreover, particular attention must be paid to potential biases embedded in genomic and clinical data, which could perpetuate health disparities if unaddressed. Ethical AI deployment also necessitates updated informed consent protocols that encompass data usage in AI training and inference, especially in federated learning settings where data governance and ownership remain complex and context-dependent.
Capacity building in healthcare systems: Strengthening healthcare institutions’ technical infrastructure and enhancing healthcare professionals’ AI proficiency are vital for the widespread adoption and successful implementation of AI technologies in the field of hepatology.
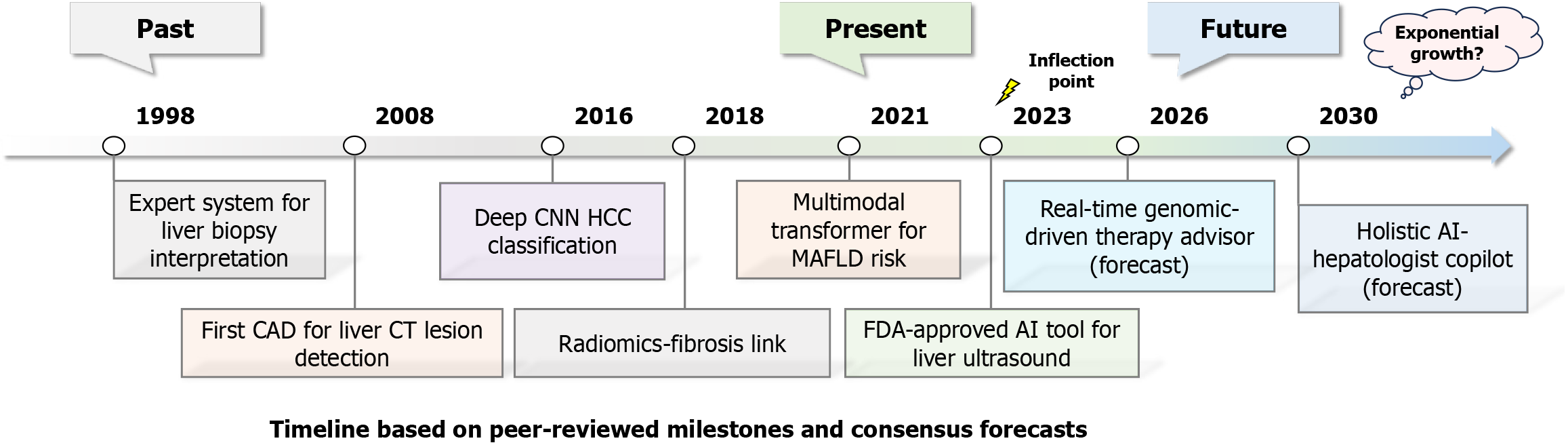
Future research directions primarily focus on data standardization and sharing, model interpretability and clinical application, multi-modal data fusion, and precision medicine (Figure 3). In terms of data standardization and sharing, the establishment of multi-center databases is critical. By integrating imaging, laboratory, genomic, and clinical data from various healthcare institutions, standardized datasets can be constructed to provide comprehensive support for AI models. Additionally, promoting the development of data-sharing platforms ensures privacy protection and ethical compliance while enhancing data utilization efficiency.
Model interpretability and clinical application are essential for the practical implementation of AI technologies. Developing transparent and interpretable AI models, such as explainable machine learning methods and visualization techniques, can enhance clinicians’ trust in these models. Moreover, strengthening interdisciplinary collaboration, developing user-friendly AI tools, and formulating relevant policies and regulations are key to advancing AI applications in clinical settings. Multi-modal data fusion is a crucial direction for improving AI model performance. By employing cross-modal alignment, attention mechanisms, and hierarchical fusion strategies, imaging, genomic, and laboratory data can be effectively integrated to enhance diagnostic and predictive accuracy. Additionally, the application of AI in liver disease basic research and translational medicine can uncover disease mechanisms, identify potential therapeutic targets, and accelerate clinical translation.
Precision medicine represents a key future development direction. By integrating patients’ genomic, phenotypic, and environmental data, AI technologies can enable precise diagnosis, predict disease progression, and develop personalized treatment plans. Furthermore, AI holds promising potential in precision medicine for liver diseases, including early prediction, optimized drug administration, and novel drug development, offering new possibilities for liver disease treatment. Future research must continue to break new ground in data standardization, model interpretability, multi-modal fusion, and precision medicine to drive the widespread application and clinical implementation of AI technologies in hepatology.
The application of AI in hepatology holds promising potential, but it also encounters multiple challenges, including data dependency, model generalization, ethical regulation, and systemic societal issues. Only through coordinated efforts across technology, ethics, policy, and society can AI achieve efficient, safe, and sustainable application in hepatology, thereby enhancing patient care and treatment outcomes.
We extend our heartfelt thanks to all the staff of the Department of Gastroenterology at The Second Affiliated Hospital of Xi’an Jiaotong University for their support and contributions to this research.
| 1. | Chen ZW, Xiao HM, Ye X, Liu K, Rios RS, Zheng KI, Jin Y, Targher G, Byrne CD, Shi J, Yan Z, Chi XL, Zheng MH. A novel radiomics signature based on T2-weighted imaging accurately predicts hepatic inflammation in individuals with biopsy-proven nonalcoholic fatty liver disease: a derivation and independent validation study. Hepatobiliary Surg Nutr. 2022;11:212-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Xia F, Wei W, Wang J, Duan Y, Wang K, Zhang C. Machine learning model for non-alcoholic steatohepatitis diagnosis based on ultrasound radiomics. BMC Med Imaging. 2024;24:221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Chen Y, Laevens BPM, Lemainque T, Müller-Franzes GA, Seibel T, Dlugosch C, Clusmann J, Koop PH, Gong R, Liu Y, Jakhar N, Cao F, Schophaus S, Raju TB, Raptis AA, van Haag F, Joy J, Loomba R, Valenti L, Kather JN, Brinker TJ, Herzog M, Costa IG, Hernando D, Schneider KM, Truhn D, Schneider CV. Deep Learning Reveals Liver MRI Features Associated With PNPLA3 I148M in Steatotic Liver Disease. Liver Int. 2025;45:e70164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Zhang X, Yang W, Li X, Zhao Y, Xie Z, Li S, Zeng Y, Hao X, Xin X, Zhang Y, Feng Z, Jiang H, Gao Z, Yin X. Generation of a High-Precision Whole Liver Panorama and Cross-Scale 3D Pathological Analysis for Hepatic Fibrosis. Adv Sci (Weinh). 2025;12:e2502744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Nakatsuka T, Tateishi R, Sato M, Hashizume N, Kamada A, Nakano H, Kabeya Y, Yonezawa S, Irie R, Tsujikawa H, Sumida Y, Yoneda M, Akuta N, Kawaguchi T, Takahashi H, Eguchi Y, Seko Y, Itoh Y, Murakami E, Chayama K, Taniai M, Tokushige K, Okanoue T, Sakamoto M, Fujishiro M, Koike K. Deep learning and digital pathology powers prediction of HCC development in steatotic liver disease. Hepatology. 2025;81:976-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 6. | Azhie A, Sharma D, Sheth P, Qazi-Arisar FA, Zaya R, Naghibzadeh M, Duan K, Fischer S, Patel K, Tsien C, Selzner N, Lilly L, Jaeckel E, Xu W, Bhat M. A deep learning framework for personalised dynamic diagnosis of graft fibrosis after liver transplantation: a retrospective, single Canadian centre, longitudinal study. Lancet Digit Health. 2023;5:e458-e466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Adamichou C, Genitsaridi I, Nikolopoulos D, Nikoloudaki M, Repa A, Bortoluzzi A, Fanouriakis A, Sidiropoulos P, Boumpas DT, Bertsias GK. Lupus or not? SLE Risk Probability Index (SLERPI): a simple, clinician-friendly machine learning-based model to assist the diagnosis of systemic lupus erythematosus. Ann Rheum Dis. 2021;80:758-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Hu Z, Hu Y, Zhang S, Dong L, Chen X, Yang H, Su L, Hou X, Huang X, Shen X, Ye C, Tu W, Chen Y, Chen Y, Cai S, Zhong J, Dong L. Machine-learning-based models assist the prediction of pulmonary embolism in autoimmune diseases: A retrospective, multicenter study. Chin Med J (Engl). 2024;137:1811-1822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Nour M, Senturk U, Polat K. Diagnosis and classification of Parkinson's disease using ensemble learning and 1D-PDCovNN. Comput Biol Med. 2023;161:107031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 10. | Wang Z, Gu Y, Huang L, Liu S, Chen Q, Yang Y, Hong G, Ning W. Construction of machine learning diagnostic models for cardiovascular pan-disease based on blood routine and biochemical detection data. Cardiovasc Diabetol. 2024;23:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 11. | Burki T. Natural language processing and detecting delirium. Lancet Respir Med. 2022;10:639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Pruneski JA, Pareek A, Nwachukwu BU, Martin RK, Kelly BT, Karlsson J, Pearle AD, Kiapour AM, Williams RJ 3rd. Natural language processing: using artificial intelligence to understand human language in orthopedics. Knee Surg Sports Traumatol Arthrosc. 2023;31:1203-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 13. | So-Armah KA, Lim JK, Lo Re V 3rd, Tate JP, Chang CH, Butt AA, Gibert CL, Rimland D, Marconi VC, Goetz MB, Ramachandran V, Brittain E, Long M, Nguyen KL, Rodriguez-Barradas MC, Budoff MJ, Tindle HA, Samet JH, Justice AC, Freiberg MS; VACS Project Team. FIB-4 stage of liver fibrosis is associated with incident heart failure with preserved, but not reduced, ejection fraction among people with and without HIV or hepatitis C. Prog Cardiovasc Dis. 2020;63:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Rodriguez LA, Schmittdiel JA, Liu L, Macdonald BA, Balasubramanian S, Chai KP, Seo SI, Mukhtar N, Levin TR, Saxena V. Hepatocellular Carcinoma in Metabolic Dysfunction-Associated Steatotic Liver Disease. JAMA Netw Open. 2024;7:e2421019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 15. | Teng S, Zheng N, Al-Huqail AA, Lu Y, Ali E, Ali HE, Zhao H. Effect of nanoparticle macroalgae in the treatment of fatty liver disease using logistic regression, and support vector machine. Environ Res. 2023;224:115426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Deng L, Xiao M. A New Automatic Hyperparameter Recommendation Approach Under Low-Rank Tensor Completion e Framework. IEEE Trans Pattern Anal Mach Intell. 2023;45:4038-4050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Bhat M, Rabindranath M, Chara BS, Simonetto DA. Artificial intelligence, machine learning, and deep learning in liver transplantation. J Hepatol. 2023;78:1216-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 18. | Hong W, Zhang Y, Wang S, Zheng D, Hsu S, Zhou J, Fan J, Zeng Z, Wang N, Ding Z, Yu M, Gao Q, Du S. Deciphering the immune modulation through deep transcriptomic profiling and therapeutic implications of DNA damage repair pattern in hepatocellular carcinoma. Cancer Lett. 2024;582:216594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 19. | Wang K, Lu X, Zhou H, Gao Y, Zheng J, Tong M, Wu C, Liu C, Huang L, Jiang T, Meng F, Lu Y, Ai H, Xie XY, Yin LP, Liang P, Tian J, Zheng R. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut. 2019;68:729-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 385] [Article Influence: 55.0] [Reference Citation Analysis (1)] |
| 20. | Ioannou GN, Tang W, Beste LA, Tincopa MA, Su GL, Van T, Tapper EB, Singal AG, Zhu J, Waljee AK. Assessment of a Deep Learning Model to Predict Hepatocellular Carcinoma in Patients With Hepatitis C Cirrhosis. JAMA Netw Open. 2020;3:e2015626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 21. | Su R, Tao X, Yan L, Liu Y, Chen CC, Li P, Li J, Miao J, Liu F, Kuai W, Hou J, Liu M, Mi Y, Xu L. Early screening, diagnosis and recurrence monitoring of hepatocellular carcinoma in patients with chronic hepatitis B based on serum N-glycomics analysis: A cohort study. Hepatology. 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Zheng R, Wang Q, Lv S, Li C, Wang C, Chen W, Wang H. Automatic Liver Tumor Segmentation on Dynamic Contrast Enhanced MRI Using 4D Information: Deep Learning Model Based on 3D Convolution and Convolutional LSTM. IEEE Trans Med Imaging. 2022;41:2965-2976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Zhang DH, Liang C, Hu SY, Huang XY, Yu L, Meng XL, Guo XJ, Zeng HY, Chen Z, Zhang L, Pei YZ, Ye M, Cai JB, Huang PX, Shi YH, Ke AW, Chen Y, Ji Y, Shi YG, Zhou J, Fan J, Yang GH, Sun QM, Shi GM, Lu JC. Application of a single-cell-RNA-based biological-inspired graph neural network in diagnosis of primary liver tumors. J Transl Med. 2024;22:883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Wang M, Yu G, Ressom HW. Integrative Analysis of Proteomic, Glycomic, and Metabolomic Data for Biomarker Discovery. IEEE J Biomed Health Inform. 2016;20:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Bannaga AS, Metzger J, Kyrou I, Voigtländer T, Book T, Melgarejo J, Latosinska A, Pejchinovski M, Staessen JA, Mischak H, Manns MP, Arasaradnam RP. Discovery, validation and sequencing of urinary peptides for diagnosis of liver fibrosis-A multicentre study. EBioMedicine. 2020;62:103083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Wang M, Wang Z, Sun H, Wang J, Shen C, Weng G, Chai X, Li H, Cao D, Hou T. Deep learning approaches for de novo drug design: An overview. Curr Opin Struct Biol. 2022;72:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 27. | Goles M, Daza A, Cabas-Mora G, Sarmiento-Varón L, Sepúlveda-Yañez J, Anvari-Kazemabad H, Davari MD, Uribe-Paredes R, Olivera-Nappa Á, Navarrete MA, Medina-Ortiz D. Peptide-based drug discovery through artificial intelligence: towards an autonomous design of therapeutic peptides. Brief Bioinform. 2024;25:bbae275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 28. | Jusoh AS, Remli MA, Mohamad MS, Cazenave T, Fong CS. How generative Artificial Intelligence can transform drug discovery? Eur J Med Chem. 2025;295:117825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 29. | Ziegelmayer S, Reischl S, Harder F, Makowski M, Braren R, Gawlitza J. Feature Robustness and Diagnostic Capabilities of Convolutional Neural Networks Against Radiomics Features in Computed Tomography Imaging. Invest Radiol. 2022;57:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Qu WF, Tian MX, Lu HW, Zhou YF, Liu WR, Tang Z, Yao Z, Huang R, Zhu GQ, Jiang XF, Tao CY, Fang Y, Gao J, Wu XL, Chen JF, Zhao QF, Yang R, Chu TH, Zhou J, Fan J, Yu JH, Shi YH. Development of a deep pathomics score for predicting hepatocellular carcinoma recurrence after liver transplantation. Hepatol Int. 2023;17:927-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Li R, Xu L, Xie K, Song J, Ma X, Chang L, Yan Q. DHT-Net: Dynamic Hierarchical Transformer Network for Liver and Tumor Segmentation. IEEE J Biomed Health Inform. 2023;27:3443-3454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Li C, Wang Y, Bai R, Zhao Z, Li W, Zhang Q, Zhang C, Yang W, Liu Q, Su N, Lu Y, Yin X, Wang F, Gu C, Yang A, Luo B, Zhou M, Shen L, Pan C, Wang Z, Wu Q, Yin J, Hou Y, Shi Y. Development of fully automated models for staging liver fibrosis using non-contrast MRI and artificial intelligence: a retrospective multicenter study. EClinicalMedicine. 2024;77:102881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 33. | Kim Y, Jung H, Kumar S, Paton RS, Kim S. Designing solvent systems using self-evolving solubility databases and graph neural networks. Chem Sci. 2024;15:923-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Pu C, Gu L, Hu Y, Han W, Xu X, Liu H, Chen Y, Zhang Y. Prediction of Human Liver Microsome Clearance with Chirality-Focused Graph Neural Networks. J Chem Inf Model. 2024;64:5427-5438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 35. | Bo Z, Song J, He Q, Chen B, Chen Z, Xie X, Shu D, Chen K, Wang Y, Chen G. Application of artificial intelligence radiomics in the diagnosis, treatment, and prognosis of hepatocellular carcinoma. Comput Biol Med. 2024;173:108337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 36. | Feng Z, Li H, Liu Q, Duan J, Zhou W, Yu X, Chen Q, Liu Z, Wang W, Rong P. CT Radiomics to Predict Macrotrabecular-Massive Subtype and Immune Status in Hepatocellular Carcinoma. Radiology. 2023;307:e221291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 104] [Reference Citation Analysis (0)] |
| 37. | Kotowski K, Kucharski D, Machura B, Adamski S, Gutierrez Becker B, Krason A, Zarudzki L, Tessier J, Nalepa J. Detecting liver cirrhosis in computed tomography scans using clinically-inspired and radiomic features. Comput Biol Med. 2023;152:106378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Zhou S, Sun D, Mao W, Liu Y, Cen W, Ye L, Liang F, Xu J, Shi H, Ji Y, Wang L, Chang W. Deep radiomics-based fusion model for prediction of bevacizumab treatment response and outcome in patients with colorectal cancer liver metastases: a multicentre cohort study. EClinicalMedicine. 2023;65:102271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Guo L, Zhu J, Wang K, Cheng KK, Xu J, Dong L, Xu X, Chen C, Shah M, Peng Z, Wang J, Cai Z, Dong J. Multimodal Image Fusion Offers Better Spatial Resolution for Mass Spectrometry Imaging. Anal Chem. 2023;95:9714-9721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/