Published online Oct 14, 2025. doi: 10.3748/wjg.v31.i38.112489
Revised: August 12, 2025
Accepted: September 16, 2025
Published online: October 14, 2025
Processing time: 77 Days and 20 Hours
In this editorial, we comment on the article by Zhang et al recently published in the World Journal of Gastroenterology. The manuscript elucidates significant novel mechanisms underlying hepatocellular carcinoma (HCC) progression. HCC is currently considered one of the major causes of global cancer-associated deaths, underscoring the critical need for novel therapeutic targets. Growing evidence underlines the role of the lipid raft protein flotillin-1 (FLOT1) in cancer, whose dysregulation drives tumor cell growth and survival. However, the regulatory role of FLOT1 on Golgi apparatus function in HCC is unknown. In this study, Zhang et al elucidated a pivotal mechanism by which FLOT1 promotes HCC pro
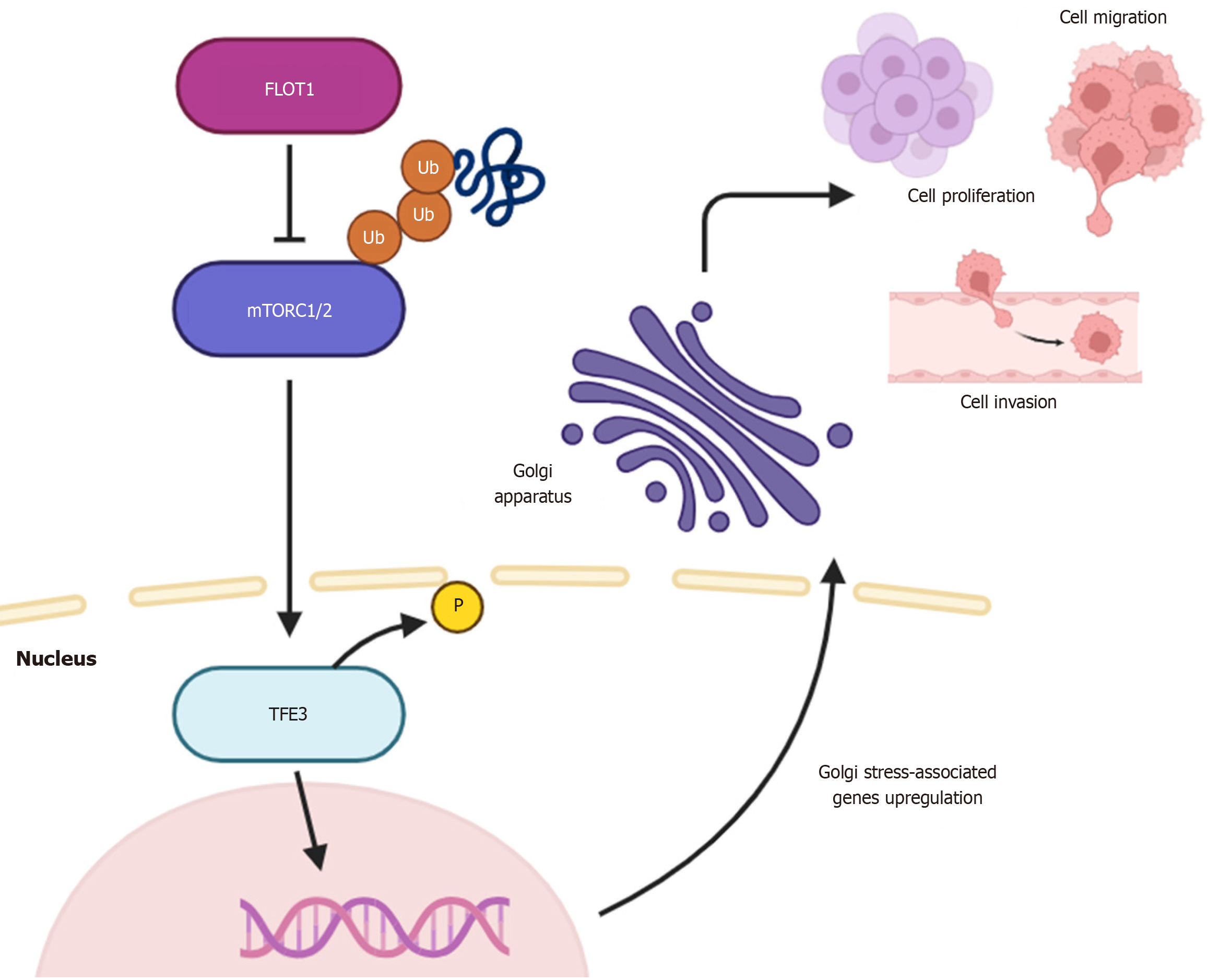
Core Tip: Hepatocellular carcinoma (HCC) is currently considered a primary contributor of cancer-associated mortality on a global scale. The authors elucidate a novel mechanism whereby the lipid raft protein flotillin-1 (FLOT1) drives HCC progression through transcription factor E3-mediated Golgi stress response. FLOT1 overexpression enhanced proliferation, migration and invasion, and suppressed apoptosis in vitro and in mice, whereas gene silencing reversed these phenotypes. Mechanistically, FLOT1 inhibits mechanistic target of rapamycin complexes 1 and 2 via ubiquitination, promoting transcription factor E3 dephosphorylation and subsequent nuclear translocation, resulting in enhanced expression of multiple genes involved in Golgi stress response. These findings advance our understanding of lipid raft-associated proteins in cancer and propose FLOT1 as a potential target for the treatment of HCC.
- Citation: Mazziotta C, Rotondo JC. Unraveling the role of flotillin-1 in driving hepatocellular carcinoma progression through transcription factor E3-mediated Golgi stress response. World J Gastroenterol 2025; 31(38): 112489
- URL: https://www.wjgnet.com/1007-9327/full/v31/i38/112489.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i38.112489
Hepatocellular carcinoma (HCC) is the predominant form of liver malignancy and represents one of the major causes of global cancer-associated deaths[1]. HCC accounts for roughly 75%-85% of hepatic cancers globally[2]. The HCC epidemiological burden is disproportionately high in East Asia and sub-Saharan Africa, which are endemic regions for chronic hepatitis B virus and hepatitis C virus infections. Additional etiological risk factors encompass hepatic cirrhosis, alcohol abuse, diabetes, non-alcoholic fatty liver disease, as well as aflatoxin B1 exposure. The 5-year survival rate of early-stage HCC patients is approximately 70%, which dramatically decreases to approximately 20% in advanced and metastatic disease[1]. Therapeutic approaches for primary localized HCC include surgery, liver transplantation, and localized ablative procedures. Transarterial chemoembolization is applied as locoregional therapy. Systemic therapies commonly include multikinase inhibitors such as sorafenib and/or lenvatinib and immunotherapeutic regimens with atezolizumab and/or bevacizumab, which have collectively improved advanced/metastatic HCC patient outcomes[2]. Despite significant advancements in diagnostic techniques and therapeutic approaches, HCC continues to be characterized by high recurrence rates, profound heterogeneity, intrinsic resistance to conventional systemic treatments, and unfavorable prognosis. Understanding the key players and molecular mechanisms involved in HCC initiation and progression is of paramount importance for the development of more effective therapies.
In this context, a recent study by Zhang et al[3] provides critical insights into the oncogenic role of the lipid raft-associated scaffolding protein flotillin-1 (FLOT1) in HCC biology. FLOT1 belongs to the flotillin family of proteins and, along with FLOT2, constitutes one of its two homologous isoforms. FLOT1 is an integral membrane protein that localizes to caveolae, which are specialized lipid raft domains on the inner surface of the cellular membrane, involved in vesicular transport and cellular shape and polarity. Through its scaffolding activity, FLOT1 serves as a platform on the plasma membrane or intracellular organelles for proteins involved in various cellular processes, including endocytosis, adhesion, actin cytoskeleton reorganization, and cell signaling[4].
Previous research has highlighted FLOT1 involvement in modulating key signaling pathways and promoting tumor progression and metastasis in several cancer types, including gastric and lung cancers. However, its mechanistic involvement in regulating Golgi apparatus stress response, whose activation is known to influence tumor cell behavior in HCC remains unknown. The study demonstrates that FLOT1 promotes HCC progression through activation of tran
Dysregulation of FLOT1/2 proteins has been described in cancer, where it contributes to oncogenesis through multiple mechanisms[5]. FLOT1/2 proteins have been reported to be frequently overexpressed in multiple cancer types including lung, nasopharyngeal, colorectal, gastric, and esophageal squamous cell carcinoma. FLOT1/2 overexpression promotes tumor cell survival, proliferation, invasion, and metastasis mainly by modulating the MEK/Raf/signal-regulated kinase (ERK), nuclear factor-κB and phosphoinositide 3-kinase/protein kinase B pathways. This protein is also involved in epithelial-mesenchymal transition, contributing to invasion, metastasis and poor prognosis[6]. In gastric cancer, FLOT1 plays a key role in tumor development and metastasis through breast cancer anti-estrogen resistance protein 1/ERK signaling. In lung cancer, increased FLOT1 expression drives diverse malignant behaviors, including enhanced growth, invasion, and migration, along with promotion of immune evasion, increased expression of programmed death ligand-1, and reduced ferroptosis, often mediated by ERK/protein kinase B pathway activation[3]. Concerning HCC, early evidence reported FLOT1 mRNA/protein overexpression in HCC cells and tissues compared to their corresponding normal liver counterparts. Moreover, high FLOT1 expression has also been correlated with unfavorable prognosis in HCC patients[7]. More recent mechanistic data obtained in HCC cells indicate that the long non-coding RNA HOX antisense intergenic RNA (HOTAIR) can indirectly regulate FLOT1 expression by competitively binding to miR-214-3p, an upstream regulator of FLOT1. The interplay between HOTAIR, miR-214-3p, and FLOT1 further influences cell proliferation, migration, and invasion in HCC[8]. For these reasons, FLOT1 is currently considered a potential prognostic marker and an important oncogene in HCC[3]. However, the mechanisms by which FLOT1 contributes to HCC tumorigenesis and progression remain only partially understood.
Zhang et al[3] integrated in vitro approaches and experiments conducted with mice to delineate the oncogenic role of FLOT1 during HCC progression. Immunohistochemical analysis of a cohort of HCC specimens revealed that FLOT1 was overexpressed in HCCs compared to adjacent non-tumorous liver tissues, while high FLOT1 Levels were related to decreased survival in HCC patients. These data support previous findings outlining a positive correlation between FLOT1 protein levels and tumor size as well as clinical and Cancer of the Liver Italian Program (CLIP) stages, vascular invasion, and increased disease recurrence. Furthermore, HCC patients with higher FLOT1 expression have been reported to experience shorter overall survival[7]. These findings cumulatively underscore the prognostic utility of FLOT1 in HCC.
Further functional experiments conducted by Zhang et al[3] demonstrated that FLOT1 overexpression can enhance cell growth, cell cycle progression, migration/invasion, and lead to anti-apoptotic effects in vitro in HCC cells. Conversely, FLOT1 silencing suppressed the malignant phenotype of HCC cells and increased the expression of apoptotic proteins including cleaved-poly(ADP-ribose) polymerase and caspase-3 both in vitro as well as in xenografts. Notably, FLOT1 predominantly localizes in lipid rafts, specialized, cholesterol- and sphingolipid-enriched membrane microdomains, while raft-based organization is fundamental for initiating apoptosis. Indeed, clusters of apoptotic signaling molecule-enriched rafts (CASMERs) promote apoptosis by clustering apoptotic signaling molecules in membrane raft microdo
Subsequent mechanistic investigation of the pathways through which FLOT1 contributes to tumorigenesis revealed that it suppresses mTORC1/2 activity via ubiquitination in HCC cells. mTORC1/2 are multiprotein complexes that regulate cell growth and metabolism. Through phosphorylation, these complexes negatively regulate TFE3, a transcription factor crucial for cellular stress responses and lysosome biogenesis. In particular, under conditions of cell starvation and Golgi stress, TFE3 rapidly undergoes dephosphorylation followed by nuclear translocation. Nuclear TFE3 binds to promoters of Golgi apparatus stress response-associated genes[3]. Notably, an emerging body of evidence highlights that modifications in Golgi dynamics, including organelle structure and activity, significantly contribute to cancer onset and development[9]. Indeed, this organelle regulates vesicular trafficking, protein secretion, glycosylation, and signal transduction pathways, whose dysregulation plays a key role in cancer. Dysfunctions in Golgi apparatus have been increasingly implicated in the adaptation of cancer cells to adverse microenvironments, promoting angiogenesis, metastasis, and therapy resistance. In HCC, overexpression of Golgi-resident proteins, such as Golgi phosphoprotein 3, has been shown to activate mTOR signaling, promoting tumor progression[10]. Golgi phosphoprotein 3 has also been correlated with more aggressive tumor phenotypes and poorer prognosis in HCC patients. Additional Golgi-resident proteins, such as the Golgi protein 73, have been shown to promote HCC cell migration and invasion[11]. Moreover, previous transcriptomic analyses of Golgi apparatus-related genes have revealed molecular subtypes of HCC with specific prognoses and therapeutic responses[12]. The study by Zhang et al[3] extends these observations on the implication of the Golgi apparatus in HCC by demonstrating that FLOT1 overexpression induces a Golgi stress program via TFE3 activation, which positively regulates stress response genes. This pathway enhances the potential of tumor cells to withstand cellular stress and maintain malignant characteristics, highlighting the critical role of the Golgi stress response in HCC biology.
Importantly, pharmacological inhibition of mTORC1/2 with Torin1 partially reversed the effects of FLOT1 knock
In conclusion, the study by Zhang et al[3] provides compelling and mechanistic evidence that FLOT1 is a key driver of HCC progression by modulating the Golgi stress response[3]. This adaptive mechanism supports rapid proliferation, migration, and invasion by maintaining secretory pathway efficiency, metabolic support, and resistance to oxidative damage. The results are likely to reflect a pathological hijacking of a homeostatic stress pathway, wherein cancer cells exploit Golgi stress adaptation to sustain tumor growth and metastasis. The findings by Zhang et al[3] significantly advance our understanding of how lipid raft-associated proteins interact with organelle stress responses to sustain the malignant phenotype of HCC cells. Further studies should aim to explore the full spectrum of FLOT1 interactors. For instance, the mechanism whereby FLOT1 promotes CASMER-mediated apoptosis remains unknown and warrants additional investigation. The identification and validation of reliable biomarkers are critical for early cancer detection[13], prognostic stratification, and the development of personalized therapeutic strategies. Evaluating the clinical utility of FLOT1 as a biomarker for HCC prognostication and therapeutic response warrants further investigation. Therapeutically, RNA interference (RNAi)-based strategies targeting FLOT1 could be developed and tested in preclinical models to assess their efficacy and safety. Indeed, RNAi-mediated silencing of FLOT1 represents a promising approach to inhibit tumor progression[14]. We therefore recommend focusing on the development of RNAi-mediated FLOT1 silencing in HCC. Targeting either FLOT1 or its downstream effectors could offer a promising avenue for therapeutic development, while combining FLOT1-targeted therapies with conventional immunotherapies may yield synergistic antitumor effects and help overcome treatment resistance. In addition, given the regulatory role of the long non-coding RNA HOTAIR on FLOT1 expression through competitive binding to miR-214-3p[8], it might be of interest to explore the role of the HOTAIR/miR-214-3p/FLOT1 axis within the context of Golgi homeostasis in HCC. Lastly, elucidating the crosstalk between Golgi stress pathways and other cellular stress responses, such as endoplasmic reticulum stress and autophagy, may uncover novel vulnerabilities that can be therapeutically exploited in HCC and other malignancies. Such multidi
| 1. | Zheng J, Wang S, Xia L, Sun Z, Chan KM, Bernards R, Qin W, Chen J, Xia Q, Jin H. Hepatocellular carcinoma: signaling pathways and therapeutic advances. Signal Transduct Target Ther. 2025;10:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 129] [Article Influence: 129.0] [Reference Citation Analysis (2)] |
| 2. | Shin H, Yu SJ. A concise review of updated global guidelines for the management of hepatocellular carcinoma: 2017-2024. J Liver Cancer. 2025;25:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 3. | Zhang L, Bai CZ, Shan JY, Xue HL, Zheng SM, Chen YL, Tang SH. Flotillin-1 promotes the progression of hepatocellular carcinoma by activating TFE3-mediated Golgi stress response via inhibition of mTORC1/2. World J Gastroenterol. 2025;31:106895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Wang R, Chen Z, Zhang Y, Xiao S, Zhang W, Hu X, Xiao Q, Liu Q, Wang X. Flotillin-1 is a prognostic biomarker for glioblastoma and promotes cancer development through enhancing invasion and altering tumour microenvironment. J Cell Mol Med. 2023;27:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Zhan Z, Ye M, Jin X. The roles of FLOT1 in human diseases (Review). Mol Med Rep. 2023;28:212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 6. | Gauthier-Rouvière C, Bodin S, Comunale F, Planchon D. Flotillin membrane domains in cancer. Cancer Metastasis Rev. 2020;39:361-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Zhang SH, Wang CJ, Shi L, Li XH, Zhou J, Song LB, Liao WT. High Expression of FLOT1 Is Associated with Progression and Poor Prognosis in Hepatocellular Carcinoma. PLoS One. 2013;8:e64709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Liu C, Shang Z, Ma Y, Ma J, Song J. HOTAIR/miR-214-3p/FLOT1 axis plays an essential role in the proliferation, migration, and invasion of hepatocellular carcinoma. Int J Clin Exp Pathol. 2019;12:50-63. [PubMed] |
| 9. | Bajaj R, Warner AN, Fradette JF, Gibbons DL. Dance of The Golgi: Understanding Golgi Dynamics in Cancer Metastasis. Cells. 2022;11:1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Liu H, Wang X, Feng B, Tang L, Li W, Zheng X, Liu Y, Peng Y, Zheng G, He Q. Golgi phosphoprotein 3 (GOLPH3) promotes hepatocellular carcinoma progression by activating mTOR signaling pathway. BMC Cancer. 2018;18:661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Wan YY. Golgi protein 73, hepatocellular carcinoma and other types of cancers. Liver Res. 2020;4:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Sun L, Liu Z, Wu Z, Ning K, Hu J, Chen Z, Wu Z, Yin X. Molecular subtype identification and signature construction based on Golgi apparatus-related genes for better prediction prognosis and immunotherapy response in hepatocellular carcinoma. Front Immunol. 2023;14:1113455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 13. | Preti M, Rotondo JC, Holzinger D, Micheletti L, Gallio N, McKay-Chopin S, Carreira C, Privitera SS, Watanabe R, Ridder R, Pawlita M, Benedetto C, Tommasino M, Gheit T. Role of human papillomavirus infection in the etiology of vulvar cancer in Italian women. Infect Agent Cancer. 2020;15:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Guan Y, Song H, Zhang G, Ai X. Overexpression of flotillin-1 is involved in proliferation and recurrence of bladder transitional cell carcinoma. Oncol Rep. 2014;32:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |