Published online Oct 7, 2025. doi: 10.3748/wjg.v31.i37.111914
Revised: August 21, 2025
Accepted: September 10, 2025
Published online: October 7, 2025
Processing time: 71 Days and 18.3 Hours
Liver failure, particularly acute-on-chronic liver failure, is associated with high mortality (50%-90%). The plasma exchange (PE) mode of the artificial liver sup
To evaluate the predictive value of serial AFP measurements in liver failure pa
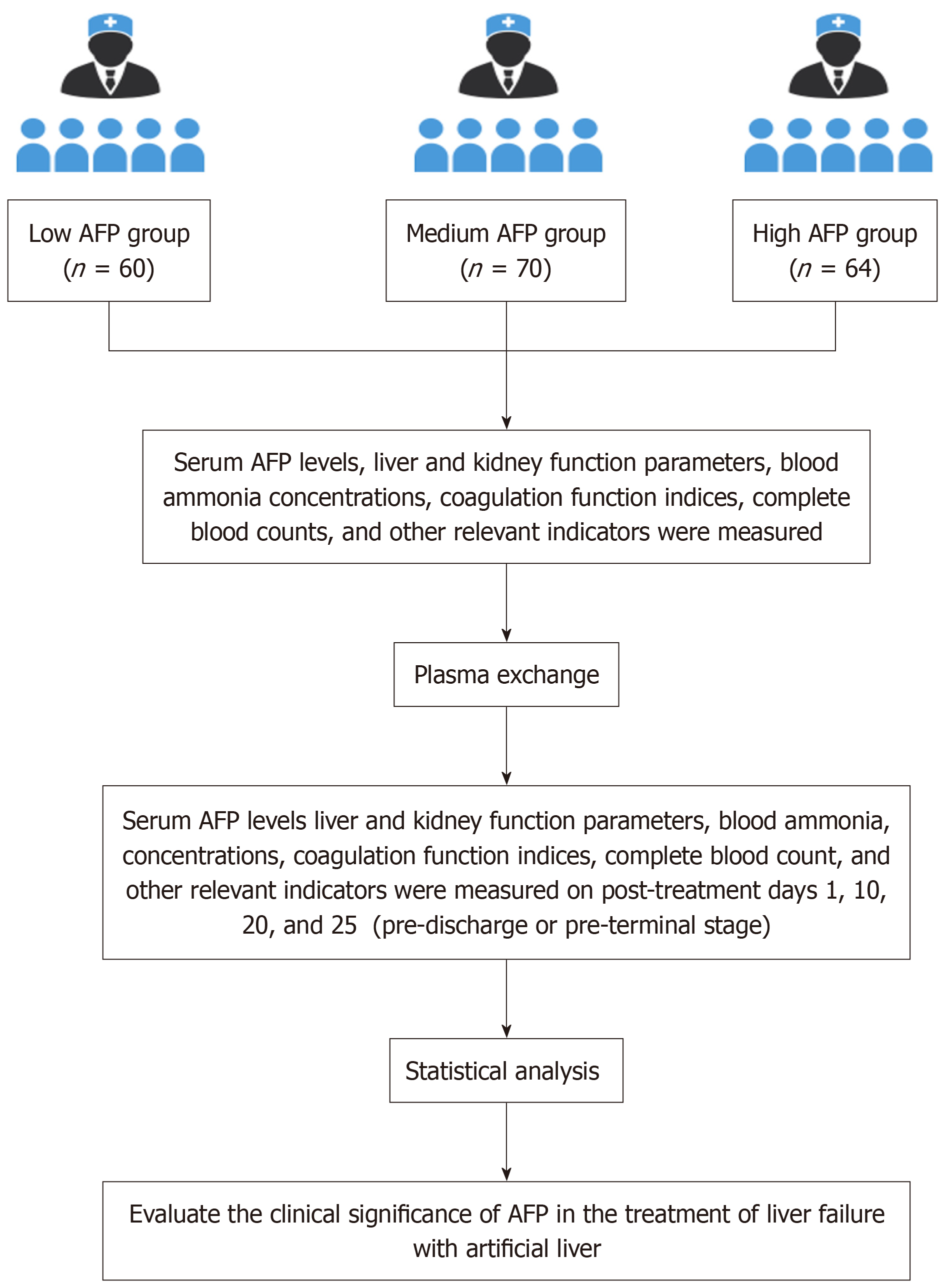
This retrospective study included 194 liver failure patients with complete AFP data, excluding those with tumors, bleeding disorders, allergies, or unstable conditions. Patients were stratified by baseline AFP into low-AFP (< 100 ng/mL, n = 60), medium-AFP (100-200 ng/mL, n = 70), and high-AFP (> 200 ng/mL, n = 64) groups. AFP was measured before PE and on days 1, 10, 20, and 25.
Stratification by baseline AFP revealed significant gradients. The high-AFP group required fewer PE sessions than the low-AFP group (2.8 ± 1.0 vs 4.2 ± 1.5) but exhibited greater post-PE AFP elevation (75.1 ± 20.3 ng/mL vs 33.1 ± 10.2 ng/mL; P < 0.001). The high-AFP group demonstrated optimal values, including the lowest ammonia, bi
AFP dynamics correlate with regenerative capacity and clinical outcomes in liver failure. Serial AFP monitoring may enhance risk stratification and support personalized therapeutic strategies.
Core Tip: This study establishes that baseline alpha-fetoprotein (AFP) stratification (< 100 ng/mL, 100-200 ng/mL, > 200 ng/mL) in liver failure patients receiving plasma exchange-based artificial liver support systems predicts regenerative capacity and clinical outcomes. High-AFP patients (> 200 ng/mL) baseline AFP levels demonstrated improved liver function recovery, fewer complications, and required fewer treatment sessions compared to those with lower levels. Critically, three-month survival was dose-dependent, with baseline AFP > 200 ng/mL providing excellent prognostic discrimination. Serial AFP monitoring enables precision artificial liver support systems therapy by identifying patients with high endogenous regenerative potential.
- Citation: Guo WB, Wang LY, Guo XJ, Yang J, Li W, Shen FY, Li YT, Yang JH, Tai WL. Prognostic value of serum alpha-fetoprotein kinetics in liver failure on artificial liver support. World J Gastroenterol 2025; 31(37): 111914
- URL: https://www.wjgnet.com/1007-9327/full/v31/i37/111914.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i37.111914
Liver failure, the ultimate manifestation of end-stage liver disease, is defined by a triple-hit mechanism involving he
In the therapeutic domain, the artificial liver support system provides unique value through dual-mode “detoxification-regeneration” interventions[4-7]. As a core non-bioartificial artificial liver support system technology, plasma exchange (PE) removes toxins (e.g., bilirubin, ammonia, interleukin-6) and replenishes hepatic proteins (e.g., albumin, coagulation factors), thereby facilitating reconstruction of a pro-regenerative microenvironment[8-11]. Randomized controlled trials have confirmed that PE significantly improves 28-day survival in patients with ACLF (71.4% vs 43.2% in controls); however, its efficacy exhibits substantial interindividual variability[12-14]. A study by Xiang et al[15] demonstrated that among patients receiving standardized PE therapy, those with hepatocyte proliferating cell nuclear antigen positivity ≥ 5% achieved a 6-month survival rate of 82.5%, whereas those with impaired regeneration (proliferating cell nuclear antigen positivity < 5%) had a markedly lower survival rate of 29.1%. These findings highlight the pivotal role of endogenous liver regenerative capacity as a prognostic threshold. Nevertheless, noninvasive assessment of regenerative potential remains an urgent unmet clinical need.
Liver regeneration is a critical determinant of survival following acute liver insults such as ACLF. Alpha-fetoprotein (AFP), a 70 kDa glycoprotein composed of 591 amino acids and predominantly secreted by fetal hepatocytes, serves not only as an oncofetal marker but also as a key indicator of hepatic regenerative activity. Its expression is dynamically reactivated during liver injury and repair, highlighting its potential utility in assessing regenerative capacity[16]. The prognostic significance of this dynamic expression in severe liver failure was elucidated in a landmark longitudinal cohort study conducted by Yuan et al[17]. Their findings demonstrated a pronounced divergence in AFP trajectories between ACLF survivors and non-survivors undergoing PE. By day 7 post-PE, median AFP levels increased significantly to 286.5 ng/mL in survivors, representing a robust 3.5-fold rise compared with the markedly lower levels observed in non-survivors (82.3 ng/mL; P < 0.001). This substantial difference highlights the association between elevated AFP levels and favorable clinical outcomes. Furthermore, peak AFP levels showed a strong positive correlation (r = 0.732) with expansion of the hepatocyte nuclear area, a well-established morphological hallmark of active cell cycle progression and proliferation[18].
Monitoring the temporal kinetics of AFP levels, particularly during the early critical phase after injury (e.g., within the first week post-PE), provides valuable insight into individual regenerative competence. Patients with persistently low or non-rising AFP levels during this period can be identified as being at high risk for disease progression and poor outcomes. Serum AFP measurement offers notable practical advantages as a continuous prognostic biomarker. As a routinely available and cost-efficient assay integrated into standard clinical biochemistry panels, it enables real-time assessment of hepatic regenerative capacity and facilitates risk stratification throughout the clinical course of liver failure. This approach supports timely and cost-effective therapeutic decision-making without imposing significant additional expenditures. Incorporating serial AFP monitoring into established prognostic frameworks, complementing conventional clinical scores such as model for end-stage liver disease and emerging technologies such as radiomic features, holds considerable promise. This integrated strategy is expected to facilitate a more comprehensive and dynamic evaluation of regenerative potential, potentially enabling earlier risk stratification and optimizing the timing of critical therapeutic interventions in the management of liver failure.
Participant selection: A total of 194 patients with liver failure, admitted to The Second Affiliated Hospital of Kunming Medical University and Baoshan People’s Hospital (Yunnan Province, China) between January 2021 and December 2023, were enrolled. All patients met the diagnostic criteria for liver failure subtypes outlined in the 2024 Chinese Guidelines for the Diagnosis and Treatment of Liver Failure (jointly issued by the Infectious Diseases and Hepatology branches of the Chinese Medical Association). Subclassification was based on the following temporal and clinical characteristics. Acute liver failure (ALF): Sudden onset (< 2 weeks), absence of pre-existing chronic liver disease, and concurrent hepatic encephalopathy ≥ grade II (West Haven criteria). Subacute liver failure (SALF): Gradual progression (2-26 weeks), no documented history of chronic liver disease prior to onset, and biochemical evidence of hepatic synthetic dysfunction [international normalized ratio (INR) ≥ 1.5] with total bilirubin ≥ 10 × upper limit of normal. ACLF: Acute deterioration of chronic liver disease within ≤ 4 weeks, presence of ≥ 1 organ failure [chronic liver failure (CLF) consortium-organ failure score ≥ 1], and exclusion of alternative decompensation triggers (e.g., variceal hemorrhage). CLF: Progressive hepatic dysfunction in cirrhotic patients, characterized by recurrent decompensation events (≥ 2 episodes of grade II-IV ascites or overt hepatic encephalopathy within 6 months) (Figure 1).
Exclusion criteria: For patients with liver failure and AFP ≥ 400 ng/mL, liver imaging examinations were performed to identify potential liver tumors[19]. Pregnancy, gonadal embryonic tumors, or other non-hepatic tumors. Severe active bleeding or disseminated intravascular coagulation. Severe allergic reaction to blood products or medications used during treatment, such as plasma, heparin, or protamine. Circulatory failure or unstable cerebral infarction.
This retrospective study included 194 patients meeting the diagnostic criteria, stratified by baseline AFP (electrochemiluminescence immunoassay; < 7 ng/mL) using established cutoffs: < 100 ng/mL (subclinical), 100-200 ng/mL (transitional), and > 200 ng/mL (robust regeneration)[20]. Patients were categorized as low-AFP (< 100 ng/mL, n = 60), medium-AFP (100-200 ng/mL, n = 70), and high-AFP (> 200 ng/mL, n = 64).
Medical treatment: All patients received protocolized care in accordance with the 2023 European Association for the Study of the Liver Clinical Practice Guidelines[21]. (1) Nutritional support: Caloric intake: 35-40 kcal/kg/day via enteral feeding (preferred) or parenteral nutrition if enteral feeding was not feasible; micronutrient supplementation: Vitamin K (10 mg IV weekly), zinc acetate (50 mg, three times a day); sodium restriction: Strict sodium restriction (< 80 mmol/day) with daily electrolyte monitoring; (2) Albumin replacement: Human albumin (20%, CSL Behring, PA, United States) 1 g/kg/day for the following indications: Spontaneous bacterial peritonitis (SBP) prophylaxis and hepatorenal syndrome management; target serum albumin level: > 30 g/L (adjusted according to 24-hour urine sodium excretion); (3) Hepatoprotective therapy: Anti-inflammatory: Glycyrrhizin (80-120 mg/day IV, adjusted according to serum potassium levels); membrane stabilization: Polyenylphosphatidylcholine (456 mg, three times a day); antioxidant: N-acetylcysteine (150 mg/kg loading dose); note: Medication was discontinued if INR > 3.0 or total bilirubin > 300 μmol/L; (4) Antiviral therapy: Entecavir (0.5 mg, once a day, Baraclude®) for HBV-DNA-positive cases. For creatinine clearance < 50 mL/minute, the dose was adjusted to 0.25 mg once a day. Confirmatory testing, including HBV genotyping, was performed prior to initiation; and (5) Complication management: Hepatorenal syndrome-acute kidney injury: Terlipressin (2 mg, every 6 hours) + albumin. SBP: Ceftriaxone (2 g, once a day) until the ascitic polymorphonuclear leukocyte count was < 250/mm3; hepatic encephalopathy: Lactulose (30 mL, titrated to 2-3 stools/day) + rifaximin (550 mg BID); variceal bleeding: Somatostatin infusion (250 μg/hour) + endoscopic band ligation within 6 hours of presentation, following the protocol outlined in the American Association for the Study of Liver Diseases 2021 guidelines)[22].
Artificial liver treatment: All patients received PE as part of the comprehensive medical treatment described above. PE treatment was performed in accordance with the expert consensus on the clinical application of artificial liver blood purification technology (2022 edition)[23].
Machine: X-10 artificial liver therapy system (Zhuhai Jianfan Biotechnology Co., Ltd., Guangdong, China). Consumables: Plasma separator (Berke Co., Ltd., CA, United States), disposable blood circuit connecting catheter (Tianjin Hanahao Medical Materials Co., Ltd., Tianjin, China), Abell double-lumen catheter (size 11.5 Fr × 16 cm) for femoral vein catheterization.
Pre-treatment venous blood tests included AFP, complete blood count, liver and kidney function tests, coagulation profile, and blood ammonia levels. Procedures were performed in an air-disinfected room with electrocardiogram monitoring and low-flow oxygen administration. Extracorporeal access was established via femoral vein puncture using a double-lumen catheter. The plasma separator and circuit were primed with 500 mL of 4% heparin saline, followed by rinsing with heparin-free saline. Intravenous dexamethasone (5 mg) and calcium gluconate were administered prophylactically.
Systemic anticoagulation was initiated with an intravenous heparin bolus (20 mg) and adjusted during treatment according to patient condition, coagulation parameters, body weight, treatment duration, transmembrane pressure, plasma flow, and prothrombin time. Blood flow was maintained at 100-120 mL/minute, with a plasma separation rate of 20-30 mL/minute. PE volume was 2000-3000 mL [approximately body weight (kg) × 40 mL] using fresh frozen plasma. Treatment duration was approximately 2-3 hours. Heparin administration was discontinued 1-1.5 hours before completion of the procedure, depending on transmembrane pressure, and neutralized with protamine sulfate. Prophylactic amikacin was administered. Treatment frequency and intervals (1-4 days) were determined by clinical status.
Venous blood samples were collected from all patients before PE treatment (on day 2 of admission), and on post-treatment days 1, 10, 20, and 25 (pre-discharge or pre-terminal stage). AFP levels were measured using electrochemiluminescence immunoassay (Maglumi 4000, Snibe Diagnostics, Shenzhen, China) with a detection limit of 0.5 ng/mL. The normal reference range for AFP was defined as < 7 ng/mL. Comprehensive clinical data were recorded for each patient. The study evaluated the correlation between dynamic changes in AFP levels and prognosis in liver failure patients, compared survival and mortality rates among different AFP-level subgroups, and assessed the predictive value and clinical significance of AFP levels for determining disease outcomes in liver failure.
Treatment-emergent adverse events during PE therapy were actively monitored and included: Hypersensitivity reactions (e.g., rash, bronchospasm); hemorrhagic complications (platelet count < 50 × 109/L or INR > 2.5); hemodynamic instability (sustained systolic blood pressure < 90 mmHg for > 10 minutes); febrile responses (core temperature > 38.5 °C); and thromboembolic events (confirmed by Doppler ultrasound or computed tomography angiography). The association with artificial liver treatment was evaluated.
Data were analyzed using SPSS version 19.0. Continuous variables were expressed as mean ± SD. The normality of distribution for all continuous variables was assessed using the Shapiro-Wilk test, with P > 0.05 indicating adherence to parametric assumptions. A paired t-test was used for within-group comparisons before and after treatment, and an independent samples t-test for between-group comparisons. Categorical variables were analyzed using the Pearson χ² test. Post hoc analyses were adjusted using the Bonferroni correction for multiple comparisons. A P value < 0.05 was considered statistically significant.
Kaplan-Meier survival curves with log-rank tests were used to compare mortality rates among AFP subgroups. The predictive performance of AFP levels for clinical outcomes was assessed using receiver operating characteristic curve analysis, with calculation of the area under the curve (AUC). Optimal prognostic thresholds were determined using Youden’s index. Sample size calculation, based on an anticipated AFP effect size (Cohen’s d = 0.8) from prior studies, with α = 0.05 and β = 0.2, indicated a requirement of at least 50 patients per group. To control for potential confounding factors, multivariate logistic regression analyses were performed. Primary outcomes (e.g., 3-month survival) were modeled as dependent variables, with AFP group as the primary independent variable. Covariates included age, sex, liver failure subtype, and baseline liver function parameters [albumin, bilirubin, and prothrombin activity (PTA)]. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) are reported.
This study enrolled 194 patients with liver failure (aged 15-65 years, mean age 40.6 ± 13.2 years), including 115 males (59.3%) and 79 females (40.7%), with a mean hospitalization duration of 23.5 ± 12.1 days (Table 1). Based on AFP levels, patients were stratified into three groups for baseline analysis: AFP < 100 ng/mL (n = 60), 100-200 ng/mL (n = 70), and > 200 ng/mL (n = 64) (Table 2). Laboratory indicators prior to treatment, including blood ammonia (μmol/L), albumin (g/L), total bilirubin (μmol/L), alanine aminotransferase (ALT) (U/L), aspartate aminotransferase (AST) (U/L), γ-glutamyl transferase (γ-GT) (U/L), and PTA (%), did not differ significantly among the three groups (P > 0.05; Table 3).
| Parameter total cases (n = 194) | P value1 | |
| Age, range, mean ± SD (years) | 15-65 (40.6 ± 13.2) | 0.382 |
| Sex | 0.724 | |
| Male | 115 (59.3) | |
| Female | 79 (40.7) | |
| Hospital stays (days) | 23.5 ± 12.1 | 0.563 |
| Group | Cases | Gender (n, male/female) | Age, mean ± SD (years) | P value1 |
| Low AFP group (< 100 ng/mL) | 60 | 36/24 | 41.6 ± 11.8 | > 0.05 |
| Medium AFP group (100-200 ng/mL) | 70 | 40/30 | 40.9 ± 10.9 | > 0.05 |
| High AFP group (> 200 ng/mL) | 64 | 39/25 | 40.5 ± 10.2 | > 0.05 |
| Parameter | Low AFP group | Medium AFP group | High AFP group | P value1 |
| Blood ammonia (μmol/L) | 188.5 ± 50.1 | 185.2 ± 48.3 | 190.3 ± 52.7 | 0.8216 |
| Albumin (g/L) | 25.8 ± 3.9 | 26.1 ± 3.7 | 25.6 ± 4.0 | 0.9308 |
| Total bilirubin (μmol/L) | 145.3 ± 35.2 | 142.6 ± 33.8 | 148.1 ± 36.5 | 0.8627 |
| ALT (U/L) | 265.4 ± 70.8 | 260.2 ± 68.1 | 268.7 ± 72.3 | 0.8795 |
| AST (U/L) | 225.6 ± 60.3 | 220.3 ± 58.4 | 229.1 ± 61.9 | 0.8998 |
| γ-GT (U/L) | 485.2 ± 120.6 | 478.3 ± 118.1 | 492.7 ± 123.8 | 0.9339 |
| PTA (%) | 28.3 ± 5.1 | 29.1 ± 5.4 | 27.8 ± 4.9 | 0.9657 |
Stratification by liver failure subtype revealed significant differences in AFP distribution across groups (all P < 0.01). Among the 24 patients with ALF, 62.5% (15/24) were categorized in the medium-AFP group (100-200 ng/mL). SALF cases (n = 38) were predominantly classified in the high-AFP group, representing 47.4% (18/38). In contrast, the ACLF cohort (n = 89) and the CLF cohort (n = 43) primarily exhibited low-AFP levels, accounting for 50.6% (45/89) and 65.1% (28/43), respectively. All subgroup comparisons were statistically significant: ALF (P = 0.004), SALF (P = 0.008), ACLF (P < 0.001), and CLF (P < 0.001; Table 4).
| Type | Cases (n) | Low AFP group | Medium AFP group | High AFP group | P value1 |
| ALF | 24 | 5 (20.8) | 15 (62.5) | 4 (16.7) | 0.004 |
| SALF | 38 | 5 (13.2) | 15 (39.5) | 18 (47.4) | 0.008 |
| ACLF | 89 | 45 (50.6) | 30 (33.7) | 14 (15. 7) | < 0.001 |
| CLF | 43 | 28 (65.1) | 10 (23.3) | 5 (11.6) | < 0.001 |
Multivariable logistic regression analysis identified AFP stratification as the most significant independent predictor of 3-month survival. Compared with the low-AFP group, the high-AFP group had a significantly lower risk of mortality (OR = 0.325, 95%CI: 0.148-0.714; P < 0.001), and the medium-AFP group also demonstrated a protective effect (OR = 0.44, 95%CI: 0.27-0.71; P < 0.001). Among liver failure subtypes, both ALF (OR = 0.36, 95%CI: 0.20-0.66; P = 0.001) and SALF (OR = 0.46, 95%CI: 0.26-0.81; P = 0.007) were associated with improved survival compared with ACLF. Several key markers of hepatic reserve independently predicted clinical outcomes: Albumin (per 1 g/L increase: OR = 0.91, 95%CI: 0.88-0.95; P < 0.001), PTA (per 1% increase: OR = 0.94, 95%CI: 0.92-0.96; P = 0.001), and blood ammonia (per 1 μmol/L increase: OR = 1.01, 95%CI: 1.00-1.01; P = 0.002). Age and bilirubin levels were also significantly associated with survival (P < 0.01), whereas sex and transaminase levels showed no statistically significant association (Table 5).
| Variable | Three-month survival status | |||
| OR1 | 95%CI | P value2 | Significance | |
| Age (per additional year) | 1.03 | 1.01-1.05 | 0.005 | |
| Gender (male vs female) | 0.81 | 0.57-1.15 | 0.241 | NS |
| AFP grouping | ||||
| Medium vs low | 0.44 | 0.27-0.71 | < 0.001 | |
| High vs low | 0.325 | 0.148-0.714 | < 0.001 | |
| Types of liver failure | ||||
| ALF vs ACLF | 0.36 | 0.20-0.66 | 0.001 | |
| SALF vs ACLF | 0.46 | 0.26-0.81 | 0.007 | |
| CLF vs ACLF | 0.64 | 0.33-1.23 | 0.178 | NS |
| Liver function indicators | ||||
| Blood ammonia (μmol/L) | 1.01 | 1.00-1.01 | 0.002 | |
| Albumin (g/L) | 0.91 | 0.88-0.95 | < 0.001 | |
| Total bilirubin (μmol/L) | 1.01 | 1.00-1.01 | 0.004 | |
| ALT (U/L) | 1.01 | 0.98-1.03 | 0.467 | NS |
| AST (U/L) | 1.00 | 0.99-1.03 | 0.226 | NS |
| γ-GT (U/L) | 1.00 | 0.99-1.01 | 0.167 | NS |
| PTA (%) | 0.94 | 0.92-0.96 | 0.001 | |
Patients were categorized into three groups based on baseline AFP levels: Low (< 100 ng/mL), medium (100-200 ng/mL), and high (> 200 ng/mL). As intended by stratification, no intergroup difference was observed at baseline (P = 0.984), but significant differences emerged from day 10 onward (P < 0.05). Time-dependent trajectories: High-AFP group: AFP levels increased steadily from 320.5 ng/mL to 395.6 ng/mL, with a linear daily increase rate of 3.8 ng/mL/day (P < 0.001). Medium-AFP group: AFP levels rose from 148.3 ng/mL to 205.4 ng/mL, corresponding to a daily increase rate of 2.8 ng/mL/day (P < 0.001), representing a less pronounced increase compared with the high-AFP group. Low-AFP group: AFP levels exhibited minimal progression, rising from 65.2 ng/mL to 98.3 ng/mL, with a daily increase rate of 1.6 ng/mL/day (P < 0.001; Table 6).
| Group | Before treatment | Day 1 after treatment | Day 10 after treatment | Day 20 after treatment | Day 25 after treatment | P value |
| Low AFP group | 65.2 ± 20.1 | 72.5 ± 18.3 | 85.4 ± 22.6 | 92.1 ± 25.4 | 98.3 ± 28.7 | < 0.001 |
| Medium AFP group | 148.3 ± 35.6 | 160.8 ± 40.2 | 175.6 ± 45.3 | 190.2 ± 50.1 | 205.4 ± 55.8 | < 0.001 |
| High AFP group | 320.5 ± 80.4 | 340.2 ± 85.6 | 365.7 ± 90.3 | 380.1 ± 95.2 | 395.6 ± 98.5 | < 0.001 |
| P value1 | 0.984 | 0.932 | 0.018 | 0.006 | 0.003 |
Variation in treatment sessions: Patients with high baseline AFP levels required significantly fewer PE treatment sessions compared with other groups (2.8 ± 1.0 vs 4.2 ± 1.5 sessions in the low-AFP group, P < 0.001 vs 3.5 ± 1.2 sessions in the medium-AFP group, P = 0.009). Additionally, the medium-AFP group underwent significantly fewer sessions than the low-AFP group (3.5 ± 1.2 vs 4.2 ± 1.5 sessions, P = 0.003).
Hierarchical AFP increase: The high-AFP group demonstrated the greatest absolute post-treatment increase in AFP levels (75.1 ± 20.3 ng/mL), which was significantly greater than that of the medium-AFP group (57.1 ± 15.6 ng/mL, P = 0.009) and the low-AFP group (33.1 ± 10.2 ng/mL, P < 0.001). A strong inverse correlation was observed between the magnitude of AFP elevation and the number of treatment sessions required (P < 0.001; Table 7).
| Group | Treatment sessions | AFP increase | P value (within-group1 | P value (between-group2 |
| Low AFP group | 4.2 ± 1.5 | 33.1 ± 10.2 | < 0.001 | Reference |
| Medium AFP group | 3.5 ± 1.2 | 57.1 ± 15.6 | < 0.001 | vs low: 0.003; vs high: 0.012 |
| High AFP group | 2.8 ± 1.0 | 75.1 ± 20.3 | < 0.001 | vs low: < 0.001; vs medium: 0.009 |
Systematic analysis of final liver biomarker measurements revealed significant differences among cohorts stratified by post-treatment AFP levels. Patients with high baseline AFP (> 200 ng/mL) consistently demonstrated superior hepatic function profiles compared with those with lower AFP levels: Ammonia levels were significantly lower in the high-AFP group than in the low-AFP group (75.8 ± 25.1 μmol/L vs 125.6 ± 45.3 μmol/L; P = 0.001). Albumin concentrations were markedly higher in the high-AFP group (35.6 ± 4.2 g/L vs 28.3 ± 4.1 g/L; P < 0.001). Total bilirubin was substantially reduced in the high-AFP group (28.7 ± 12.6 μmol/L vs 68.5 ± 22.1 μmol/L; P < 0.001).
Markers of hepatocellular injury showed progressive improvement with increasing AFP levels: ALT decreased stepwise from the low-AFP group to the high-AFP group (180.5 ± 60.2 U/L to 120.8 ± 48.3 U/L; low vs high, P = 0.006). AST exhibited a similar decline (155.4 ± 50.7 U/L to 95.3 ± 38.4 U/L; low vs high, P < 0.001). γ-GT also demonstrated a concentration-dependent reduction (320.6 ± 105.4 U/L to 185.2 ± 75.3 U/L; low vs high, P = 0.002). A distinct pattern was observed for PTA: The medium-AFP group showed significantly impaired coagulation function (35.2% ± 8.6%), which was markedly lower than both the low-AFP group (55.4% ± 10.2%; P < 0.001) and the high-AFP group (72.8% ± 12.4%; P < 0.001; Table 8).
| Parameter | Low AFP group | Medium AFP group | High AFP group | Overall P value (ANOVA)1 | Post-hoc test (Tukey HSD)2 |
| Blood ammonia (μmol/L) | 125.6 ± 45.3 | 98.4 ± 30.2 | 75.8 ± 25.1 | 0.003 | Low vs med: 0.045 |
| Low vs high: 0.001 | |||||
| Med vs high: 0.357 | |||||
| Albumin (g/L) | 28.3 ± 4.1 | 32.5 ± 3.8 | 35.6 ± 4.2 | 0.001 | Low vs med: 0.003 |
| Low vs high: < 0.001 | |||||
| Med vs high: 0.032 | |||||
| Total bilirubin (μmol/L) | 68.5 ± 22.1 | 45.2 ± 18.3 | 28.7 ± 12.6 | < 0.001 | Low vs med: 0.001 |
| Low vs high: < 0.001 | |||||
| Med vs high: 0.017 | |||||
| ALT (U/L) | 180.5 ± 60.2 | 150.3 ± 55.6 | 120.8 ± 48.3 | 0.012 | Low vs med: 0.213 |
| Low vs high: 0.006 | |||||
| Med vs high: 0.058 | |||||
| AST (U/L) | 155.4 ± 50.7 | 120.6 ± 45.2 | 95.3 ± 38.4 | 0.005 | Low vs med: 0.045 |
| Low vs high: < 0.001 | |||||
| Med vs high: 0.038 | |||||
| γ-GT (U/L) | 320.6 ± 105.4 | 240.8 ± 90.5 | 185.2 ± 75.3 | 0.008 | Low vs med: 0.041 |
| Low vs high: 0.002 | |||||
| Med vs high: 0.128 | |||||
| PTA (%) | 55.4 ± 10.2 | 35.2 ± 8.6 | 72.8 ± 12.4 | < 0.001 | Low vs med: < 0.001 |
| Low vs high: 0.032 | |||||
| Med vs high: < 0.001 |
Significant differences in complication rates were observed across AFP-stratified groups. The low-AFP group consistently demonstrated the highest incidence of adverse events: Hypersensitivity reactions occurred in 12.5% of patients (vs 3.1% in the high-AFP group; P = 0.038). Hypotension was observed in 18.8% (vs 6.3% in the high-AFP group; P = 0.009), and SBP occurred in 25.0% (vs 9.4% in the high-AFP group; P = 0.003; Table 9).
| Complication type | Low AFP group | Medium AFP group | High AFP group | P value1 | Post-hoc test (Bonferroni correction)2 |
| Allergic reaction | 12.5 | 6.3 | 3.1 | 0.042 | Low vs high: 0.038 |
| Low vs med: 0.215 | |||||
| Med vs high: 0.417 | |||||
| Hypotension | 18.8 | 12.5 | 6.3 | 0.023 | Low vs high: 0.009 |
| Low vs med: 0.137 | |||||
| Med vs high: 0.254 | |||||
| SBP | 25.0 | 15.6 | 9.4 | 0.011 | Low vs high: 0.003 |
| Low vs med: 0.062 | |||||
| Med vs high: 0.168 |
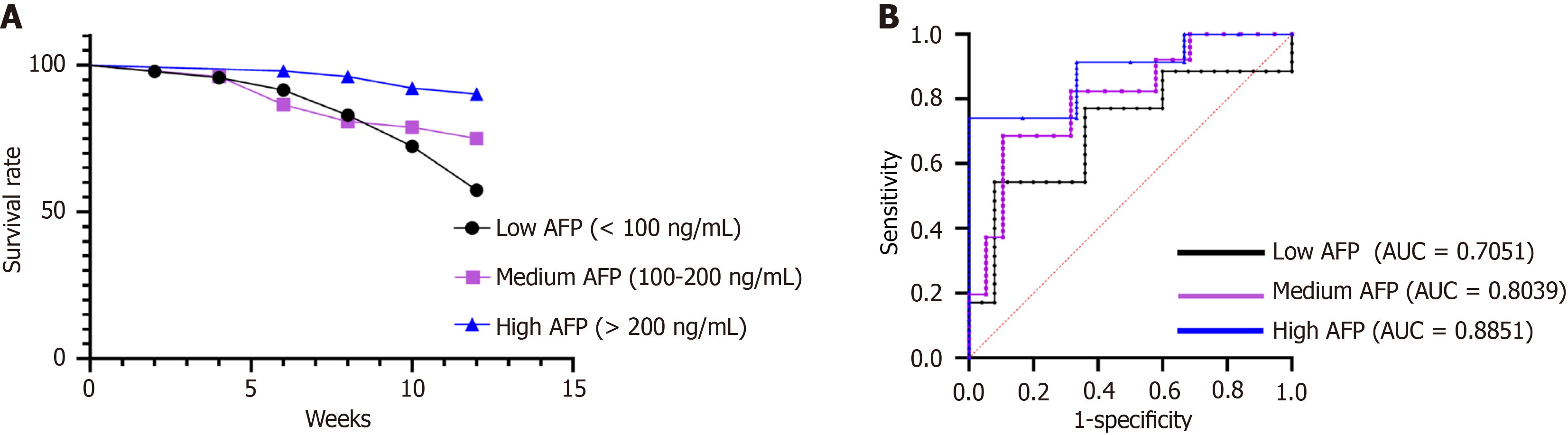
Survival rates differed significantly across AFP strata. The low-AFP group had survival rates of 70.0% at discharge and 56.7% at 3 months. The medium-AFP group showed significantly higher rates of 81.4% at discharge and 72.9% at 3 months (P < 0.001). The high-AFP group demonstrated the highest survival, with 96.9% at discharge and 90.6% at 3 months (P < 0.001; Table 10, Figure 2A). Based on pre-treatment AFP values, 3-month post-treatment predictive discrimination was greatest in the high-AFP group (AUC = 0.8851, cutoff value = 0.7414), followed by the medium-AFP group (AUC = 0.8039, cutoff value = 0.5810). The low-AFP group had relatively weaker predictive ability (AUC = 0.7051, cutoff value = 0.4629) (Figure 2B).
Baseline characteristics of the 194 patients were comparable across AFP strata in terms of demographics and hospitalization (all P > 0.05), controlling for potential confounding factors. Etiologies reflected the patterns reported in the Asia-Pacific region[24,25], with ACLF (45.9%) and CLF (22.1%) predominating. Notably, the CLF and ACLF cohorts were largely characterized by low-AFP levels (65.1% and 50.6%, respectively), suggesting impaired hepatic regeneration. In contrast, SALF cases exhibited a predominance of high-AFP (47.4%), indicative of preserved regenerative capacity, whereas ALF patients were primarily categorized within the medium-AFP group (62.5%), consistent with intermediate regenerative dynamics. Multivariate analysis confirmed that AFP retains significant prognostic value, thereby esta
Dynamic AFP trajectories reflect regenerative potential: Serial measurement of AFP following PE therapy revealed a distinct temporal pattern. The high-AFP group demonstrated sustained post-treatment increases (320.5 ± 48.2 ng/mL to 395.6 ± 53.7 ng/mL; P < 0.05), potentially attributable to receptor-mediated co-activation of the Wnt/β-catenin and signal transducer and activator of transcription 3 pathways-mechanisms known to regulate hepatocyte progenitor expansion and differentiation[26]. These kinetic changes align with preclinical evidence linking elevated AFP levels to hepatic progenitor cell proliferation[27]. Importantly, patients in the high-AFP group achieved significantly greater AFP increments (75.1 ng/mL) with fewer PE sessions (2.8 ± 1.0 vs 4.2 ± 1.5; P = 0.008). This inverse correlation between AFP response and therapeutic intensity supports the “AFP threshold hypothesis”, in which AFP levels exceeding 200 ng/mL indicate competent endogenous capacity to orchestrate liver regeneration via paracrine signaling and niche remodeling[20].
Elevated AFP levels promote multidimensional hepatic restoration: Synthetic function progressively improved, with albumin levels rising from 28.3 ± 3.1 g/L in the low-AFP group to 35.6 ± 4.2 g/L in the high-AFP group (P = 0.001). Detoxification capacity was significantly enhanced in the high-AFP group compared with the low-AFP group, as evidenced by reductions in ammonia (125.6 ± 18.7 μmol/L vs 75.8 ± 12.3 μmol/L; P = 0.003) and bilirubin (68.5 ± 10.2 μmol/L vs 28.7 ± 6.5 μmol/L; P < 0.001), suggesting AFP-mediated upregulation of urea cycle enzymes[28]. Longitudinal kinetics further demonstrated AFP-dependent restoration: High-AFP patients showed greater albumin increase (+8.7 ± 2.1 g/L vs +3.2 ± 1.5 g/L in low-AFP; P = 0.003), bilirubin decline (119.4 ± 38.6 μmol/L vs 76.8 ± 41.6 μmol/L in low-AFP; P < 0.001), and accelerated γ-GT reduction (-57.6 ± 18.4 U/L, P = 0.005 vs baseline). Coagulation function was markedly enhanced with elevated AFP (PTA: 72.8% ± 12.4% vs 55.4% ± 10.2% in low-AFP; P < 0.001), consistent with known AFP-coagulation interactions[29].
Cytoprotective effects were also evident, with significant reductions in γ-GT (320.6 ± 45.1 U/L to 185.2 ± 30.8 U/L; P = 0.008), ALT (180.5 ± 25.3 U/L to 120.8 ± 18.9 U/L; P = 0.012), and AST (P < 0.001 across groups), indicating attenuated hepatocyte necrosis and biliary repair. Notably, the medium-AFP group exhibited reduced PTA (35.2% ± 8.6%), potentially reflecting imbalanced coagulation factor synthesis during rapid hepatocyte proliferation, where accelerated consumption transiently outpaces production, particularly for short half-life factors such as factor VII (t1/2 = 6 hours)[30,31]. This transient synthetic dysfunction highlights the complexity of coagulation dynamics during hepatic regeneration and warrants further investigation.
Clinical significance of complications and survival: Patients with high AFP levels exhibited a dose-dependent reduction in complications compared with their low-AFP counterparts: Allergic reactions (3.1% vs 12.5%; P = 0.038), hypotension (6.3% vs 18.8%; P = 0.009), and SBP (9.4% vs 25.0%; P = 0.003), with the medium-AFP group demonstrating intermediate rates. Notably, no patients underwent liver transplantation during follow-up, confirming that all outcomes reflect native liver pathophysiology. This protective association persisted after statistical adjustment. The inverse association with SBP aligns with evidence that hepatocyte regeneration enhances innate immunity[32].
Furthermore, the survival gradient, from 56.7% in the low-AFP group to 72.9% in the medium-AFP group and 90.6% in the high-AFP group at 3 months (P < 0.001), paralleled progressive improvements in prognostic discrimination (AUC: 0.7051, 0.8039, 0.8851). This concordance supports the conclusion that baseline AFP intrinsically stratifies both survival probability and prognostic precision.
Although this study demonstrated adequate statistical power, external validation in larger, prospective, multicenter cohorts remains essential. Future research should integrate serial AFP kinetics with complementary regenerative biomarkers, particularly epithelial cell adhesion molecule positive hepatic progenitor cells and dynamic microRNA profiles such as microRNA-122, to develop multidimensional predictive models. These models should be evaluated in parallel against established prognostic instruments (e.g., model for end-stage liver disease and sequential organ failure assessment scores) to quantify incremental clinical value using net reclassification improvement analyses. Furthermore, mechanism-based interventions targeting AFP-associated pathways (e.g., pharmacological Wnt/β-catenin agonists) should undergo rigorous preclinical evaluation as promising candidates for regenerative medicine applications.
This study establishes serial AFP measurement as a key biomarker for patients with liver failure undergoing PE. AFP enables effective stratification of regenerative capacity to guide initial PE intensity, predicts the kinetics of functional recovery after PE, identifies individuals at high risk for complications or mortality, and highlights candidates for therapy de-escalation. Importantly, dynamic AFP monitoring (days 1/10/20/25) provides real-time guidance for therapeutic escalation or de-escalation. Incorporating AFP dynamics into clinical decision-making enhances prognostic accuracy, optimizes resource utilization, and improves survival outcomes.
| 1. | Liver Failure and Artificial Liver Group; Chinese Society of Infectious Diseases; Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. [Guidelines for diagnosis and treatment of liver failure (2024 version)]. Zhonghua Gan Zang Bing Za Zhi. 2025;33:18-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 2. | Tong JJ, Zhao W, Mu XY, Xu X, Su HB, Liu XY, Chen J, Zhai XR, Wang Y, Hu JH. Predictive value of the Chinese group on the study of severe hepatitis B-acute-on-chronic liver failure score in the short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure. Chin Med J (Engl). 2019;132:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Schulz M, Trebicka J. Acute-on-chronic liver failure: a global disease. Gut. 2022;71:5-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Ballester MP, Elshabrawi A, Jalan R. Extracorporeal liver support and liver transplantation for acute-on-chronic liver failure. Liver Int. 2025;45:e15647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Duan ZP. [Concerns and preventive interventions in the treatment of pre-hepatic failure]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Zhu B, Xin SJ, You SL. [Advances in early warning and treatment of pre-hepatic failure]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Li LJ, Yang Q, Huang JR, Xu XW, Chen YM, Fu SZ. Effect of artificial liver support system on patients with severe viral hepatitis: a study of four hundred cases. World J Gastroenterol. 2004;10:2984-2988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Yang YF, Wei LL, Zhang N, Huang P, Yang YJ, Wang LR. [The efficacy of artificial liver support system treatment on hepatic failure in China: a meta-analysis]. Zhonghua Gan Zang Bing Za Zhi. 2006;14:732-734. [PubMed] |
| 9. | Chen LG, Guleng B, Ren JL, Chen JM, Wang L. Artificial liver support system in treatment of liver failure after acute poisoning. World J Emerg Med. 2011;2:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Lan X, Hong C, Zhang X, Zhou L, Li Y, Zhang C, Mo X, Zhou J, Li B, Qi T, He Q, Luo W, Lai Q, Ji Y, Xu Y, Liu J, Zhou F, Chen J. Artificial Liver Support System Improves One-Year Prognosis of Patients With Hepatitis B Virus-Associated Acute-on-Chronic Liver Failure. J Gastroenterol Hepatol. 2025;40:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Yang CF, Liu JW, Jin LM, Li YM. Association of duration and etiology with the effect of the artificial liver support system in pediatric acute liver failure. Front Pediatr. 2022;10:951443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Xiao LL, Xu XW, Huang KZ, Zhao YL, Zhang LJ, Li LJ. Artificial Liver Support System Improves Short-Term Outcomes of Patients with HBV-Associated Acute-on-Chronic Liver Failure: A Propensity Score Analysis. Biomed Res Int. 2019;2019:3757149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Huang Y, Ju T, Zhang H, Cao D, Li X, Yang J, Yan D. Lower level of IL-28A as a predictive index of the artificial liver support system in effective treatment of patients with HBV-ACLF. J Clin Lab Anal. 2022;36:e24766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Zeng Y, Gan D, Zhang K, Long T, He Y, Zhou R, Liu S, Xiong M. The impact of artificial liver support system on intestinal microbiota and serum bile acid profiles in patients with acute-on-chronic liver failure: a prospective cohort study. Hepatol Int. 2024;18:1540-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Xiang Y, Li R, Cai J, Jiang Q. Three Artificial Liver Models of Treatment of Acute-on-Chronic Liver Failure. Ther Clin Risk Manag. 2024;20:731-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Kakisaka K, Kataoka K, Onodera M, Suzuki A, Endo K, Tatemichi Y, Kuroda H, Ishida K, Takikawa Y. Alpha-fetoprotein: A biomarker for the recruitment of progenitor cells in the liver in patients with acute liver injury or failure. Hepatol Res. 2015;45:E12-E20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Yuan G, Zhou Y, Liu J, Hu C, Huang H, Ren Y, Yu W, Guo Y, Zhang YY, Zhou Y. AFP specificity for HCC surveillance is increased by mitigating liver injury among treated chronic hepatitis B patients with elevated AFP. Int J Clin Exp Pathol. 2019;12:1315-1323. [PubMed] |
| 18. | Xie Z, Violetta L, Chen E, Huang K, Wu D, Xu X, Ouyang X, Zhao Y, Li L. A prognostic model for hepatitis B acute-on-chronic liver failure patients treated using a plasma exchange-centered liver support system. J Clin Apher. 2020;35:94-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Kim KM, Sinn DH, Jung SH, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. The recommended treatment algorithms of the BCLC and HKLC staging systems: does following these always improve survival rates for HCC patients? Liver Int. 2016;36:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Jiang M, Ren J, Belmonte JCI, Liu GH. Hepatocyte reprogramming in liver regeneration: Biological mechanisms and applications. FEBS J. 2023;290:5674-5688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on acute-on-chronic liver failure. J Hepatol. 2023;79:461-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 209] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 22. | Yim HJ, Kim TH, Suh SJ, Yim SY, Jung YK, Seo YS, Kang SH, Kim MY, Baik SK, Kim HS, Kim YS, Park SY, Kim BI, Park JY, Heo J, Sohn JH, Heo NY, Han KH, Um SH. Response-Guided Therapy With Cefotaxime, Ceftriaxone, or Ciprofloxacin for Spontaneous Bacterial Peritonitis: A Randomized Trial: A Validation Study of 2021 AASLD Practice Guidance for SBP. Am J Gastroenterol. 2023;118:654-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 23. | Severe Liver Disease and Artificial Liver Group; Chinese Society of Hepatology; Chinese Medical Association. [Expert Consensus on Clinical Application of Artificial Liver Blood Purification Technology (2022 Edition)]. Linchuang Gandanbing Zazhi. 2022;38:767-775. [DOI] [Full Text] |
| 24. | Kojima H, Nakamura K, Kupiec-Weglinski JW. Therapeutic targets for liver regeneration after acute severe injury: a preclinical overview. Expert Opin Ther Targets. 2020;24:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Jiao Y, Lu W, Xu P, Shi H, Chen D, Chen Y, Shi H, Ma Y. Hepatocyte-derived exosome may be as a biomarker of liver regeneration and prognostic valuation in patients with acute-on-chronic liver failure. Hepatol Int. 2021;15:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Zhang C, Sun C, Zhao Y, Ye B, Yu G. Signaling pathways of liver regeneration: Biological mechanisms and implications. iScience. 2024;27:108683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 27. | Chen F, Schönberger K, Tchorz JS. Distinct hepatocyte identities in liver homeostasis and regeneration. JHEP Rep. 2023;5:100779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 28. | Satilmis B, Akbulut S, Sahin TT, Dalda Y, Tuncer A, Kucukakcali Z, Ogut Z, Yilmaz S. Assessment of Liver Regeneration in Patients Who Have Undergone Living Donor Hepatectomy for Living Donor Liver Transplantation. Vaccines (Basel). 2023;11:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Hussain A, Ali G, Afzal MA, Tayyeb A, Tariq MA, Akram SJ, Ahmad FJ, Akram J. Phase-dependent expression profiling and quantification of several growth factors in liver regeneration after partial hepatectomy. Pak Biomed J. 2021;3. [DOI] [Full Text] |
| 30. | Grama A, Sîrbe C, Burac L, Benţa G, Bordea MA, Pop TL. Coagulation Factors as Predictive Markers of Poor Outcomes in Children with Acute Liver Failure. Clin Lab. 2022;68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Chen L, Li S, Nie J, Zhao J, Yu S, Li Y, Peng J. Bmal1 Regulates Coagulation Factor Biosynthesis in Mouse Liver in Streptococcus oralis Infection. Front Cell Infect Microbiol. 2020;10:530190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Li B, Qin Y, Qiu Z, Ji J, Jiang X. A cohort study of hepatectomy-related complications and prediction model for postoperative liver failure after major liver resection in 1,441 patients without obstructive jaundice. Ann Transl Med. 2021;9:305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/