Published online Oct 7, 2025. doi: 10.3748/wjg.v31.i37.107665
Revised: June 18, 2025
Accepted: August 22, 2025
Published online: October 7, 2025
Processing time: 181 Days and 22 Hours
Metabolic dysfunction-associated steatotic liver disease is the most prevalent chronic liver condition, affecting over one-third of the global population, with cirrhosis present in up to 3.3% of cases. Early detection of advanced liver disease in at-risk populations can enable timely intervention, prevent progression, and reduce complications. This review focuses on the current recommendations for early detection of advanced liver disease, evaluates the evidence for the per
Core Tip: Early detection of liver fibrosis in community settings is essential for timely intervention and to prevent liver related events. Although non-invasive testing strategies are likely cost-effective, their adoption by non-hepatology specialists is limited. Key challenges include the use of overly broad target populations and suboptimal selection or application of non-invasive tests (NITs). Optimizing these pathways by integrating better NITs and refining referral algorithms can improve risk stratification, minimize unnecessary specialist referrals, reduce the burden on healthcare systems, and facilitate timely, multidisciplinary care for individuals at the highest risk for liver-related adverse outcomes.
- Citation: Pustjens J, Brouwer WP, Ayada I, Janssen HLA, van Kleef LA. Considerations and clinical utility of referral pathways for early detection of liver disease in at-risk populations. World J Gastroenterol 2025; 31(37): 107665
- URL: https://www.wjgnet.com/1007-9327/full/v31/i37/107665.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i37.107665
Liver disease is becoming increasingly prevalent and poses a significant global problem, with metabolic dysfunction-associated steatotic liver disease (MASLD) being the most common chronic liver disease. Currently, more than 1 in 3 individuals have MASLD, and among them, 3.3% have cirrhosis, which is mostly irreversible[1,2]. In recent years, several guidelines have emphasized the importance of early detection of liver fibrosis to enable timely interventions and prevent progression to advanced liver disease and its complications[3-7]. However, the feasibility of these strategies remains contentious due to the immense strain they place on healthcare systems, the limitations of diagnostic tools, and system level barriers[8-13].
Awareness of liver disease is poor among the general population and knowledge is limited among primary care professionals, while the prevalence of MASLD and advanced liver disease is high, raising the question of whether screening is required[1,12,14,15]. The Wilson and Jungner criteria are commonly used to assess whether screening for a disease is justified[16]. These criteria evaluate broad aspects related to the disease itself, the healthcare setting, diagnostic methods, treatment options, and cost-effectiveness (Table 1). Given the typically long asymptomatic phase preceding cirrhosis, a condition associated with high morbidity and mortality, screening for advanced liver disease might prevent symptomatic disease[17-19]. Moreover, with pharmaceutical treatment now available for fibrotic metabolic dysfunction-associated steatohepatitis (MASH) in the United States and lifestyle interventions being potentially effective when adhered to in a pre-cirrhotic stage, early detection may offer a crucial window for intervention and patient education[20,21].
| Wilson and Jungner criteria | |
| Disease | The condition sought should be an important health problem |
| The natural history of the condition, including development from latent to declared disease, should be adequately understood | |
| There should be a recognizable latent or early symptomatic stage | |
| Diagnosis | There should be a suitable test or examination |
| The test should be acceptable to the population | |
| Case-finding should be a continuing process and not a “once and for all” project | |
| Treatment | There should be an agreed policy on whom to treat as patients |
| There should be an accepted treatment for patients with recognized disease | |
| Setting | Facilities for diagnosis and treatment should be available |
| Cost-effectiveness | The cost of case-finding should be economically balanced in relation to possible expenditure on medical care as a whole |
However, several challenges remain: (1) Ensuring adequate facilities for screening and treatment; (2) Determining optimal evaluation and re-evaluation strategies including the choice of non-invasive tests (NITs); and (3) Evaluating and improving cost-effectiveness[22]. Preliminary studies indicate that fibrosis-4 (FIB-4)-based screening strategies may be cost-effective[23-25]. However, real-world data, particularly regarding the long-term impact of such programs, remains limited. With ongoing advances in NITs and the anticipated availability of more effective pharmaceutical agents, the cost-effectiveness of screening is expected to improve. These developments may help overcome the remaining barriers to implementing advanced liver disease screening[26].
While referral pathways can be optimized, the success of screening programs ultimately depends on patient adherence to follow-up testing and specialist care. Concerningly, a German general health screening study found that only 50% of individuals identified as at risk of cirrhosis sought specialist care[27]. This is particularly troubling if screening for advanced liver disease is integrated into a multidisciplinary framework, as in that study, where follow-up attendance was low. Although adherence rates may differ in liver health-specific screening programs as illustrated by vibration controlled transient elastography (VCTE) measurement alongside retina screening (80% attended follow-up visit), monitoring follow-up participation is essential when evaluating referral pathways[28]. Engagement with screening and adherence to follow-up visits might be improved by community-based education, use of telehealth, and artificial intelligence, where possible. The impact of these tools for referral pathway efficacy needs to be proven.
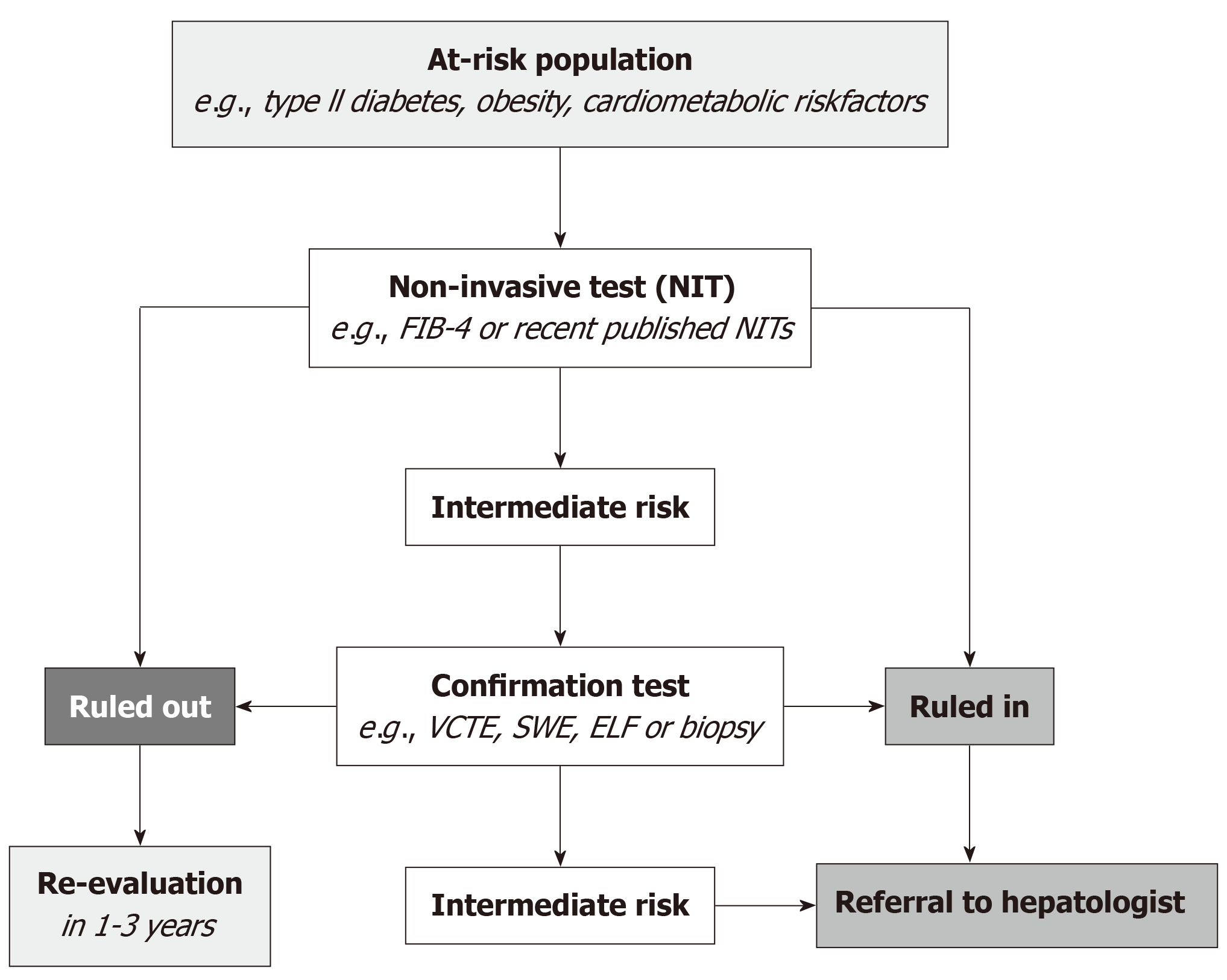
Since the European Association for the Study of the Liver (EASL) NIT guideline in 2021, subsequent guidelines and several position papers have recommended early detection of advanced liver disease in at-risk populations, largely motivated by the anticipated availability of pharmacological therapies and the growing disease burden[3-7,29]. Figure 1 depicts a typical referral pathway. Although not all Wilson and Jungner criteria are fully satisfied, the broad endorsement of early detection of advanced liver disease in at-risk populations underscores the anticipated magnitude of the liver disease burden.
Screening for advanced liver disease is not feasible in the general population and should instead be targeted to individuals at risk, making it more accurately described as case-finding. Careful initial selection is essential to avoid overburdening already strained healthcare systems and to improve cost-effectiveness by reducing false positives in both primary and validation testing. However, when defining the target population, the yield of the program, particularly its sensitivity, may already be affected[30-32].
The target population for each guideline is summarized in Table 2. Although the guidelines differ slightly, there is general consensus on case-finding for the following subgroups: (1) Type 2 diabetes; (2) Obesity with ≥ 1 other metabolic dysfunction criteria known as metabolically complicated obesity; and (3) Persistent elevated liver enzymes. The metabolic dysfunction criteria in the referral strategies align with the metabolic dysfunction criteria required for MASLD diagnosis and include hypertension, dyslipidemia, and pre-diabetes, among others[33]. Although the European MASLD guideline does not, other guidelines recommend case-finding in individuals with ≥ 2 metabolic dysfunction criteria, even in the absence of abdominal obesity. Similarly, guidelines differ in how elevated liver enzymes are addressed, with varying transaminase cutoff values. The American Association for the Study of Liver Diseases (AASLD) guideline further highlights that first-degree relatives of patients with MASLD cirrhosis, as well as individuals with metabolic dysfunction-associated alcohol-related liver disease, should be considered for case-finding given the particularly high prevalence of advanced fibrosis in these subgroups[6].
| Metabolic dysfunction | Elevated ALT | Steatosis | |||
| T2DM | Obesity + ≥ 1 other criteria | ≥ 2 criteria | |||
| 2021 EASL NIT clinical practice guideline | + | +1 | +1 | +1 | - |
| 2021 AGA clinical care pathway | + | + | + | + | + |
| 2023 AASLD practice guidance | + | +1 | +1 | +2 | + |
| 2024 EASL-EASD-EASO clinical practice guideline | + | + | - | +3 | + |
| 2025 APASL clinical practice guideline | + | +4 | + | + | + |
Although evidence exists on the prevalence of advanced liver disease for the main criteria, such as type 2 diabetes, metabolic dysfunction, elevated liver enzymes, steatosis (up to 22%), more detailed data on prevalence within the specific subgroups that differ between guidelines remains limited[34,35]. Particularly relevant is the non-obese group with ≥ 2 metabolic dysfunction criteria in the absence of type 2 diabetes. Further evidence is needed to determine the size of this subgroup and to assess whether they require screening or can be safely excluded, thereby improving the feasibility of screening programs.
Application of the AASLD or American Gastroenterological Association selection criteria makes 70% of the United States adult population eligible for screening due to the high prevalence of metabolic dysfunction in this population[10,11]. Although this number might be substantially lower in countries with lower obesity rates, it is important to investigate whether the target population can be safely refined. Recent data underscore the importance of alcohol consumption (even within normal range) on disease progression and potential interactions with metabolic dysfunction[36-38]. Similarly, systemic inflammation is crucial in the progression from MASLD to more advanced disease (e.g., MASH and eventually fibrosis) and might, therefore, have predictive value[39]. However, adding further complexity to the screening algorithm may reduce its clinical utility and should be avoided when incorporating alcohol use or inflammation provides only marginal benefit.
Current guidelines do not suggest appropriate age ranges for screening. The clinical benefit and utility of liver fibrosis screening algorithms in patients aged over 65 should ultimately be evaluated through cost-effectiveness studies[40]. These studies have not yet been conducted in community-dwelling older adults, in whom neither steatosis nor liver stiffness measurement (LSM) ≥ 8 kPa is associated with all-cause mortality, unlike in younger and middle-aged populations[41-46]. Although these findings require further validation, this would advocate against screening for liver disease in older adult populations. The absence of increased mortality in older adults with steatosis or LSM ≥ 8 kPa may be explained by healthy survivor bias, as well as the presence of cardiovascular risk management programs targeting this subgroup. This aims at reducing cardiovascular mortality, the primary cause of death in MASLD patients[47]. Other screening programs, such as those for colorectal cancer or breast cancer, typically stop at age 75, which may be considered the upper age limit for liver disease screening[48,49]. Additionally, treatment availability is a prerequisite for the feasibility of screening[16]. Although Resmetirom was investigated among individuals aged ≥ 18 years, no subgroup analysis by age was performed. Assuming a normal distribution, only 11% (36-37 individuals per treatment arm) of this study population was aged > 70 years[20]. Therefore, potential screening and treatment initiation above this age should be performed with caution, and additional evidence is required. Therefore, we recommend that screening programs focus on young to middle-aged populations, while evidence on treatment efficacy in other age groups for long-term outcomes is still awaited.
Although a chronically elevated alanine aminotransferase (ALT) level is an important indicator for screening, it has insufficient discriminative accuracy to rule out clinically significant liver disease[50]. To address this critical clinical need, various NITs have been developed, are readily available [e.g., FIB-4, nonalcoholic fatty liver disease fibrosis score (NFS) and LSM] and have demonstrated a similar prognostic value for future liver-related events compared to histologically assessed fibrosis grades[51]. This supports the use of NITs, particularly in a screening or case-finding setting where more invasive approaches, such as liver biopsy, are not feasible.
The FIB-4 is the cornerstone of currently recommended referral pathways and consists of readily available parameters: Aspartate aminotransferase, ALT, platelets, and age. The FIB-4 is inexpensive and well-known and, therefore, has been selected as the first-line test[3-7]. The recommended cut-offs are consistent across guidelines: 1.3 to rule out disease and 2.67 for direct referral to a hepatologist, while values between 1.3 and 2.67 require a confirmation test, which may also be performed by other healthcare providers (Table 3)[3-7].
| Rule out | Rule in | |
| 2021 EASL NIT clinical practice guideline | FIB-4: < 1.3 | FIB-4: ≥ 2.67 |
| 2021 AGA clinical care pathway | FIB-4: < 1.3 (2.0 aged ≥ 65 years) | FIB-4: ≥ 2.67 |
| 2023 AASLD practice guidance | FIB-4: < 1.3 (< 2.0 aged ≥ 65 years) | FIB-4: ≥ 2.67 |
| 2024 EASL-EASD-EASO clinical practice guideline | FIB-4: < 1.3 (2.0 aged ≥ 65 years) | FIB-4: ≥ 2.67 |
| 2025 APASL clinical practice guideline | FIB-4: 1.3 NFS1 | FIB-4: ≥ 2.67 NFS1 |
However, incorporating age as a parameter in NITs, such as the FIB-4, raises important performance issues, as highlighted by several studies[8,52-59]. Although including age as a linear covariate generally improves accuracy, it compromises performance in both younger and older populations. To overcome this issue, an age-dependent cut-off of 2.0 instead of 1.3 has been proposed and applied by most guidelines. Although this cut-off helps reduce the number of false positives in older adult populations, it decreases sensitivity by one-third and is therefore debated[56]. Furthermore, although this suboptimal solution can be used for older adults, it does not resolve the poor sensitivity in individuals < 35 years. Therefore, alternatives need to be considered[6].
Despite being inexpensive and easy to perform, the widespread adoption of FIB-4 in referral pathways was not evidence-based when implemented. Originally developed to identify ≥ F3 fibrosis in patients co-infected with human immunodeficiency virus and hepatitis C, its diagnostic accuracy for detecting elevated LSM in the target population of these screening algorithms appears limited, as consistently reported in multiple studies following the inclusion of FIB-4 in the guidelines[8-11,60]. The poor performance of FIB-4 in the target screening population contrasts with its results in MASLD patients currently under hepatologist care and undergoing liver biopsy in secondary or tertiary hospital settings[61]. However, it should be noted that this is a highly selected population that was already identified for referral on other grounds, and therefore does not reflect the overall MASLD population. This is illustrated by the fact that these patients exhibit advanced liver fibrosis in 20% of cases, which is far more prevalent than in the population-based setting where approximately 5% is expected. Importantly, NIT performance depends on the a priori chance of advanced liver disease, which thus strongly depends on the line of care for which it is used (i.e., primary care vs secondary or tertiary care).
Several promising NITs have recently become available that aid in risk stratification and appear to outperform currently available NITs (Table 4)[62-69]. These new scores typically incorporate parameters of metabolic dysfunction, a key driver of fibrosis progression. A step-wise approach, in which a series of NITs is applied sequentially, may ultimately be more cost-effective and provide higher accuracy than using a single NIT followed by confirmation with LSM or enhanced liver fibrosis (ELF). However, evidence from a screening setting is still needed[56,70,71].
| Components | Target | Derivation population | |
| SAFE | Age, BMI, diabetes, AST, ALT, globulin, platelets | ≥ F2 fibrosis | MASLD patients |
| LRS | Age, sex, fasting glucose, cholesterol, AST, ALT, GGT and platelets | LSM (score correlates with expected LSM) | General population/primary care population |
| MAF-5 | BMI, waist circumference, diabetes AST and platelets | ≥ LSM 8 kPa | General population |
| FIB-9 | AST, ALT, GGT, ALP, bilirubin, albumin, platelets, prothrombin index and urea | ≥ F2 fibrosis | MASLD patients |
| LiverPRO | Age, AST, GGT, alkaline phosphatase, total cholesterol, sodium, INR, bilirubin, albumin, platelets | ≥ F2 fibrosis | At-risk metALD population |
| acMASH | AST, creatine | MASH | MASLD patients |
| CORE | Age, sex, GGT, AST, ALT | Liver related events | General population |
| CLivD | Age, sex, alcohol use, waist-hip ratio, diabetes, smoking, with or without GGT values | Fatal and non-fatal advanced liver disease | General population |
Head-to-head comparison of NITs based on sensitivity, specificity, and predictive values are challenging due to differences in their diagnostic purposes and derivation from different populations (Table 4). However, in a study directly comparing the diagnostic accuracy of ten NITs as first-line tests in a general population with metabolic dysfunction, the metabolic dysfunction-associated fibrosis 5 (MAF-5) score performed best for predicting LSM ≥ 8 kPa, LSM ≥ 12 kPa, at-risk MASH and advanced fibrosis. The steatosis-associated fibrosis estimator score was the most accurate for identifying cirrhosis. In contrast, FIB-4, the guideline-recommended first-line test, showed poor performance for pre-cirrhotic disease but was effective for cirrhosis[72]. For example, to achieve 80% sensitivity for LSM ≥ 8 kPa, MAF-5 required fewer referrals (42%) than FIB-4 (77%), with higher specificity (62% vs 24%) and positive predictive values of 6-15%. Additional details, including results for other tests, are provided in Table 5.
| Cut-off | Specificity (%) | NPV | PPV | |
| FIB-4 | 0.73 | 24 | 0.93 | 0.08 |
| SAFE | -7.04 | 52 | 0.97 | 0.12 |
| LRS | 4.98 | 46 | 0.97 | 0.11 |
| MAF-5 | -0.37 | 62 | 0.97 | 0.15 |
| CORE | 0.0018 | 37 | 0.96 | 0.10 |
Although liver biopsy remains the gold standard for diagnosing and staging fibrosis, it is not suitable as an initial confirmatory test due to its invasive nature and the relatively low prevalence of advanced liver disease following primary screening[10,11]. VCTE plays an important role in confirmation, with LSM < 8 kPa serving as a threshold to rule out advanced liver disease across all guidelines, requiring no further specialist evaluation (Table 6). Conversely, LSM ≥ 8 kPa warrants specialist follow-up, and repeat VCTE within 3 years may be considered for values ≤ 12 kPa, thereby reducing workload (Table 5)[3-7]. This approach is particularly noteworthy, as approximately 40% of individuals with an LSM ≥ 8 kPa had LSM < 8 kPa upon retesting without any intervention.
| Rule out | Rule in | |
| 2021 EASL NIT clinical practice guideline | LSM: < 8 kPa | LSM: ≥ 8 kPa |
| Alternatives: ELF, FibroMeter, Fibrotest | Alternatives: ELF, FibroMeter, Fibrotest | |
| 2021 AGA clinical care pathway | LSM: < 8 kPa | LSM: ≥ 12 kPa |
| Alternatives: SWE, ultrasound | Alternatives: SWE, ultrasound | |
| 2023 AASLD practice guidance | LSM: < 8 kPa | LSM: ≥ 8 kPa |
| Alternatives: ELF | ||
| 2024 EASL-EASD-EASO clinical practice guideline | LSM: < 8 kPa | LSM: ≥ 8 kPa |
| Alternatives: MRE, SWE or ELF with adjusted thresholds | ||
| 2025 APASL clinical practice guideline | Not mentioned | LSM: ≥ 12 kPa, SWE ≥ 8 kPa, MRE ≥ 3.6 kPa, ELF ≥ 9.8, ADAPT ≥ 6.328 |
Alternative confirmation tests when VCTE is not available vary across the guidelines but include ELF, FibroMeter, Fibrotest, shear wave elastography and magnetic resonance elastography (MRE). ELF has been adopted in the United Kingdom as a primary confirmation test.
MRE offers a highly accurate diagnosis of advanced liver fibrosis[73]. A major advantage is its reduced susceptibility to sampling bias compared to liver biopsy or VCTE, as it evaluates the entire liver volume rather than a small sample. This minimizes the risk of missing fibrosis in a heterogeneously affected liver, where localized sampling may fail to detect disease. However, MRE’s high cost, limited availability and long scan times restrict its widespread use in clinical practice. Similarly, ELF, which is based upon three serum biomarkers (hyaluronic acid, procollagen III, tissue inhibitor of metalloproteinases-1), demonstrates good diagnostic performance for advanced fibrosis[74]. However, its limited availability outside the United Kingdom due to its reliance on highly specialized laboratory measurements hinders routine adoption. It should be noted that the correlation between imaging-based and laboratory-based tests is generally weak to moderate, and confirmation using ELF score or VCTE may select different patient populations[75,76]. Therefore, widespread implementation in screening programs requires careful evaluation, and comparisons of efficacy with VCTE-based referral pathways are warranted.
Once screened, at-risk individuals should undergo periodic reassessment with additional testing, which is a key requirement for effective screening programs[16]. The guidelines recommend reassessment between 1 and 3 years of initial assessment (Table 7). The AASLD guideline is more specific and recommends 1-2 years in individuals with type 2 diabetes mellitus (T2DM) or ≥ 2 metabolic risk factors and 2-3 years if these conditions are not present. However, almost the entire target population meets the AASLD criteria for early re-evaluation, as only those who opted for screening due to presence of steatosis or elevated ALT (in the absence of metabolic dysfunction) can be considered for re-evaluation in 2-3 years. Considering the natural disease history, where it typically takes 7 years to progress to the next stage of disease, this screening interval might be too conservative. However, up to 20% of patients are fast progressors, where these shorter intervals are warranted[77,78]. Additionally, due to false negative results with the initial screening test, one may opt for a second test earlier than the average time required for disease progression to maintain trust in screening programs[79]. Although recommendations are provided by the guidelines, data on which intervals are safe in a screening setting are currently lacking and may differ based on the indication for screening and previous test results. Current ongoing projects, such as LiverScreen and LiverAIM, will further investigate what intervals are safe for re-evaluation in a screening setting[22].
| Interval | Early re-evaluation | Screening test | |
| 2021 EASL NIT clinical practice guideline | 1-3 years | FIB-4 | |
| 2021 AGA clinical care pathway | 2-3 years | FIB-4 | |
| 2023 AASLD practice guidance | 2-3 years | After 1-2 years in individuals with T2DM or ≥ 2 metabolic risk factors | FIB-4 |
| 2024 EASL-EASD-EASO clinical practice guideline | 1-3 years | Within 1 year when FIB-4 was indeterminate and management of comorbidities was intensified, whilst VCTE was not performed | FIB-4 |
| 2025 APASL clinical practice guideline | 2-3 years | FIB-4, NFS |
Previous studies indicated the value of repeated testing. For example, studies of dynamic changes in FIB-4 have shown that individuals initially classified as intermediate risk who transitioned to the low-risk group within 5 years were not at increased risk of liver-related events. Nonetheless, almost 50% of all liver-related events occurred in the 67% of the general Swedish population that had low FIB-4 on two occasions[80]. This is inferior to the results for MAF-5 from the United Kingdom biobank. There, just 25% of the incident cirrhosis, liver cancer and liver-related mortality occurred in the 70% of individuals with MAF-5 < 1 (low-risk and indeterminate risk). Moreover, like FIB-4, dynamic changes were associated with risk changes overtime[81]. However, another study reported only weak associations with changes in FIB-4, aspartate aminotransferase to platelet ratio index and NFS with disease progression in MASLD patients[82].
Re-evaluation for liver disease may eventually be a dynamic process similar to the colon cancer screening test where risk factors are considered together with findings during colonoscopy in case of a positive screening test[83,84]. If there is a subgroup where extended screening intervals for significant liver disease appear to be safe, this would increase screening program feasibility.
Several challenges in NIT development can affect performance in screening programs. Key decisions include defining the target outcome, such as increased LSM, histology-based advanced fibrosis, or liver-related events including hepatocellular carcinoma and decompensated cirrhosis, and the population in which the model is trained (e.g., general vs hospital-based population). Ideally, the development setting should match the intended screening setting; for example, an NIT intended for population screening would ideally be developed in a general population with available liver biopsy data. Because this ideal scenario is not feasible, compromises must be made, which can affect the utility of the score in screening programs and should be considered during implementation. Most NITs have been developed in hospital-based populations, as biopsy data are generally unavailable in the general population. However, these populations represent only the "tip of the iceberg", with more advanced liver disease, limiting the generalizability of these NITs to the broader screening population. On the other hand, the MAF-5 and liver risk score were developed in a more general population setting and were trained on increased liver stiffness due to lack of biopsy[63,64]. Interestingly, CORE, a new risk score measuring gamma-glutamyl transferase, aspartate aminotransferase, and ALT, used a Swedish registry and did not consider fibrosis, but major adverse liver-related outcomes, events that need to be prevented by screening programs[68]. Due to differences in populations where NITs are developed and are employed, differences might occur between performance in the development populations and the actual performance in a screening setting. Therefore, the study population in which the NIT is developed should be considered when deployed in referral strategies and is preferably similar to the target screening population.
Screening programs for advanced liver disease initially increase costs but become cost-effective, not necessarily cost-saving, long-term by the expected reduced mortality and advanced fibrosis rates, thereby lowering long-term expenses[85]. Several studies have used Markov models to evaluate the long-term costs and cost per quality-adjusted life year (QALY) of various screening strategies[23,24,86,87]. In a study on participants with type 2 diabetes, all investigated NITs were associated with improved QALYs, with the most substantial gains from VCTE, followed by FIB-4, ELF, and NFS[23]. Similarly, a Korean study assessing a sequential approach of FIB-4 followed by VCTE in at-risk populations (T2DM, obesity, metabolic syndrome), reported incremental cost of 298 dollars and a 0.0199 QALY gain per patient. This corresponds to a cost of 14949 dollars per QALY gained, which is well below the national willingness-to-pay threshold of 25000 dollars per QALY in Korea, indicating cost-effectiveness. When the broader benefits of treatment, such as reductions in cardiovascular and cancer-related morbidity were incorporated, cost-effectiveness further improved, with an incremental cost-effectiveness ratio of 12749 dollars per QALY. These findings indicate that if liver fibrosis screening with currently available NITs were implemented in primary care, it would likely be cost effective. Notably, upcoming pharmacological treatments may contribute even more to cost-effectiveness compared to the current standard of care, particularly because lifestyle interventions are notoriously difficult for patients to adhere to[88,89].
Besides economical costs, the social and psychological burden of liver disease screening should not be underestimated, particularly given the high false positive rate associated with current NITs. At present, no available test yields more true positives than false positives. Insights from breast cancer screening programs have shown that false positive results can have lasting psychosocial consequences and reduce willingness to participate in future screening rounds[90,91]. While data on the psychological impact of liver disease screening are limited, studies indicate that more than half of individuals newly diagnosed with MASLD report symptoms of anxiety[92]. This may be in part linked to stigmas surrounding liver disease. Many individuals with chronic liver disease express fear of being labeled as alcoholics[93]. Therefore, the potential for false positive results must be clearly communicated to patients and, ideally minimized through optimized screening strategies[94].
Although MASLD is an important risk factor for liver-related complications and mortality, only a subset of individuals will progress to advanced liver disease[17,19]. Among MASLD patients, cardiovascular disease is the primary cause of death, which illustrates that MASLD management cannot be separated from cardiovascular risk management, and a more holistic management approach is warranted[18].
The updated 2025 American Diabetes Association guideline now includes a section on mitigating the risk of MASLD and MASH, recommending assessment of liver health and consideration of incretin-based therapies, which may offer benefits in these conditions[95]. Moreover, the new obesity definition now includes MASLD with fibrosis as one of the comorbidities requiring investigation[96]. Similarly, a Delphi consensus paper on the management of MASLD in cardiovascular disease agreed on screening for MASLD and fibrosis in type 2 diabetes, metabolic syndrome, overweight/obesity and argued for a screening pathway of MASLD and fibrosis in cardiovascular disease management using imaging and or NITs. Moreover, they also urged screening for cardiovascular disease in MASLD patients[18]. Altogether, there is momentum in increasing awareness of liver health by health care providers, particularly among those who treat metabolic dysfunction.
In the future, liver disease screening may even be integrated into cardiovascular risk management. For example, NIT-based screening could be implemented as reflex tests in those with cardiometabolic disorders. A recent study proposed an innovative approach in which VCTE measurement was performed following diabetic retinopathy screening, resulting in a high participation rate for fibrosis screening[28]. However, this approach deals with a selected population that was already engaged in a screening program. Another study implemented automatic fibrosis score (FIB-4 and aspartate aminotransferase to platelet ratio index) calculations and electronic reminder messages in a randomized controlled setting for those with type II diabetes attending medical or diabetes clinics[97]. Implementation of this care pathway increased appropriate referral for hepatology assessment or further fibrosis tests in patients with increased fibrosis scores from 3.1% to 33.3%. These results suggest that incorporating NIT-based reflex testing could enhance early liver fibrosis detection. Unfortunately, implementation of these clinical care pathways remains in its early stages, although important steps have been made.
Early detection of advanced liver disease in at-risk individuals is important both for patient education and for preventing future liver-related events, and it is likely already cost-effective in its current form. Although referral strategies have been successfully developed, they are not yet widely adopted by non-hepatology healthcare providers. Remaining challenges include overly broad target populations, suboptimal selection of the initial NIT from a screening perspective, and limited data on safe screening intervals. These obstacles may be addressed through early reassessment of existing referral pathways and the integration of recently developed NITs.
| 1. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 2011] [Article Influence: 670.3] [Reference Citation Analysis (3)] |
| 2. | Owrangi S, Paik JM, Golabi P, de Avila L, Hashida R, Nader A, Paik A, Henry L, Younossi ZM. Meta-Analysis: Global Prevalence and Mortality of Cirrhosis in Metabolic Dysfunction-Associated Steatotic Liver Disease. Aliment Pharmacol Ther. 2025;61:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 969] [Article Influence: 484.5] [Reference Citation Analysis (1)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1299] [Article Influence: 259.8] [Reference Citation Analysis (1)] |
| 5. | Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, Abdelmalek MF, Harrison SA, Loomba R, Mantzoros CS, Bugianesi E, Eckel RH, Kaplan LM, El-Serag HB, Cusi K. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2021;161:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 443] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 6. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1465] [Cited by in RCA: 1586] [Article Influence: 528.7] [Reference Citation Analysis (1)] |
| 7. | Eslam M, Fan JG, Yu ML, Wong VW, Cua IH, Liu CJ, Tanwandee T, Gani R, Seto WK, Alam S, Young DY, Hamid S, Zheng MH, Kawaguchi T, Chan WK, Payawal D, Tan SS, Goh GB, Strasser SI, Viet HD, Kao JH, Kim W, Kim SU, Keating SE, Yilmaz Y, Kamani L, Wang CC, Fouad Y, Abbas Z, Treeprasertsuk S, Thanapirom K, Al Mahtab M, Lkhagvaa U, Baatarkhuu O, Choudhury AK, Stedman CAM, Chowdhury A, Dokmeci AK, Wang FS, Lin HC, Huang JF, Howell J, Jia J, Alboraie M, Roberts SK, Yoneda M, Ghazinian H, Mirijanyan A, Nan Y, Lesmana CRA, Adams LA, Shiha G, Kumar M, Örmeci N, Wei L, Lau G, Omata M, Sarin SK, George J. The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic dysfunction-associated fatty liver disease. Hepatol Int. 2025;19:261-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 8. | van Kleef LA, Sonneveld MJ, de Man RA, de Knegt RJ. Poor performance of FIB-4 in elderly individuals at risk for chronic liver disease - implications for the clinical utility of the EASL NIT guideline. J Hepatol. 2022;76:245-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Ajmera V, Tesfai K, Sandoval E, Lopez S, Cervantes V, Madamba E, Bettencourt R, Manousou P, Richards L, Loomba R. Validation of AGA clinical care pathway and AASLD practice guidance for nonalcoholic fatty liver disease in a prospective cohort of patients with type 2 diabetes. Hepatology. 2024;79:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Udompap P, Therneau TM, Canning RE, Benson JT, Allen AM. Performance of American Gastroenterological Association Clinical Care Pathway for the risk stratification of patients with nonalcoholic fatty liver disease in the US population. Hepatology. 2023;77:931-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 11. | Chang M, Chang D, Kodali S, Harrison SA, Ghobrial M, Alkhouri N, Noureddin M. Degree of Discordance Between FIB-4 and Transient Elastography: An Application of Current Guidelines on General Population Cohort. Clin Gastroenterol Hepatol. 2024;22:1453-1461.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Tsochatzis EA, Valenti L, Thiele M, Péloquin S, Lazure P, Masson MH, Allen AM, Lazarus JV, Noureddin M, Rinella M, Tacke F, Murray S. Use of non-invasive diagnostic tools for metabolic dysfunction-associated steatohepatitis: A qualitative exploration of challenges and barriers. Liver Int. 2024;44:1990-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 13. | Bech KT, Lindvig KP, Thiele M, Castera L. Algorithms for Early Detection of Silent Liver Fibrosis in the Primary Care Setting. Semin Liver Dis. 2024;44:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Alqahtani SA, Paik JM, Biswas R, Arshad T, Henry L, Younossi ZM. Poor Awareness of Liver Disease Among Adults With NAFLD in the United States. Hepatol Commun. 2021;5:1833-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Younossi ZM, Ong JP, Takahashi H, Yilmaz Y, Eguc Hi Y, El Kassas M, Buti M, Diago M, Zheng MH, Fan JG, Yu ML, Wai-Sun Wong V, Alswat K, Chan WK, Mendez-Sanchez N, Burra P, Bugianesi E, Duseja AK, George J, Papatheodoridis GV, Saeed H, Castera L, Arrese M, Kugelmas M, Romero-Gomez M, Alqahtani S, Ziayee M, Lam B, Younossi I, Racila A, Henry L, Stepanova M; Global Nonalcoholic Steatohepatitis Council. A Global Survey of Physicians Knowledge About Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2022;20:e1456-e1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (34)] |
| 16. | Hall K. Max Wilson and the Principles and Practice of Screening for Disease. Int J Neonatal Screen. 2020;6:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Israelsen M, Francque S, Tsochatzis EA, Krag A. Steatotic liver disease. Lancet. 2024;404:1761-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 169] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 18. | Chew NWS, Mehta A, Goh RSJ, Zhang A, Chen Y, Chong B, Chew HSJ, Shabbir A, Brown A, Dimitriadis GK, Huang DQ, Foo R, le Roux CW, Figtree GA, Fudim M, Pandey A, Mamas MA, Hausenloy DJ, Richards AM, Nicholls SJ, Chan MY, Muthiah MD, Sanyal A, Sperling LS. Cardiovascular-Liver-Metabolic Health: Recommendations in Screening, Diagnosis, and Management of Metabolic Dysfunction-Associated Steatotic Liver Disease in Cardiovascular Disease via Modified Delphi Approach. Circulation. 2025;151:98-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 19. | Huang DQ, Wong VWS, Rinella ME, Boursier J, Lazarus JV, Yki-Järvinen H, Loomba R. Metabolic dysfunction-associated steatotic liver disease in adults. Nat Rev Dis Primers. 2025;11:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 103] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 20. | Harrison SA, Taub R, Neff GW, Lucas KJ, Labriola D, Moussa SE, Alkhouri N, Bashir MR. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29:2919-2928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 283] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 21. | Kjaergaard M, Lindvig KP, Thorhauge KH, Johansen S, Hansen JK, Andersen P, Hansen CD, Schnefeld HL, Bech KT, Torp N, Israelsen M, Detlefsen S, Graupera I, Gines P, Krag A, Thiele M. Screening for Fibrosis Promotes Lifestyle Changes: A Prospective Cohort Study in 4796 Individuals. Clin Gastroenterol Hepatol. 2024;22:1037-1047.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 22. | Ginès P, Castera L, Lammert F, Graupera I, Serra-Burriel M, Allen AM, Wong VW, Hartmann P, Thiele M, Caballeria L, de Knegt RJ, Grgurevic I, Augustin S, Tsochatzis EA, Schattenberg JM, Guha IN, Martini A, Morillas RM, Garcia-Retortillo M, de Koning HJ, Fabrellas N, Pich J, Ma AT, Diaz MA, Roulot D, Newsome PN, Manns M, Kamath PS, Krag A; LiverScreen Consortium Investigators. Population screening for liver fibrosis: Toward early diagnosis and intervention for chronic liver diseases. Hepatology. 2022;75:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 187] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 23. | Forlano R, Stanic T, Jayawardana S, Mullish BH, Yee M, Mossialos E, Goldin R, Petta S, Tsochatzis E, Thursz M, Manousou P. A prospective study on the prevalence of MASLD in people with type-2 diabetes in the community. Cost effectiveness of screening strategies. Liver Int. 2024;44:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Park H, Yoon EL, Kim M, Kwon SH, Kim D, Cheung R, Kim HL, Jun DW. Cost-effectiveness study of FIB-4 followed by transient elastography screening strategy for advanced hepatic fibrosis in a NAFLD at-risk population. Liver Int. 2024;44:944-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Serra-Burriel M, Graupera I, Torán P, Thiele M, Roulot D, Wai-Sun Wong V, Neil Guha I, Fabrellas N, Arslanow A, Expósito C, Hernández R, Lai-Hung Wong G, Harman D, Darwish Murad S, Krag A, Pera G, Angeli P, Galle P, Aithal GP, Caballeria L, Castera L, Ginès P, Lammert F; investigators of the LiverScreen Consortium. Transient elastography for screening of liver fibrosis: Cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J Hepatol. 2019;71:1141-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 26. | Newsome PN, Sanyal AJ, Engebretsen KA, Kliers I, Østergaard L, Vanni D, Bugianesi E, Rinella ME, Roden M, Ratziu V. Semaglutide 2.4 mg in Participants With Metabolic Dysfunction-Associated Steatohepatitis: Baseline Characteristics and Design of the Phase 3 ESSENCE Trial. Aliment Pharmacol Ther. 2024;60:1525-1533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 88] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 27. | Labenz C, Arslanow A, Nguyen-Tat M, Nagel M, Wörns MA, Reichert MC, Heil FJ, Mainz D, Zimper G, Römer B, Binder H, Farin-Glattacker E, Fichtner U, Graf E, Stelzer D, Van Ewijk R, Ortner J, Velthuis L, Lammert F, Galle PR. Structured Early detection of Asymptomatic Liver Cirrhosis: Results of the population-based liver screening program SEAL. J Hepatol. 2022;77:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Lindfors A, Strandberg R, Hagström H. Screening for advanced liver fibrosis due to metabolic dysfunction-associated steatotic liver disease alongside retina scanning in people with type 2 diabetes: a cross-sectional study. Lancet Gastroenterol Hepatol. 2025;10:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 29. | Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, Pryke R, Hutchinson SJ, Sangro B, Martin NK, Cecchini M, Dirac MA, Belloni A, Serra-Burriel M, Ponsioen CY, Sheena B, Lerouge A, Devaux M, Scott N, Hellard M, Verkade HJ, Sturm E, Marchesini G, Yki-Järvinen H, Byrne CD, Targher G, Tur-Sinai A, Barrett D, Ninburg M, Reic T, Taylor A, Rhodes T, Treloar C, Petersen C, Schramm C, Flisiak R, Simonova MY, Pares A, Johnson P, Cucchetti A, Graupera I, Lionis C, Pose E, Fabrellas N, Ma AT, Mendive JM, Mazzaferro V, Rutter H, Cortez-Pinto H, Kelly D, Burton R, Lazarus JV, Ginès P, Buti M, Newsome PN, Burra P, Manns MP. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 448] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 30. | Park H, Yoon EL, Kim M, Cho S, Kim JH, Jun DW, Nah EH. Selecting the Target Population for Screening of Hepatic Fibrosis in Primary Care Centers in Korea. J Clin Med. 2022;11:1474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Åberg F, Jula A, Färkkilä M, Salomaa V, Erlund I, Männistö S, Vihervaara T, Perola M, Lundqvist A, Männistö V. Comparison of various strategies to define the optimal target population for liver fibrosis screening: A population-based cohort study. United European Gastroenterol J. 2022;10:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 32. | Dietrich CG, Rau M, Geier A. Screening for nonalcoholic fatty liver disease-when, who and how? World J Gastroenterol. 2021;27:5803-5821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 33. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1676] [Cited by in RCA: 1817] [Article Influence: 605.7] [Reference Citation Analysis (2)] |
| 34. | Pustjens J, van Kleef LA, Janssen HLA, de Knegt RJ, Brouwer WP. Differential prevalence and prognostic value of metabolic syndrome components among patients with MASLD. JHEP Rep. 2024;6:101193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 35. | Man S, Deng Y, Ma Y, Fu J, Bao H, Yu C, Lv J, Liu H, Wang B, Li L. Prevalence of Liver Steatosis and Fibrosis in the General Population and Various High-Risk Populations: A Nationwide Study With 5.7 Million Adults in China. Gastroenterology. 2023;165:1025-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 142] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 36. | Marti-Aguado D, Calleja JL, Vilar-Gomez E, Iruzubieta P, Rodríguez-Duque JC, Del Barrio M, Puchades L, Rivera-Esteban J, Perelló C, Puente A, Gomez-Medina C, Escudero-García D, Serra MA, Bataller R, Crespo J, Arias-Loste MT. Low-to-moderate alcohol consumption is associated with increased fibrosis in individuals with metabolic dysfunction-associated steatotic liver disease. J Hepatol. 2024;81:930-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 102] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 37. | van Kleef LA, de Knegt RJ, Brouwer WP. Metabolic dysfunction-associated fatty liver disease and excessive alcohol consumption are both independent risk factors for mortality. Hepatology. 2023;77:942-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 38. | Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3:1260-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | Wiering L, Tacke F. Treating inflammation to combat non-alcoholic fatty liver disease. J Endocrinol. 2023;256:e220194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | European Association for the Study of the Liver. Clinical Practice Guideline Panel; Chair:; EASL Governing Board representative:; Panel members:. Reply to: Correspondence on "EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update". J Hepatol. 2022;76:251-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, Schwimmer JB. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263-2271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 42. | Golabi P, Paik J, Reddy R, Bugianesi E, Trimble G, Younossi ZM. Prevalence and long-term outcomes of non-alcoholic fatty liver disease among elderly individuals from the United States. BMC Gastroenterol. 2019;19:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 43. | van Kleef LA, Sonneveld MJ, Zhu F, Ikram MA, Kavousi M, de Knegt RJ. Liver stiffness is associated with excess mortality in the general population driven by heart failure: The Rotterdam Study. Liver Int. 2023;43:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 44. | van Kleef LA, Sonneveld MJ, Kavousi M, Ikram MA, de Man RA, de Knegt RJ. Fatty liver disease is not associated with increased mortality in the elderly: A prospective cohort study. Hepatology. 2023;77:585-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 45. | Ciardullo S, Muraca E, Zerbini F, Perseghin G. Liver stiffness is associated with all-cause mortality in patients with NAFLD: A systematic review and meta-analysis. Liver Int. 2023;43:2604-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Vilar-Gomez E, Vuppalanchi R, Gawrieh S, Samala N, Chalasani N. CAP and LSM as determined by VCTE are independent predictors of all-cause mortality in the US adult population. Hepatology. 2023;77:1241-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 47. | Mellemkjær A, Kjær MB, Haldrup D, Grønbæk H, Thomsen KL. Management of cardiovascular risk in patients with metabolic dysfunction-associated steatotic liver disease. Eur J Intern Med. 2024;122:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 48. | Pokharel R, Lin YS, McFerran E, O'Mahony JF. A Systematic Review of Cost-Effectiveness Analyses of Colorectal Cancer Screening in Europe: Have Studies Included Optimal Screening Intensities? Appl Health Econ Health Policy. 2023;21:701-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 49. | Evans A. The pros and cons of breast screening in older women. Ann Breast Surg. 2024;8:10-10. [DOI] [Full Text] |
| 50. | Lindvig KP, Hansen TL, Madsen BS, Kjaergaard M, Møller L, Detlefsen S, Krag A, Thiele M. Diagnostic accuracy of routine liver function tests to identify patients with significant and advanced alcohol-related liver fibrosis. Scand J Gastroenterol. 2021;56:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Mózes FE, Lee JA, Vali Y, Alzoubi O, Staufer K, Trauner M, Paternostro R, Stauber RE, Holleboom AG, van Dijk AM, Mak AL, Boursier J, de Saint Loup M, Shima T, Bugianesi E, Gaia S, Armandi A, Shalimar, Lupșor-Platon M, Wong VW, Li G, Wong GL, Cobbold J, Karlas T, Wiegand J, Sebastiani G, Tsochatzis E, Liguori A, Yoneda M, Nakajima A, Hagström H, Akbari C, Hirooka M, Chan WK, Mahadeva S, Rajaram R, Zheng MH, George J, Eslam M, Petta S, Pennisi G, Viganò M, Ridolfo S, Aithal GP, Palaniyappan N, Lee DH, Ekstedt M, Nasr P, Cassinotto C, de Lédinghen V, Berzigotti A, Mendoza YP, Noureddin M, Truong E, Fournier-Poizat C, Geier A, Martic M, Tuthill T, Anstee QM, Harrison SA, Bossuyt PM, Pavlides M; LITMUS investigators. Performance of non-invasive tests and histology for the prediction of clinical outcomes in patients with non-alcoholic fatty liver disease: an individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 52. | van Kleef LA, de Knegt RJ, Ayada I, Pan Q, Brouwer WP. The Steatosis-associated fibrosis estimator (SAFE) score: validation in the general US population. Hepatol Commun. 2023;7:e0075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 53. | McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, Francque S, Van Gaal L, Schattenberg JM, Tiniakos D, Burt A, Bugianesi E, Ratziu V, Day CP, Anstee QM. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 710] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 54. | Sugiyama A, Kurisu A, E B, Ouoba S, Ko K, Rakhimov A, Akita T, Harakawa T, Sako T, Koshiyama M, Kumada T, Tanaka J. Distribution of FIB-4 index in the general population: analysis of 75,666 residents who underwent health checkups. BMC Gastroenterol. 2022;22:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 55. | Vali Y, van Dijk AM, Lee J, Boursier J, Ratziu V, Yunis C, Schattenberg JM, Valenti L, Gomez MR, Schuppan D, Petta S, Allison M, Hartman ML, Porthan K, Dufour JF, Bugianesi E, Gastadelli A, Derdak Z, Fournier-Poizat C, Shumbayawonda E, Kalutkiewicz M, Yki-Jarvinen H, Ekstedt M, Geier A, Trylesinski A, Francque S, Brass C, Pavlides M, Holleboom AG, Nieuwdorp M, Anstee QM, Bossuyt PM; LITMUS investigators. Precision in Liver Diagnosis: Varied Accuracy Across Subgroups and the Need for Variable Thresholds in Diagnosis of MASLD. Liver Int. 2025;45:e16240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 56. | Boursier J, Guillaume M, Leroy V, Irlès M, Roux M, Lannes A, Foucher J, Zuberbuhler F, Delabaudière C, Barthelon J, Michalak S, Hiriart JB, Peron JM, Gerster T, Le Bail B, Riou J, Hunault G, Merrouche W, Oberti F, Pelade L, Fouchard I, Bureau C, Calès P, de Ledinghen V. New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J Hepatol. 2019;71:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 57. | van Kleef LA, Pustjens J, Janssen HLA, Brouwer WP. Diagnostic Accuracy of the LiverRisk Score to Detect Increased Liver Stiffness Among a United States General Population and Subgroups. J Clin Exp Hepatol. 2025;15:102512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | De Vincentis A, Tavaglione F, Namba S, Kanai M, Okada Y, Kamatani Y, Maurotti S, Pedone C, Antonelli Incalzi R, Valenti L, Romeo S, Vespasiani-Gentilucci U. Poor accuracy and sustainability of the first-step FIB4 EASL pathway for stratifying steatotic liver disease risk in the general population. Aliment Pharmacol Ther. 2024;59:1402-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Wernberg CW, Indira Chandran V, Lauridsen MM, Skytthe MK, Hansen CD, Hansen JK, Grønkjær LL, Jacobsen BG, Di Caterino T, Detlefsen S, Thiele M, Guiliani AM, Villesen IF, Leeming DJ, Karsdal M, Graversen JH, Krag A. Ability of soluble TREM2 and PRO-C3 as biomarkers to predict changes in MASLD activity. JHEP Rep. 2025;7:101432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 60. | Graupera I, Thiele M, Serra-Burriel M, Caballeria L, Roulot D, Wong GL, Fabrellas N, Guha IN, Arslanow A, Expósito C, Hernández R, Aithal GP, Galle PR, Pera G, Wong VW, Lammert F, Ginès P, Castera L, Krag A; Investigators of the LiverScreen Consortium. Low Accuracy of FIB-4 and NAFLD Fibrosis Scores for Screening for Liver Fibrosis in the Population. Clin Gastroenterol Hepatol. 2022;20:2567-2576.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 197] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 61. | Mózes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, Fournier C, Staufer K, Stauber RE, Bugianesi E, Younes R, Gaia S, Lupșor-Platon M, Petta S, Shima T, Okanoue T, Mahadeva S, Chan WK, Eddowes PJ, Hirschfield GM, Newsome PN, Wong VW, de Ledinghen V, Fan J, Shen F, Cobbold JF, Sumida Y, Okajima A, Schattenberg JM, Labenz C, Kim W, Lee MS, Wiegand J, Karlas T, Yılmaz Y, Aithal GP, Palaniyappan N, Cassinotto C, Aggarwal S, Garg H, Ooi GJ, Nakajima A, Yoneda M, Ziol M, Barget N, Geier A, Tuthill T, Brosnan MJ, Anstee QM, Neubauer S, Harrison SA, Bossuyt PM, Pavlides M; LITMUS Investigators. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. 2022;71:1006-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 353] [Article Influence: 88.3] [Reference Citation Analysis (2)] |
| 62. | Sripongpun P, Kim WR, Mannalithara A, Charu V, Vidovszky A, Asch S, Desai M, Kim SH, Kwong AJ. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. Hepatology. 2023;77:256-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 63. | Serra-Burriel M, Juanola A, Serra-Burriel F, Thiele M, Graupera I, Pose E, Pera G, Grgurevic I, Caballeria L, Piano S, van Kleef L, Reichert M, Roulot D, Pericàs JM, Schattenberg JM, Tsochatztis EA, Guha IN, Garcia-Retortillo M, Hernández R, Hoyo J, Fuentes M, Expósito C, Martínez A, Such P, Madir A, Detlefsen S, Tonon M, Martini A, Ma AT, Pich J, Bonfill E, Juan M, Soria A, Carol M, Gratacós-Ginès J, Morillas RM, Toran P, Navarrete JM, Torrejón A, Fournier C, Llorca A, Arslanow A, de Koning HJ, Cucchietti F, Manns M, Newsome PN, Hernáez R, Allen A, Angeli P, de Knegt RJ, Karlsen TH, Galle P, Wong VW, Fabrellas N, Castera L, Krag A, Lammert F, Kamath PS, Ginès P; LiverScreen Consortium Investigators. Development, validation, and prognostic evaluation of a risk score for long-term liver-related outcomes in the general population: a multicohort study. Lancet. 2023;402:988-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 64. | van Kleef LA, Francque SM, Prieto-Ortiz JE, Sonneveld MJ, Sanchez-Luque CB, Prieto-Ortiz RG, Kwanten WJ, Vonghia L, Verrijken A, De Block C, Gadi Z, Janssen HLA, de Knegt RJ, Brouwer WP. Metabolic Dysfunction-Associated Fibrosis 5 (MAF-5) Score Predicts Liver Fibrosis Risk and Outcome in the General Population With Metabolic Dysfunction. Gastroenterology. 2024;167:357-367.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 65. | Feng G, Mózes FE, Ji D, Treeprasertsuk S, Okanoue T, Shima T, Liang H, Tsochatzis E, Chen J, Schattenberg JM, Labenz C, Mahadeva S, Chan WK, Chi X, Delamarre A, de Lédinghen V, Petta S, Bugianesi E, Hagström H, Boursier J, Calleja JL, Goh GB, Gallego-Durán R, Sanyal AJ, Fan JG, Castéra L, Lai M, Harrison SA, Romero-Gomez M, Kim SU, Zhu Y, Ooi G, Shi J, Yoneda M, Nakajima A, Zhang J, Lupsor-Platon M, Zhong B, Cobbold JFL, Ye CY, Eddowes PJ, Newsome P, Li J, George J, He F, Song MJ, Tang H, Fan Y, Jia J, Xu L, Lin S, Li Y, Lu Z, Nan Y, Niu J, Yan X, Zhou Y, Liu C, Deng H, Ye Q, Zeng QL, Li L, Wang J, Yang S, Lin H, Lee HW, Yip TC, Fournier-Poizat C, Wong GL, Pennisi G, Armandi A, Liu WY, Shang Y, de Saint-Loup M, Llop E, Teh KKJ, Lara-Romero C, Asgharpour A, Mahgoub S, Chan MS, Canivet CM, Ji F, Xin Y, Chai J, Dong Z, Targher G, Byrne CD, He N, Mi M, Ye F, Wong VW, Pavlides M, Zheng MH. acFibroMASH Index for the Diagnosis of Fibrotic MASH and Prediction of Liver-related Events: An International Multicenter Study. Clin Gastroenterol Hepatol. 2025;23:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 66. | Calès P, Canivet CM, Costentin C, Lannes A, Oberti F, Fouchard I, Hunault G, de Lédinghen V, Boursier J. A new generation of non-invasive tests of liver fibrosis with improved accuracy in MASLD. J Hepatol. 2025;82:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 67. | Lindvig KP, Thorhauge KH, Hansen JK, Kjærgaard M, Hansen CD, Johansen S, Lyngbeck E, Israelsen M, Andersen P, Bech KT, Torp N, Schnefeld HL, Detlefsen S, Möller S, Graupera I, Trelle MB, Antonsen S, Harris R, Kårhus LL, Bjørnsbo KS, Brøns C, Hansen T, Geier A, Wedemeyer H, Zeuzem S, Schattenberg JM, Ginès P, Guha IN, Krag A, Thiele M. Development, validation, and prognostic evaluation of LiverPRO for the prediction of significant liver fibrosis in primary care: a prospective cohort study. Lancet Gastroenterol Hepatol. 2025;10:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 68. | Strandberg R, Talbäck M, Hammar N, Hagström H. OS-057-YI CORE: a new risk score measuring GGT, AST, and ALT outperforms FIB-4 when predicting the risk of cirrhosis in a primary care setting. J Hepatol. 2024;80:S40. [DOI] [Full Text] |
| 69. | Coste P, Llop E, Perelló C, Hernández M, López M, Abad J, Ferre C, Martínez JL, Fernández N, Calleja JL. Comparison of non-invasive fibrosis scores to predict increased liver stiffness in the general population with unknown liver disease: Searching for the primary physician's best friend. Dig Liver Dis. 2022;54:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Kjaergaard M, Lindvig KP, Thorhauge KH, Andersen P, Hansen JK, Kastrup N, Jensen JM, Hansen CD, Johansen S, Israelsen M, Torp N, Trelle MB, Shan S, Detlefsen S, Antonsen S, Andersen JE, Graupera I, Ginés P, Thiele M, Krag A. Using the ELF test, FIB-4 and NAFLD fibrosis score to screen the population for liver disease. J Hepatol. 2023;79:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 150] [Reference Citation Analysis (0)] |
| 71. | Eslam M, Wong GL, Hashem AM, Chan HL, Nielsen MJ, Leeming DJ, Chan AW, Chen Y, Duffin KL, Karsdal M, Schattenberg JM, George J, Wong VW. A Sequential Algorithm Combining ADAPT and Liver Stiffness Can Stage Metabolic-Associated Fatty Liver Disease in Hospital-Based and Primary Care Patients. Am J Gastroenterol. 2021;116:984-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 72. | van Kleef LA, Pustjens J, Schattenberg JM, Holleboom AG, Castro Cabezas M, Tushuizen ME, de Knegt RJ, Ikram MA, Janssen HLA, Francque SM, Brouwer WP. Comparison of diagnostic accuracy and utility of non-invasive tests for clinically significant liver disease in a general population with metabolic dysfunction. Hepatology. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Selvaraj EA, Mózes FE, Jayaswal ANA, Zafarmand MH, Vali Y, Lee JA, Levick CK, Young LAJ, Palaniyappan N, Liu CH, Aithal GP, Romero-Gómez M, Brosnan MJ, Tuthill TA, Anstee QM, Neubauer S, Harrison SA, Bossuyt PM, Pavlides M; LITMUS Investigators. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J Hepatol. 2021;75:770-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 251] [Article Influence: 50.2] [Reference Citation Analysis (3)] |
| 74. | Zoncapè M, Liguori A, Tsochatzis EA. Non-invasive testing and risk-stratification in patients with MASLD. Eur J Intern Med. 2024;122:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 75. | Uzlova N, Mnozil Stridova K, Merta D, Rychlik I, Frankova S. Transient Elastography as the First-Line Assessment of Liver Fibrosis and Its Correlation with Serum Markers. Medicina (Kaunas). 2023;59:752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 76. | Reinson T, Patel J, Mathews M, Fountain D, Buchanan RM, Byrne CD. Performance of the Enhanced Liver Fibrosis Score, Comparison with Vibration-controlled Transient Elastography Data, and Development of a Simple Algorithm to Predict Significant Liver Fibrosis in a Community-based Liver Service: A Retrospective Evaluation. J Clin Transl Hepatol. 2023;11:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 77. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643-54.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1302] [Article Influence: 118.4] [Reference Citation Analysis (1)] |
| 78. | Hagström H, Shang Y, Hegmar H, Nasr P. Natural history and progression of metabolic dysfunction-associated steatotic liver disease. Lancet Gastroenterol Hepatol. 2024;9:944-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 138] [Reference Citation Analysis (0)] |
| 79. | Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K. False-negative results in screening programmes: systematic review of impact and implications. Health Technol Assess. 2000;4:1-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Hagström H, Talbäck M, Andreasson A, Walldius G, Hammar N. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol. 2020;73:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 81. | Liu S, Jiang X, Fu J, Wong VW, Zhong VW, Qi X. Baseline and Dynamic MAF-5 Score to Predict Liver Fibrosis and Liver-Related Events in General Population With MASLD. Clin Gastroenterol Hepatol. 2025;23:365-368.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 82. | Balkhed W, Åberg FO, Nasr P, Ekstedt M, Kechagias S. Repeated measurements of non-invasive fibrosis tests to monitor the progression of non-alcoholic fatty liver disease: A long-term follow-up study. Liver Int. 2022;42:1545-1556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 539] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 84. | Bénard F, Barkun AN, Martel M, von Renteln D. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations. World J Gastroenterol. 2018;24:124-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 228] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (7)] |
| 85. | Younossi ZM, Paik JM, Henry L, Stepanova M, Nader F. Pharmaco-Economic Assessment of Screening Strategies for High-Risk MASLD in Primary Care. Liver Int. 2025;45:e16119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 86. | Choo BP, Goh GBB, Chia SY, Oh HC, Tan NC, Tan JYL, Ang TL, Bee YM, Wong YJ. Non-alcoholic fatty liver disease screening in type 2 diabetes mellitus: A cost-effectiveness and price threshold analysis. Ann Acad Med Singap. 2022;51:686-694. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 87. | Gruneau L, Kechagias S, Sandström P, Ekstedt M, Henriksson M. Cost-effectiveness analysis of noninvasive tests to identify advanced fibrosis in non-alcoholic fatty liver disease. Hepatol Commun. 2023;7:e00191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 88. | Middleton KR, Anton SD, Perri MG. Long-Term Adherence to Health Behavior Change. Am J Lifestyle Med. 2013;7:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 318] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 89. | De Bacquer D, Astin F, Kotseva K, Pogosova N, De Smedt D, De Backer G, Rydén L, Wood D, Jennings C; EUROASPIRE IV and V surveys of the European Observational Research Programme of the European Society of Cardiology. Poor adherence to lifestyle recommendations in patients with coronary heart disease: results from the EUROASPIRE surveys. Eur J Prev Cardiol. 2022;29:383-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 90. | Miglioretti DL, Abraham L, Sprague BL, Lee CI, Bissell MCS, Ho TH, Bowles EJA, Henderson LM, Hubbard RA, Tosteson ANA, Kerlikowske K. Association Between False-Positive Results and Return to Screening Mammography in the Breast Cancer Surveillance Consortium Cohort. Ann Intern Med. 2024;177:1297-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 91. | Gram EG, Siersma V, Brodersen JB. Long-term psychosocial consequences of false-positive screening mammography: a cohort study with follow-up of 12-14 years in Denmark. BMJ Open. 2023;13:e072188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 92. | Allen AM, Kim WR, Carrieri P, Canning R, Ou FS, Benson J, Olson JL, Venkatesh SK, Li J, Yin M, Eslami M, Ehman RL, Hunter Berg J, Lazarus JV. Population perspectives on benefits and harms of screening for metabolic dysfunction-associated steatotic liver disease. Hepatology. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Burnham B, Wallington S, Jillson IA, Trandafili H, Shetty K, Wang J, Loffredo CA. Knowledge, attitudes, and beliefs of patients with chronic liver disease. Am J Health Behav. 2014;38:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 94. | Shiha G, Korenjak M, Eskridge W, Casanovas T, Velez-Moller P, Högström S, Richardson B, Munoz C, Sigurðardóttir S, Coulibaly A, Milan M, Bautista F, Leung NWY, Mooney V, Obekpa S, Bech E, Polavarapu N, Hamed AE, Radiani T, Purwanto E, Bright B, Ali M, Dovia CK, McColaugh L, Koulla Y, Dufour JF, Soliman R, Eslam M. Redefining fatty liver disease: an international patient perspective. Lancet Gastroenterol Hepatol. 2021;6:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 95. | American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S181-S206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 279] [Article Influence: 279.0] [Reference Citation Analysis (0)] |
| 96. | Rubino F, Cummings DE, Eckel RH, Cohen RV, Wilding JPH, Brown WA, Stanford FC, Batterham RL, Farooqi IS, Farpour-Lambert NJ, le Roux CW, Sattar N, Baur LA, Morrison KM, Misra A, Kadowaki T, Tham KW, Sumithran P, Garvey WT, Kirwan JP, Fernández-Real JM, Corkey BE, Toplak H, Kokkinos A, Kushner RF, Branca F, Valabhji J, Blüher M, Bornstein SR, Grill HJ, Ravussin E, Gregg E, Al Busaidi NB, Alfaris NF, Al Ozairi E, Carlsson LMS, Clément K, Després JP, Dixon JB, Galea G, Kaplan LM, Laferrère B, Laville M, Lim S, Luna Fuentes JR, Mooney VM, Nadglowski J Jr, Urudinachi A, Olszanecka-Glinianowicz M, Pan A, Pattou F, Schauer PR, Tschöp MH, van der Merwe MT, Vettor R, Mingrone G. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025;13:221-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 662] [Article Influence: 662.0] [Reference Citation Analysis (1)] |
| 97. | Zhang X, Yip TC, Wong GL, Leow WX, Liang LY, Lim LL, Li G, Ibrahim L, Lin H, Lai JCT, Chim AM, Chan HLY, Kong AP, Chan WK, Wong VW. Clinical care pathway to detect advanced liver disease in patients with type 2 diabetes through automated fibrosis score calculation and electronic reminder messages: a randomised controlled trial. Gut. 2023;72:2364-2371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/