INTRODUCTION
Colorectal cancer (CRC) is the most common digestive tract tumor with high morbidity and mortality. CRC accounts for approximately 10% of all cancers and is the second leading cause of all cancer deaths[1]. According to the China Cancer Statistics Report in 2024[2], the incidence and mortality of CRC in China rank second and fourth of all malignant tumors, respectively. In the early stage, CRC patients have no symptoms or atypical symptoms, so most newly diagnosed patients have progressed to the middle or late stage with metastasis and lack effective treatment[3].
The treatment of CRC is mainly based on traditional modalities, including surgery, radiotherapy and chemotherapy. The high recurrence rate and high mortality rate indicate that current treatments need to be further optimized. In the past few years, new approaches such as targeted therapy, immunotherapy, and nanomedicine have opened new doors for the treatment of CRC, and many have shown good results in clinical trials. The success of new approaches combined with traditional chemotherapy in the treatment of refractory tumors will provide promising prospects for patients with advanced CRC. Recently, a new concept of immunogenic cell death (ICD) has emerged[4,5]. ICD is a regulated form of cell death that can activate both innate and adaptive immune responses through the release of damage-associated molecular patterns (DAMPs), which can be recognized by pattern-recognition receptors (PRRs). Recently, many studies have shed light on the potential role of ICD features as novel biomarkers in CRC[6]. For example, calreticulin[7] and high mobility group box-1 (HMGB1)[8], well-known DAMPs and hallmarks of ICD, are significantly increased in CRC tissues and have been investigated as biomarkers[9].
DAMPs include a variety of small molecule proteins and metabolic molecules released by damaged, stressed or dying cells, which bind to corresponding PRRs on the cell membrane to activate a series of signaling pathways and regulate the expression of inflammatory factors, and even induce cell apoptosis, pyroptosis and other types of programmed cell death[10]. DAMPs such as surface-exposed calreticulin, HMGB1 and heat shock protein 90 are closely related to anti-tumor immunity and are vital for the ICD of cancer cells[7,8,11,12]. Therefore, the role of DAMPs in CRC deserves further investigation and identifying DAMPs that could inhibit tumorigenesis may provide a promising option for cancer treatment.
Intracellular peroxiredoxin 1 (Prdx1), with no immunological functions inside the cell before its secretion, is a small molecule protein with antioxidant function[13,14]. Prdx1 has been found to be highly expressed in multiple organs. In intestinal tissues, Prdx1 is highly expressed in a variety of cells, especially in epithelial cells[15-17]. In the physiological state, Prdx1 is mainly located in the cytoplasm and its main function is to remove excessive peroxides and maintain a stable intracellular redox state by relying on its own cysteine residues[18]. Interestingly, more studies have demonstrated that extracellular Prdx1 released by stressed cells is a novel DAMP with significant pro-inflammatory effects[19-24]. Our previous studies revealed that extracellular Prdx1 acts as an important DAMP to promote inflammation in acute liver injury and sepsis[25,26]. Mechanistically, Prdx1 can activate the toll-like receptor 4 (TLR4)/NLPR3/Caspase-1 signaling pathway to induce the production of a variety of pro-inflammatory cytokines.
Many studies have demonstrated that Prdx1 is highly expressed in various tumor tissues compared with healthy tissues[27-31]. Using the online Gene Expression Profiling Interactive Analysis tool, our preliminary analysis in The Cancer Genome Atlas database showed that Prdx1 is highly expressed in CRC tissues compared with adjacent normal tissues (Supplementary Figure 1A). In addition, through an analysis of the Gene Expression Omnibus database (GSE106582), we further verified that the expression of Prdx1 in CRC tissues is higher than in adjacent normal tissues (Supplementary Figure 1B). In CRC, highly-expressed Prdx1 in tumor tissues was identified as an important inflammatory marker[32]. However, whether Prdx1 as a DAMP has anti-tumor activity in CRC is unknown. Based on the importance of DAMPs, the role of Prdx1 in CRC deserves further investigation.
To address this gap, the present study verified the expression of Prdx1 in tumor tissues in patients with CRC and subsequently used human recombinant Prdx1 (rPrdx1) to reveal its effects on the malignant biological behaviors of CRC cells by multiple cellular and molecular biological methods, which may provide a new treatment option for CRC.
MATERIALS AND METHODS
Patients and clinical specimens
The Ethics Committee of the Second Xiangya Hospital of Central South University, Hunan Province, China approved our study protocol (Ethics No. LYEC2025-K0057). A total of 60 CRC patients who underwent surgery at the Second Xiangya Hospital of Central South University were included after obtaining informed consent. Following ethical approval, clinical samples were collected. Tumor tissue and adjacent non-tumor tissue samples were obtained from each CRC patient. The diagnosis of CRC was histopathologically confirmed in each patient sample and none of the patients had received chemotherapy or radiotherapy before surgery. Immunohistochemical (IHC) staining was performed as previously described[25]. Briefly, the tissue specimens were fixed in 4% neutral buffered formalin, and embedded in paraffin. For IHC staining of Prdx1 (#ab15571, Abcam), endogenous peroxidase activity was blocked for 30 minutes, and the tissues were subjected to antigen retrieval with 6.5 mmol/L citrate buffer (pH 6.0) for 20 minutes in a microwave, after which the slides were incubated with a primary antibody (1:500 for Prdx1) overnight at 4 °C. After staining with diaminobenzidine, the tissues were counterstained with hematoxylin. ImageJ software was used for quantitative analysis of IHC images. Tumor tissues and matched normal tissues were stored at -80 °C for western blotting and quantitative real-time polymerase chain reaction (qRT-PCR).
Cell culture and treatment
Three types of human CRC cells, including SW480, RKO and HCT116, were purchased from the Cell Bank of Type Culture Collection (China Academy of Sciences, Shanghai, China). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco) at 37 °C in a humidified atmosphere with 5% CO2. For Prdx1 stimulation, CRC cells were treated with rPrdx1 (#P3465, Abnova) for 6-24 hours depending on the purpose of the experiments. To investigate the downstream signaling pathway induced by Prdx1 in CRC cells, the following inhibitors or agonist were used in vitro: Camptothecin (apoptosis activator, #S1288, Selleck), MCC950 (NLRP3 inflammasome inhibitor, #S7809, Selleck), Ac-FLTD-CMK (GSDMD inhibitor, #S9817, Selleck).
Cell viability assay
MTT cell proliferation and cytotoxicity assay is a classical method for cell viability detection. Succinate dehydrogenase in the mitochondria of living cells can reduce exogenous MTT to water-insoluble blue-purple crystals of Formazan, which are deposited in cells. Dimethyl sulfoxide (DMSO) was used to dissolve Formazan in living cells. The optical density (OD) value was measured at 570 nm with a microplate reader, which is proportional to the number of living cells. To each well of the 96-well plate 100 μL of culture medium containing 5000 cells was added, and according to the experimental requirements, different doses of rPrdx1 were added. Ten μL MTT was added to each well, and the cells were incubated for another 4 hours. Then 100 μL DMSO was added to each well and the cells were incubated until the Formazan was completely dissolved. The OD value was measured at 570 nm.
Cell wound healing assay and colony formation assay
Cells were seeded in 6-well culture plates at a density of approximately 5 × 105 cells per well and incubated for 24 hours (80%-90% confluence). A 10 μL plastic pipette tip was used to make a scratch in each well, and the cells were then cultured in DMEM with 2% FBS. The wound and cell migration were photographed at time points of 0, 6, 12, 24 and 48 hours after the scratch. Three microscopy fields (× 200) were randomly selected at each time point, and cell motility was quantified by measuring the distance between the cell edges. Cells were seeded in 6-well plates at a density of 500 per well and the medium was replaced every 3-4 days. The colonies were counted and analyzed after approximately two weeks. The experiment was performed with at least three replicates for each cell line.
Transwell assay of cell migration and invasion
Four hundred μL serum-free DMEM medium containing approximately 2 × 104 cells was added to the upper 8 μm pore Transwell chamber (#3422, Corning), and the lower chambers were filled with 750 μL DMEM medium without cells. For the cell invasion assay, the Transwell chambers were coated with Matrigel (#354234, Corning). The cells were then incubated at 37 °C for 24-36 hours. For quantitative observation, the cells in both the upper chamber and lower chambers were fixed with 4% paraformaldehyde for 20 minutes, and then stained with 0.1% crystal violet solution for 10 minutes at room temperature. Finally, the cells were counted in five randomly selected fields under a microscope (× 200).
Tumorigenesis assay in nude mice
Four-week-old male BALB/c mice were housed in the Laboratory Animal Centre of the Second Xiangya Hospital of Central South University under specific pathogen-free conditions. For tumor growth, mice were randomly divided into two groups (six mice per group): RKO group and RKO + rPrdx1 group. All mice in the two groups were subcutaneously injected with RKO cells (5 × 106) into the right flank at five weeks of age. Additionally, mice in RKO + rPrdx1 group were injected weekly with rPrdx1 (10 μg/kg) via the tail vein. Tumor nodules were examined every five days and mice were sacrificed 34 days after RKO cell injection. The tumor volume (mm3) was calculated using the following formula: Volume = 1/2 × L × W2, where L represents length and W represents width. All animal care and operation were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
RNA extraction and qRT-PCR
Total RNA was extracted from tumor tissue and adjacent non-tumor tissue samples by Trizol solution (TaKaRa). qRT-PCR was conducted using the PrimeScript RT Reagent Kit (TaKaRa) and SYBR Premix Ex TaqTM II (TaKaRa) following the manufacturer’s instructions. Relative gene expression was normalized to the expression of β-actin. The data were analyzed by the 2-ΔΔCt method. The specific primers for Prdx1 and β-actin are as follows: Prdx1 forward primer: 5'-GCCGCTCTGTGGATGAGATTA-3', Prdx1 reverse primer: 5'-AGCTGGACACACTTCACCAT-3'; β-actin forward primer: 5'-CACTGTCGAGTCGCGTCC-3', β-actin reverse primer: 5'-TCATCCATGGCGAACTGGTG-3'.
Western blotting
Western blotting was conducted according to the methods outlined in our previous study. Protein lysates from tissues or cells were prepared, loaded onto SDS-PAGE gel for electrophoresis, then transferred onto a PVDF membrane, which was blocked with 5% skim milk for 1 hour and blotted according to standard methods using the following antibodies: Prdx1 (#ab15571, Abcam), GSDMD (#sc-393581, Santa Cruz), GSDME (#ab215191, Abcam), PARP (#5625, CST), p-MLKL (#ab187091, Abcam), NLRP3 (#AG-20B-0014, Adipogen), Caspase-1 (#sc-56036, Santa Cruz), Caspase-3 (#9662, CST), Caspase-4 (#ab238124, Abcam), Caspase-5 (#ab40887, Abcam) and β-actin (#3700S, CST). Some experiments have lost their complete western blotting images. Hence, in the future we will strengthen the data management to improve data archiving.
Lactate dehydrogenase release assay and ELISA
Disruption of the cell membrane structure leads to the release of lactate dehydrogenase (LDH) from the cytoplasm into the culture medium. The release of LDH is considered an important indicator of cell membrane integrity. Cellular supernatant levels of interleukin (IL)-1β (#DLB50, R&D Systems) and IL-18 (#DL180, R&D Systems) were measured by ELISA kits. The LDH assay (#C0016, Beyotime Biotech) and ELISA were conducted according to the manufacturer's instructions.
TUNEL assay
RKO and SW480 cells were washed once with 1 × PBS and fixed with 4% paraformaldehyde for 30 minutes. The cells were then washed three times with 1 × PBS. Subsequently, 1 × PBS containing 0.3% Triton X-100 was added to each well and incubated for 5 minutes at room temperature. After that, the cells were washed three times with 1 × PBS, and 50 μL of newly prepared TUNEL assay solution was added to the wells and incubated at 37 °C in the dark for 60 minutes. The cells were washed three times with 1 × PBS and then blocked with anti-fade mounting medium and observed under a fluorescence microscope.
Transmission electron microscopy
Transmission electron microscopy (TEM) is an important method for observing the morphology of cell death (apoptosis, pyroptosis, ferroptosis and necrosis, etc.), which clearly shows the changes in cell structure. When the growth of RKO and SW480 cells in 6-well plates reached the logarithmic phase (70%-80% confluence), the number of cells in each well was up to 106/mL. The culture medium was aspirated and digested with trypsin. The cells were transferred into a 1.5 mL apical-bottom Eppendorf (EP) tube and centrifuged for 5 minutes at 800-2000 rpm to form a cell precipitate approximately 0.5-1 mm in height. The solution in the EP tube was aspirated, and 500 μL of 2.5% glutaraldehyde was gently added into the tube along the wall. The sample was left at room temperature for 1 hour and then placed in a 4 °C refrigerator overnight. Glutaraldehyde was then discarded and the tube was filled with cold 1 × PBS, and the samples were observed under TEM.
Statistical analysis
All data are expressed as the mean ± SD. Statistical analysis was performed with SPSS 22.0 software (SPSS, Chicago, IL, United States) and Microsoft Excel 2016 (Microsoft, Redmond, WA, United States). Comparisons between groups were estimated using the Student’s t-test. Additionally, comparisons between multiple groups were analyzed with one-way ANOVA. The Bonferroni test was used to adjust the significance for two comparisons after one-way ANOVA. A P value < 0.05 was considered statistically significant.
RESULTS
Prdx1 is upregulated in CRC tissues
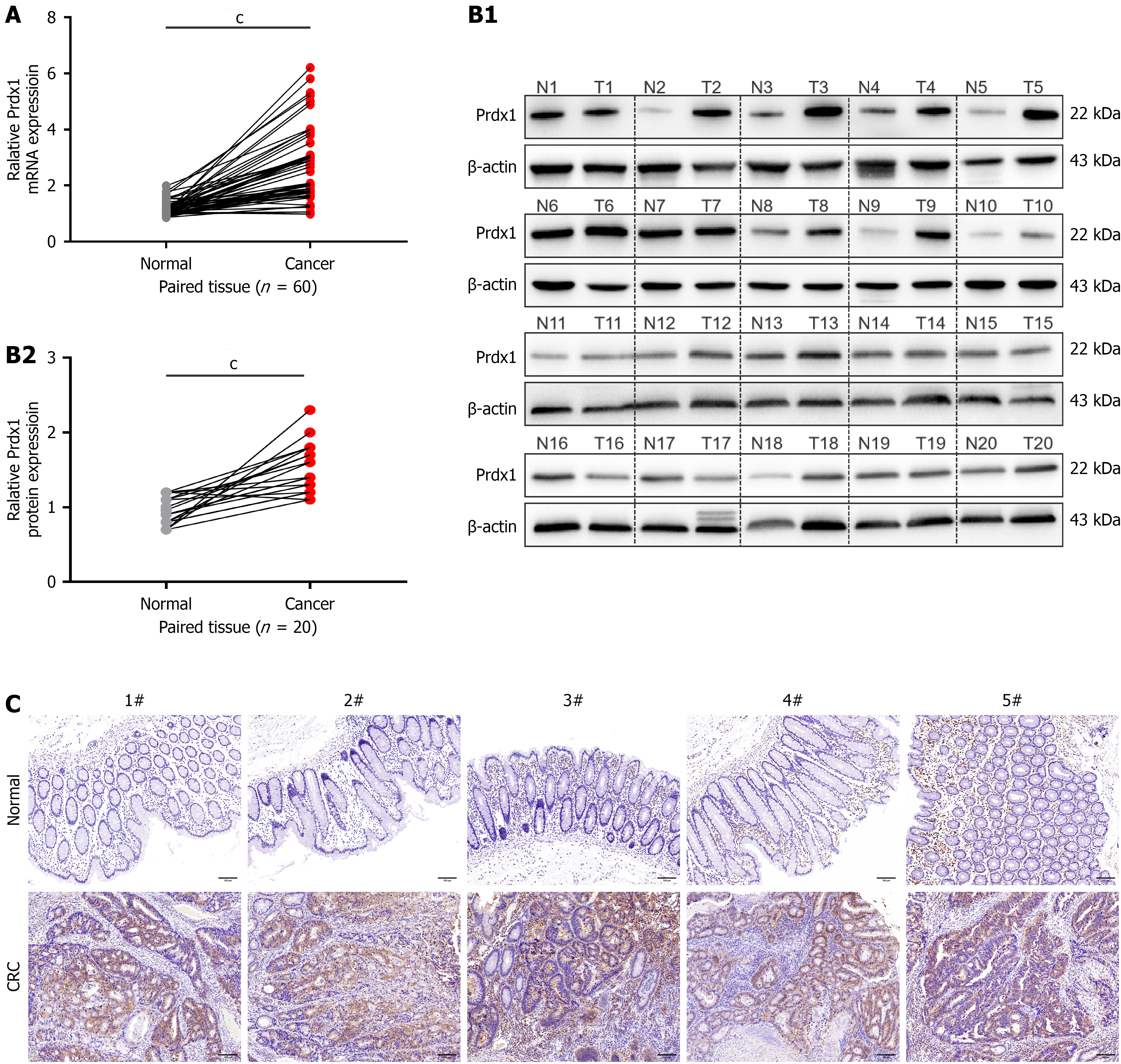
To further determine that Prdx1 expression is increased in CRC tissues, qRT-PCR and western blotting were performed using samples from CRC patients including tumors and matched adjacent normal tissues. The results showed that the mRNA and protein levels of Prdx1 were significantly upregulated in most CRC tissues compared with the adjacent normal samples (Figure 1). The above findings suggest that Prdx1 may play an important role in CRC.
Figure 1 Peroxiredoxin 1 is up-regulated in colorectal cancer tissues.
A: The mRNA expression of peroxiredoxin 1 (Prdx1) was detected by quantitative real-time polymerase chain reaction in tumor tissues and adjacent normal tissues from patients with colorectal cancer (CRC), n = 60/group; B: The protein expression of Prdx1 was detected by western blotting in tumor tissues and adjacent normal tissues from patients with CRC, n = 20/group; C: Immunohistochemistry staining for Prdx1 in tumor tissues and adjacent normal tissues from patients with CRC, each representative image is from one sample (n = 20/group), scale bar: 100 μm (200 ×). Data are expressed as the mean ± SD; cP < 0.001. Prdx1: Peroxiredoxin 1; CRC: Colorectal cancer.
rPrdx1 inhibits the viability and motility of RKO and SW480 cells
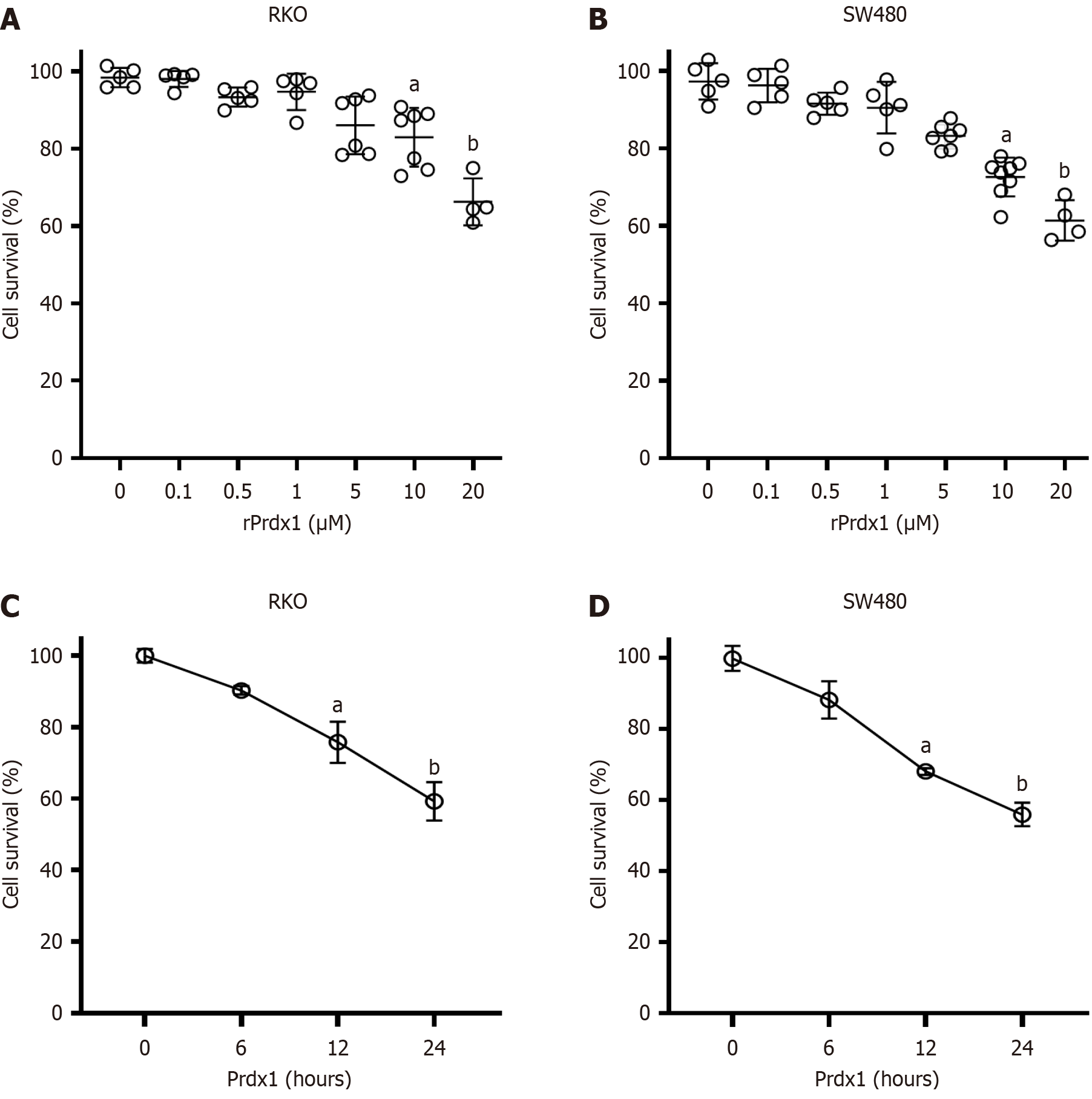
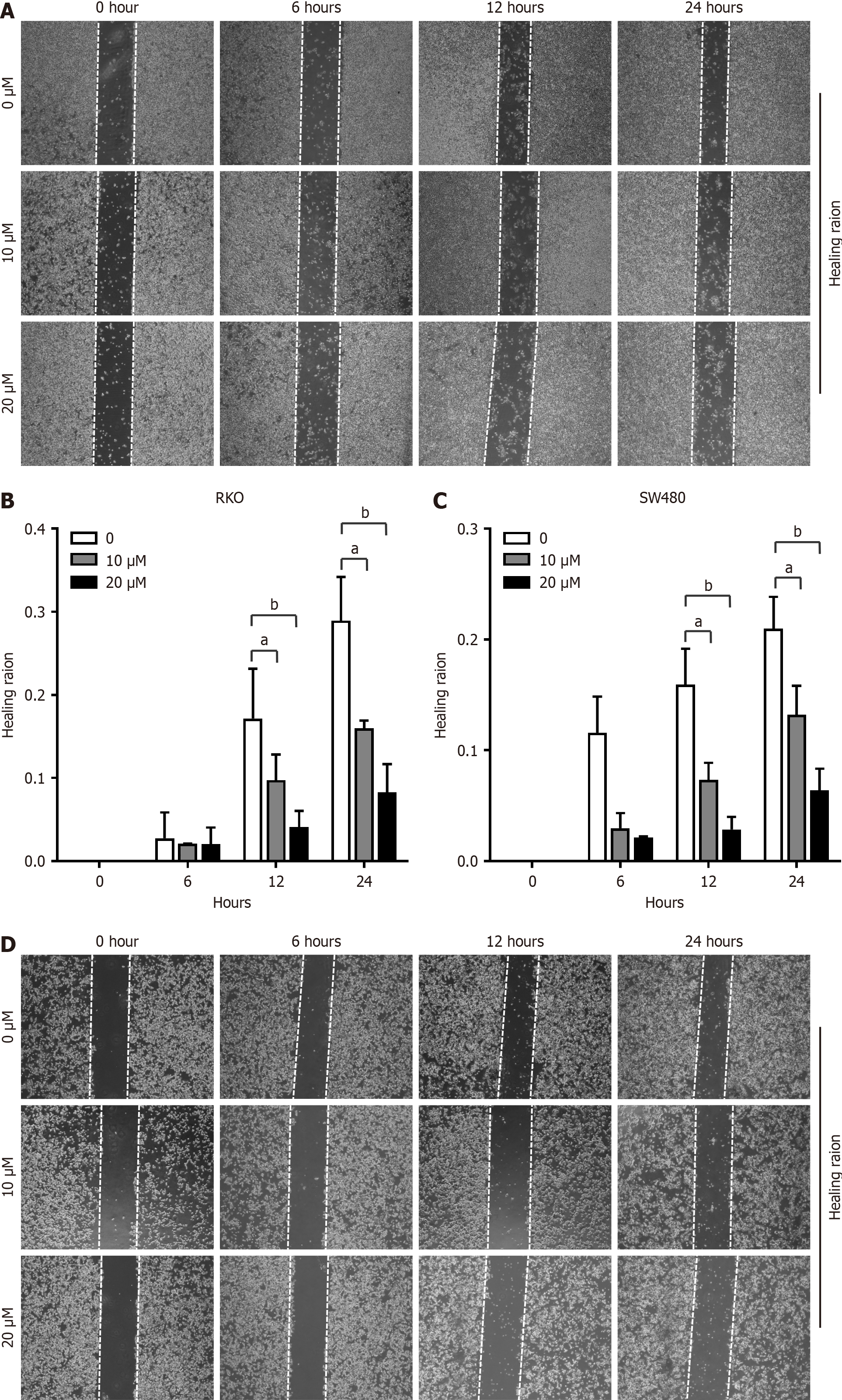
Increased expression of Prdx1 in CRC has been identified as an inflammatory marker[32], while its role in the tumorigenesis of CRC remains unknown. To investigate the effects of extracellular Prdx1 on the viability of colonic cancer cells, we used rPrdx1 to stimulate RKO, SW480 and HCT116 cell lines in vitro. Obviously, rPrdx1 significantly induced RKO and SW480 cell death, and the survival rate decreased with increased concentration and prolonged stimulation with rPrdx1 (Figure 2A and B). After 24 hours stimulation with rPrdx1 at the dose of 20 μM, the survival rate of RKO and SW480 cells was significantly reduced to only 59.2% and 55.9%, respectively (Figure 2C and D). However, rPrdx1 did not influence the viability of HCT116 cells (Supplementary Figure 2A and B). In addition, results from the cell wound healing assay also showed that rPrdx1 significantly inhibited the motility of RKO and SW480 cells (Figure 3), but had no effect on HCT116 cells (Supplementary Figure 2C). Taken together, these results demonstrate that rPrdx1 could inhibit the viability and motility of RKO and SW480 cells, but not HCT116 cells. Therefore, we only used RKO and SW480 cells in the following experiments.
Figure 2 Recombinant peroxiredoxin 1 inhibits the viability of RKO and SW480 cells.
A and B: Survival of RKO and SW480 cells detected by MTT under recombinant peroxiredoxin 1 (rPrdx1) stimulation for 24 hours at different doses; C and D: Survival of RKO and SW480 cells detected by MTT under 20 μM rPrdx1 stimulation for different time periods. This experiment was repeated three times in independent experiments. Data are expressed as the mean ± SD; aP < 0.05 vs control group (without rPrdx1); bP < 0.01 vs control group (without rPrdx1). Prdx1: Peroxiredoxin 1; rPrdx1: Recombinant peroxiredoxin 1.
Figure 3 Recombinant peroxiredoxin 1 inhibits motility of RKO and SW480 cells.
A and B: Motility of RKO cells measured by the wound healing assay under different doses of recombinant peroxiredoxin 1 (rPrdx1) stimulation for 6, 12 and 24 hours, respectively; C and D: Motility of SW480 cells measured by the wound healing assay under different doses of rPrdx1 stimulation for 6, 12 and 24 hours, respectively. These experiments were repeated three times in independent experiments. Data are expressed as the mean ± SD. aP < 0.05 vs the control group (without rPrdx1); bP < 0.01 vs the control group (without rPrdx1).
rPrdx1 inhibits the proliferation and invasion of RKO and SW480 cells
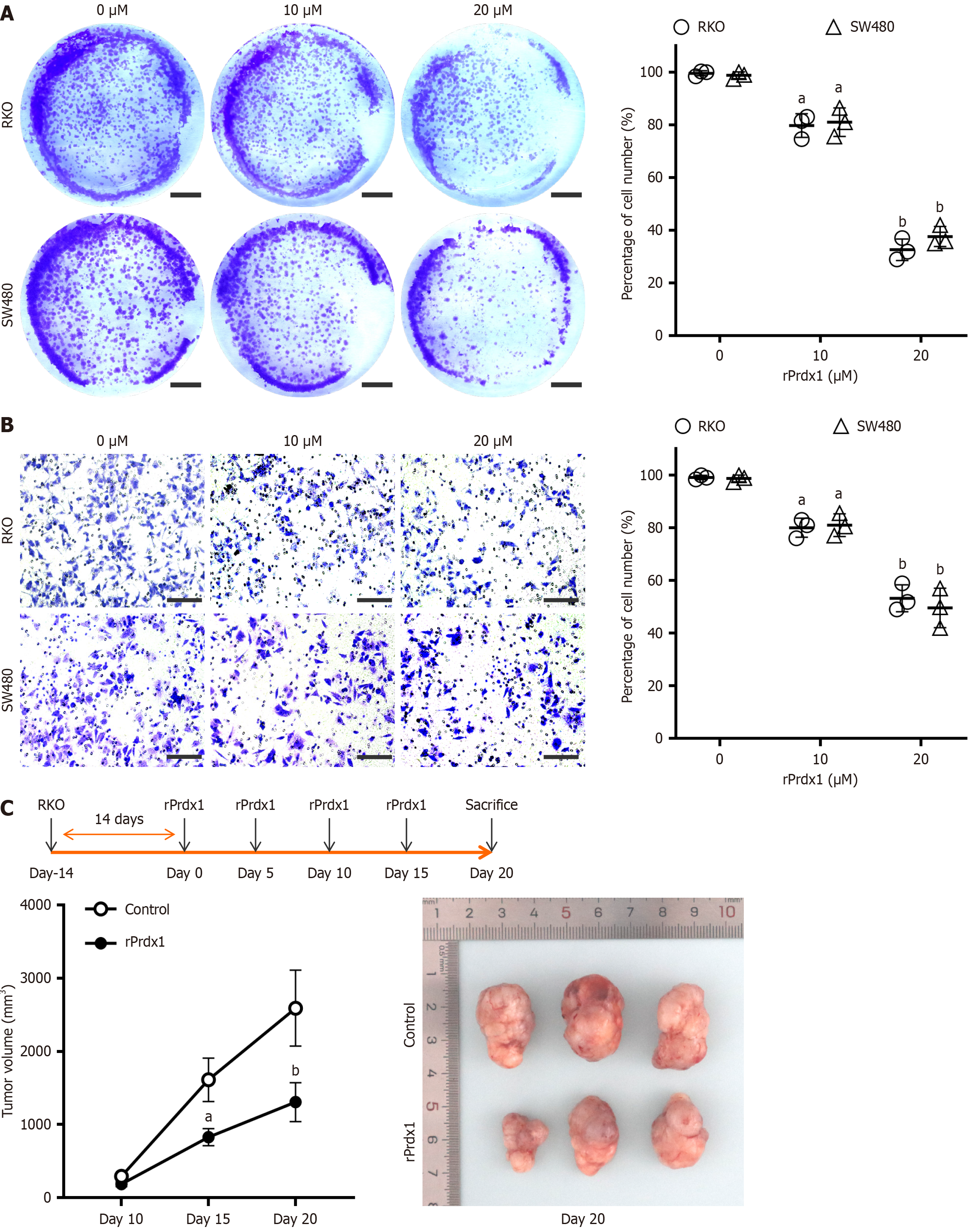
To further investigate the biological function of Prdx1 in CRC cells in vitro, we used rPrdx1 to stimulate RKO and SW480 cells. With increased rPrdx1 concentration, the proliferation and invasion ability of RKO and SW480 cells gradually decreased as shown by the colony formation assay and Transwell assay (Figure 4A and B). In addition, we found that intravenous injection of rPrdx1 in mice with subcutaneous tumor formation significantly inhibited the growth of tumor tissue (Figure 4C).
Figure 4 Recombinant peroxiredoxin 1 inhibits proliferation and invasion of RKO and SW480 cells.
A: Representative images of proliferation ability detected by the colony formation assay under stimulation by 10 μM or 20 μM recombinant peroxiredoxin 1 (rPrdx1) for 24 hours, scale bar: 1 cm; B: Representative images of invasion ability detected by the Transwell assay with stimulation by 10 μM or 20 μM rPrdx1 for 24 hours, scale bar: 200 μm (200 ×); the experiments for the colony formation assay and Transwell assay were repeated three times in independent experiments; C: Tumor volume analyses showed the inhibitory effect of intravenous injection of rPrdx1 on subcutaneous tumor formation in nude mice (n = 6/group). Bonferroni correction was used to adjust the significance for multiple comparisons after two-way RM ANOVA. Data are expressed as the mean ± SD. aP < 0.05 vs control group (without rPrdx1); bP < 0.01 vs control group (without rPrdx1). rPrdx1: Recombinant peroxiredoxin 1.
rPrdx1 induces CRC cell pyroptosis
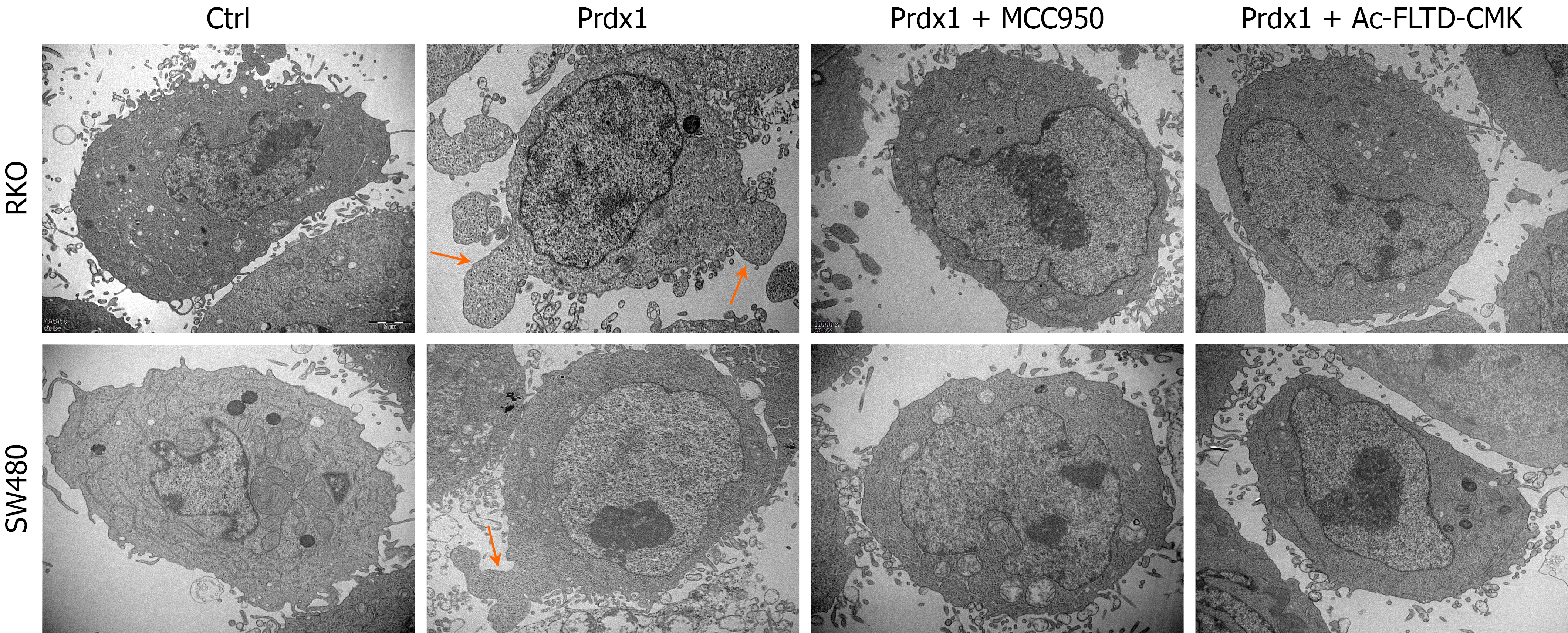
In the above studies, Prdx1 as an important DAMP, was able to induce RKO and SW480 cell death and subsequently inhibit their malignant biological behaviors, such as proliferation and invasion. However, the mechanism of Prdx1-induced cell death requires further exploration. Currently, the types of programmed cell death induced by DAMPs mainly include apoptosis, necroptosis, and pyroptosis. To clarify the types of Prdx1-induced RKO and SW480 cell death, we used TEM to observe the morphological characteristics of RKO and SW480 cells under rPrdx1 stimulation. After stimulation with rPrdx1, RKO and SW480 cells presented significant characteristics of pyroptosis, which indicated that the cell membrane locally expanded to form pyroptosis bodies, without the characteristics of cell shrinkage, karyopyknosis, karyolysis and mitochondrial abnormalities (Figure 5). TUNEL assay further demonstrated that rPrdx1 did not induce RKO and SW480 cell apoptosis (Supplementary Figure 3A). Western blotting results also showed that rPrdx1 did not induce expression of the markers of apoptosis (cleaved-Caspase3 and cleaved-PARP) and the marker of necroptosis (p-MLKL) in RKO and SW480 cells (Supplementary Figure 3B and C), which further suggested that rPrdx1 did not induce apoptosis and necroptosis in RKO and SW480 cells.
Figure 5 Recombinant peroxiredoxin 1 induces pyroptosis of RKO and SW480 cells.
Representative images of pyroptosis induced by recombinant peroxiredoxin 1 (rPrdx1) without or with NLRP3 inflammasome inhibitor (MCC950) and GSDMD inhibitor (Ac-FLTD-CMK) treatment (n = 3 per group), scale bar: 20 μm (× 10000). rPrdx1: 20 μM for 24 hours. MCC950: 10 μM for 24 hours. Ac-FLTD-CMK: 10 μM for 24 hours. Prdx1: Peroxiredoxin 1.
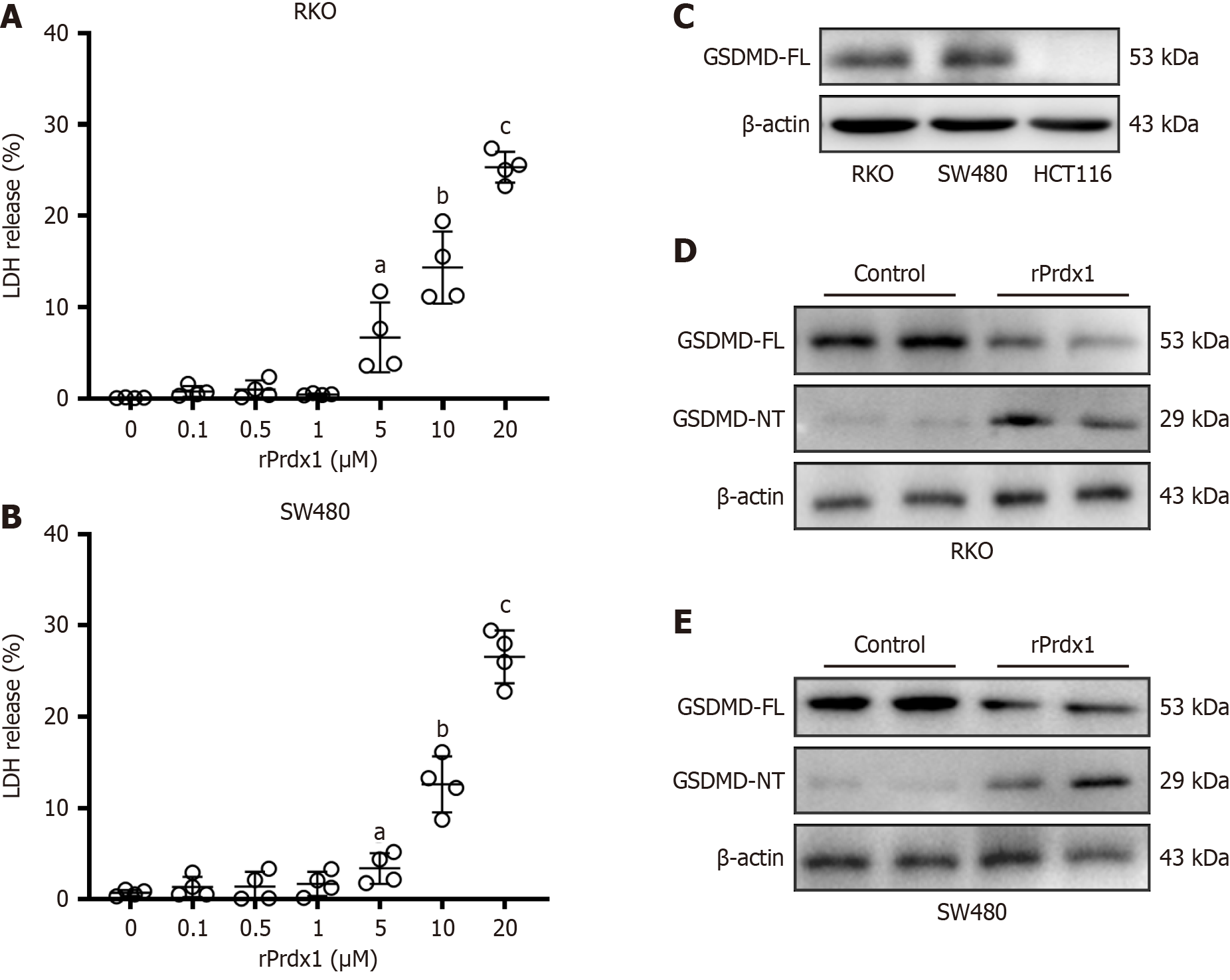
In addition, rPrdx1 induced RKO and SW480 cells to release LDH (Figure 6A and B), a marker of cell lysis, which supported the TEM results that rPrdx1 can induce pyroptosis. Notably, rPrdx1 did not induce HCT116 cells to release LDH (Supplementary Figure 2D), which is consistent with the above-mentioned cell viability result that rPrdx1 did not induce HCT116 cell death (Supplementary Figure 2A and B). Gasdermin D (GSDMD) is a key executor of pyroptosis, and the cleaved GSDMD N-terminal (NT) fragment is able to form membrane pores to trigger pyroptosis. Our results showed that GSDMD was highly expressed in RKO and SW480 cells but not expressed in HCT116 cells (Figure 6C). Furthermore, Prdx1 induced the expression of GSDMD-NT in RKO and SW480 cells (Figure 6D and E). However, we found that Prdx1 did not induce the expression of GSDME-NT (Supplementary Figure 4A and B), another important executor of pyroptosis in CRC. Hence, these findings suggest that Prdx1 can only induce RKO and SW480 cell pyroptosis, but not HCT116 cells, through the up-regulation of GSDMD-NT.
Figure 6 Recombinant peroxiredoxin 1 induces LDH release and up-regulation of GSDMD-NT in RKO and SW480 cells.
A: LDH release from RKO cells induced by different doses of recombinant peroxiredoxin 1 (rPrdx1) stimulation for 24 hours; B: LDH release from SW480 cells induced by different doses of rPrdx1 stimulation for 24 hours; C: GSDMD full-length (FL) expression in RKO, SW480 and HCT-116 cells detected by western blotting; D: GSDMD-FL and GSDMD N-terminal (NT) expression detected by western blotting in RKO cells under 20 μM rPrdx1 stimulation for 24 hours; E: GSDMD-FL and GSDMD-NT expression detected by western blotting in SW480 cells under 20 μM rPrdx1 stimulation for 24 hours. All these experiments were repeated three or four times in independent experiments. Data are expressed as the mean ± SD; aP < 0.05 vs control group (without rPrdx1); bP < 0.01 vs control group (without rPrdx1); cP < 0.001 vs control group (without rPrdx1). rPrdx1: Recombinant peroxiredoxin 1.
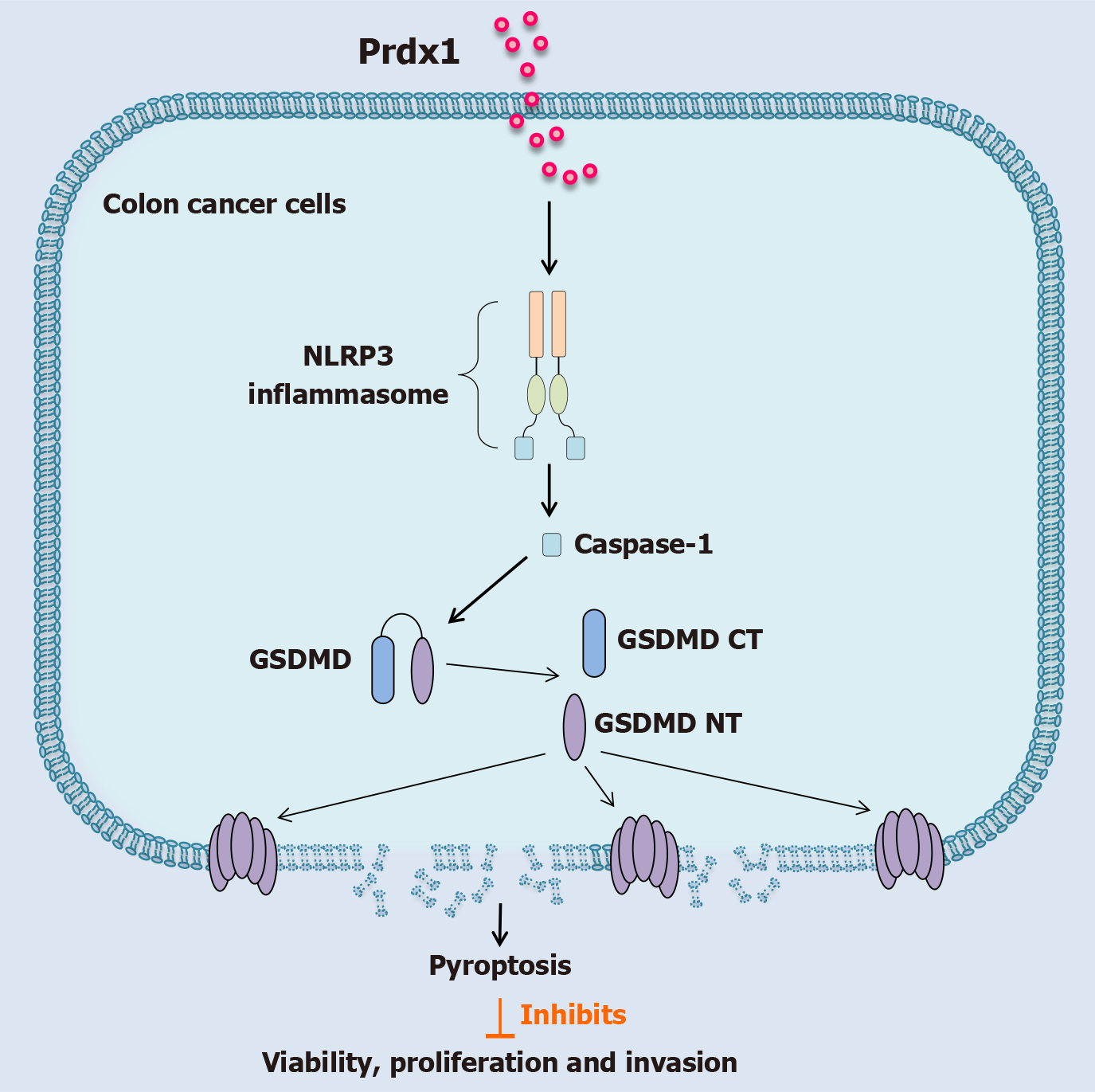
Overall, above findings reveal that Prdx1 can induce RKO and SW480 cell death and inhibit their malignant biological behaviors by promoting pyroptosis rather than apoptosis and necroptosis.
rPrdx1 activates the NLRP3/GSDMD pathway to induce CRC cell pyroptosis
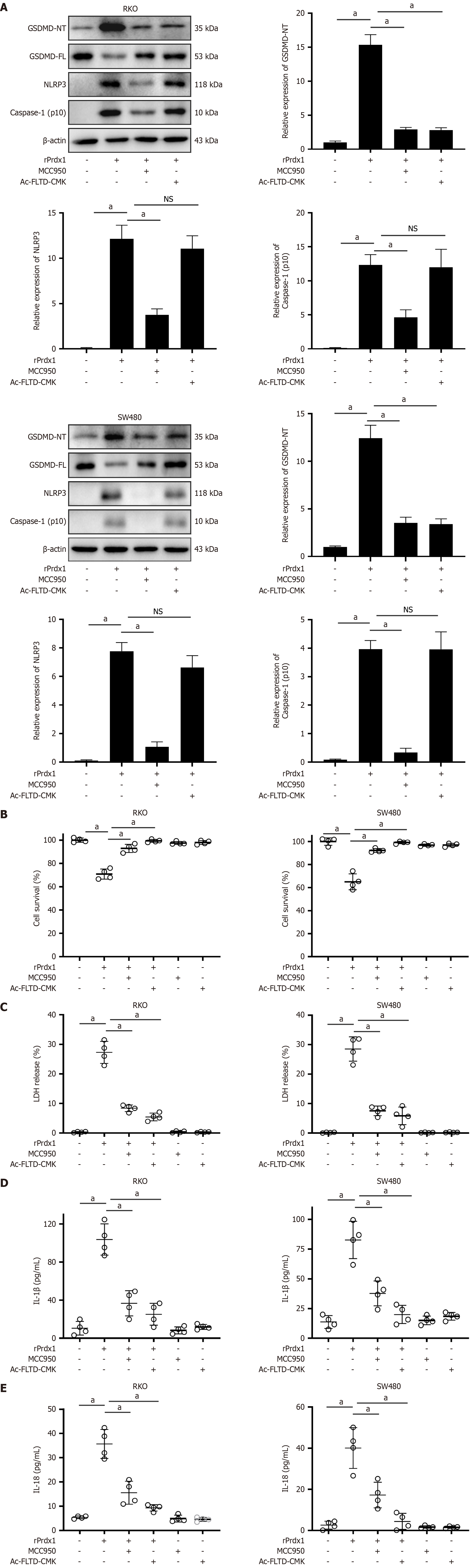
Pyroptosis is a prototype of highly pro-inflammatory programmed cell death that is mainly activated by the inflammasome/Caspase-1-dependent canonical pathway and Caspase-4/5/11-dependent non-canonical pathway[33,34]. The NLRP3 inflammasome is the most intensively studied inflammasome to activate Caspases. Our previous study demonstrated that Prdx1 activates the NLRP3 inflammasome/Caspase-1 pathway to induce the production of IL-1β[25]; therefore, we hypothesized that Prdx1 might induce RKO and SW480 cell pyroptosis by activating the NLRP3 inflammasome/Caspase-1/GSDMD pathway. To address this question, we used NLRP3 inflammasome inhibitor (MCC950) and GSDMD inhibitor (Ac-FLTD-CMK) to determine the mechanism of pyroptosis induced by Prdx1. Of note, MCC950 and Ac-FLTD-CMK significantly inhibited RKO and SW480 pyroptosis induced by rPrdx1 (Figure 5). Additionally, MCC950 and Ac-FLTD-CMK significantly suppressed the expression of cleaved-Caspase-1 and GSDMD-NT (Figure 7A), reduced LDH release and increased the viability of RKO and SW480 cells (Figure 7B and C), and inhibited the production of IL-1β and IL-18 (Figure 7D and E), markers of pyroptosis. Moreover, to exclude the effect of the non-canonical pathway, we detected the expression of Caspase-4/5 and found that Prdx1 did not induce the expression of cleaved Caspase-4/5 (Supplementary Figure 4C and D). Taken together, these findings indicate that rPrdx1 induces RKO and SW480 cell pyroptosis by activating the NLRP3/Caspase-1/GSDMD pathway.
Figure 7 Recombinant peroxiredoxin 1 activates the NLRP3/GSDMD pathway to induce pyroptosis of RKO and SW480 cells.
A: The expression of GSDMD-FL, GSDMD-NT, NLRP3 and Caspase-1 (p10) in RKO and SW480 cells detected by western blotting under recombinant peroxiredoxin 1 (rPrdx1) stimulation; B: Survival of RKO and SW480 cells detected by MTT under rPrdx1 stimulation; C: LDH release from RKO and SW480 cells induced by rPrdx1; D: IL-1β release from RKO and SW480 cells induced by rPrdx1; E: IL-18 release from RKO and SW480 cells induced by rPrdx1. rPrdx1: 20 μM for 24 hours. MCC950: 10 μM for 24 hours. Ac-FLTD-CMK: 10 μM for 24 hours. All these experiments were repeated three or four times in independent experiments. Data are expressed as the mean ± SD. aP < 0.05; NS: No significance; rPrdx1: Recombinant peroxiredoxin 1.
DISCUSSION
Many studies have found that the expression of Prdx1 is significantly up-regulated in multiple cancers, such as liver cancer[29], esophageal cancer[35], breast cancer[31], lung cancer[28], and prostate cancer[20]. The high expression of Prdx1 is considered a tumor marker[36]. Our study found that the mRNA and protein expression of Prdx1 in CRC tissues was significantly increased compared with adjacent normal samples, suggesting that Prdx1 may play an important role in CRC. From in vivo and in vitro experiments, the findings revealed the anti-tumor effect of extracellular rPrdx1 on CRC cells.
Although a few studies have shown that silencing intracellular Prdx1 in SW480 cells significantly inhibits tumor cell proliferation and migration[37], the role of extracellular Prdx1 as a DAMP in CRC remains unclear. The main function of intracellular Prdx1 is to remove excessive peroxides, which is dependent on its own cysteine residues to maintain a stable redox state. Notably, intracellular Prdx1 can be secreted into the extracellular space under various stimuli. Currently, the mechanism of Prdx1 secretion remains unclear, but Prdx1 acting as an important DAMP by binding TLR4 to induce the production of pro-inflammatory factors has been well established. In our opinion, deletion of intracellular Prdx1 inhibits tumorigenesis and is likely related to an increase in reactive oxygen species leading to oxidative stress, which results in the killing of tumor cells. In our study, extracellular Prdx1 can significantly inhibit the malignant biological behavior of RKO and SW480 cells by inducing pyroptosis of tumor cells, and the anti-tumor effect becomes more obvious with increased concentration and prolonged stimulation by rPrdx1. In addition, Prdx1 did not inhibit the malignant biology of HCT116 cells. The anti-tumor activity of Prdx1 on different CRC cell lines may be closely related to the origin of different cell lines, malignancy degree, and gene mutation status. As our study showed that Prdx1 induces pyroptosis in CRC cells, which is closely related to the expression of GSDMD in special cell types, it remains to be further explored whether CRC patients with high expression of GSDMD are more sensitive to Prdx1 stimulation and undergo pyroptosis, and whether GSDMD is closely related to the prognosis of CRC patients. In our future research, we will also conduct in-depth studies on these aforementioned issues. Further clarification of the mechanism by which Prdx1 inhibits the malignant biological behavior of CRC cells will be helpful in providing more options for the immunotherapy of CRC.
Cell death occurs in a variety of ways, depending on the cell state and triggering stimuli. Currently, programmed cell death is the most widely and complicated type of cell death, which includes pyroptosis, apoptosis and necroptosis and ferroptosis. Emerging studies have shown that a variety of chemotherapy drugs and DAMPs can induce pyroptosis of CRC cells through Gasdermins[8,38-41]. Pyroptosis of tumor cells not only significantly inhibits the migration, invasion and proliferation of tumor cells, but also improves the anti-tumor activity of immune cells in the tumor microenvironment, thus enhancing the killing effect on residual tumor cells[42,43]. In this study, we excluded the effects of Prdx1 on apoptosis, necroptosis, apoptosis and ferroptosis, and confirmed the role of Prdx1 in inducing pyroptosis of RKO and SW480 cells. In addition, we found that GSDMD inhibitors significantly inhibited Prdx1-induced pyroptosis and Prdx1 did not induce the increase of GSDME-NT in RKO and SW480 cells. As a key executor of pyroptosis, GSDMD is highly expressed in RKO and SW480 cells but not expressed in HCT116 cells, which may provide a reasonable explanation for Prdx1 not inducing HCT116 cell death. Due the lack of sufficient in vivo mechanistic experiment validation of Prdx1-induced pyroptosis in our study, hence the in vitro results would limit mechanistic explanation. In addition, immune microenvironment analysis is essential to substantiate ICD; however, our study did not design any related experiments to address this issue. We have also noted this limitation and will prioritize this experiment in our ongoing research.
Prdx1 can induce macrophages to produce inflammatory factors by activating the NLRP3 inflammasome/Caspase-1 pathway[25]. Therefore, we speculated that Prdx1 might act on GSDMD through the NLRP3 inflammasome/Caspase-1 pathway, and then further induce cell pyroptosis. However, in sepsis/acute liver injury, Prdx1 promotes inflammation by activating NF-κB or the NLRP3/Caspase-1 pathway in macrophages to produce inflammatory cytokines. Hence, activating different cell types (tumor cells or macrophages) possibly led to microenvironment-dependent distinct outcomes. The NLRP3 inflammasome inhibitor almost completely inhibited Prdx1-induced pyroptosis of RKO and SW480 cells and the expression of Caspase-1 and IL-1β. Our results strongly suggest that Prdx1-induced pyroptosis is dependent on NLRP3 inflammasome/Caspase-1/GSDMD activation. TLR4 and NLRP3 are important PRRs expressed by cells. DAMPs usually activate a variety of PRRs, which is the pathophysiological basis for their complex biological effects. Studies have shown that the key PRRs activated by DAMPs may differ in different diseases. Taking the widely studied HMGB-1 as an example, the receptor activated by HMGB-1 in tumors is mainly TLR4[44]; however, the receptor activated by HMGB-1 in acute liver failure is mainly RAGE rather than TLR4[45]. Our study demonstrated that Prdx1 is able to activate the NLRP3 inflammasome/Caspase-1/GSDMD pathway, but the PRRs of Prdx1 in CRC cells are unknown. Although the previous researches support that Prdx1 can activate TLR4, our study did not investigate the Prdx1’s corresponding receptor expressed on hepatocyte. Therefore, this is an obvious limitation in our study. To clarify the molecular bridge connecting Prdx1 to NLRP3 inflammasome, the key receptor activated by Prdx1 in CRC cells deserves further investigation. Of noting, although Prdx1 induces pyroptosis of CRC cells, pyroptosis is usually accompanied by significant pro-inflammatory effects. Therefore, it remains to clarify that whether Prdx1 can exacerbate the systemic inflammatory risk in CRC patients and ultimately affect their prognosis. Additionally, inflammatory cells such as macrophages can express GSDMD. Thus, it is necessary to further explore whether Prdx1 can induce the pyroptosis of inflammatory cells with tumor-killing effects, which is important for the targeted delivery strategies.