Published online Sep 21, 2025. doi: 10.3748/wjg.v31.i35.109987
Revised: June 20, 2025
Accepted: August 13, 2025
Published online: September 21, 2025
Processing time: 114 Days and 3.7 Hours
Severe alcoholic hepatitis (SAH) carries a 90-day mortality rate approaching 50%. Management includes corticosteroids, nutritional support, and early liver transplantation in selected cases. However, the mid-term impact of available therapies remains unclear. This systematic review provides a critical evaluation of treatments for SAH, specifically focusing on survival or mortality at 90 days as an essential window that captures short- and mid-term outcomes. The 90-day window is clinically significant, as it reflects the remission of systemic inflammation, early liver recovery, and minimizes confounding long-term behaviors such as alcohol relapse.
To review the effect of different treatments for SAH on survival and mortality at 90 days.
A systematic search of PubMed and EMBASE (last updated March 2025) was performed without language restrictions, focusing on studies published in the last decade. Study selection and data extraction were performed independently by at least two reviewers. Risk of bias was assessed using RoB 2.0 and Risk-of Bias in Non-Randomized Studies of Interventions tools. Due to heterogeneity in study designs and interventions, a meta-analysis was not feasible. A qualitative synthesis was conducted using narrative summaries and evidence tables.
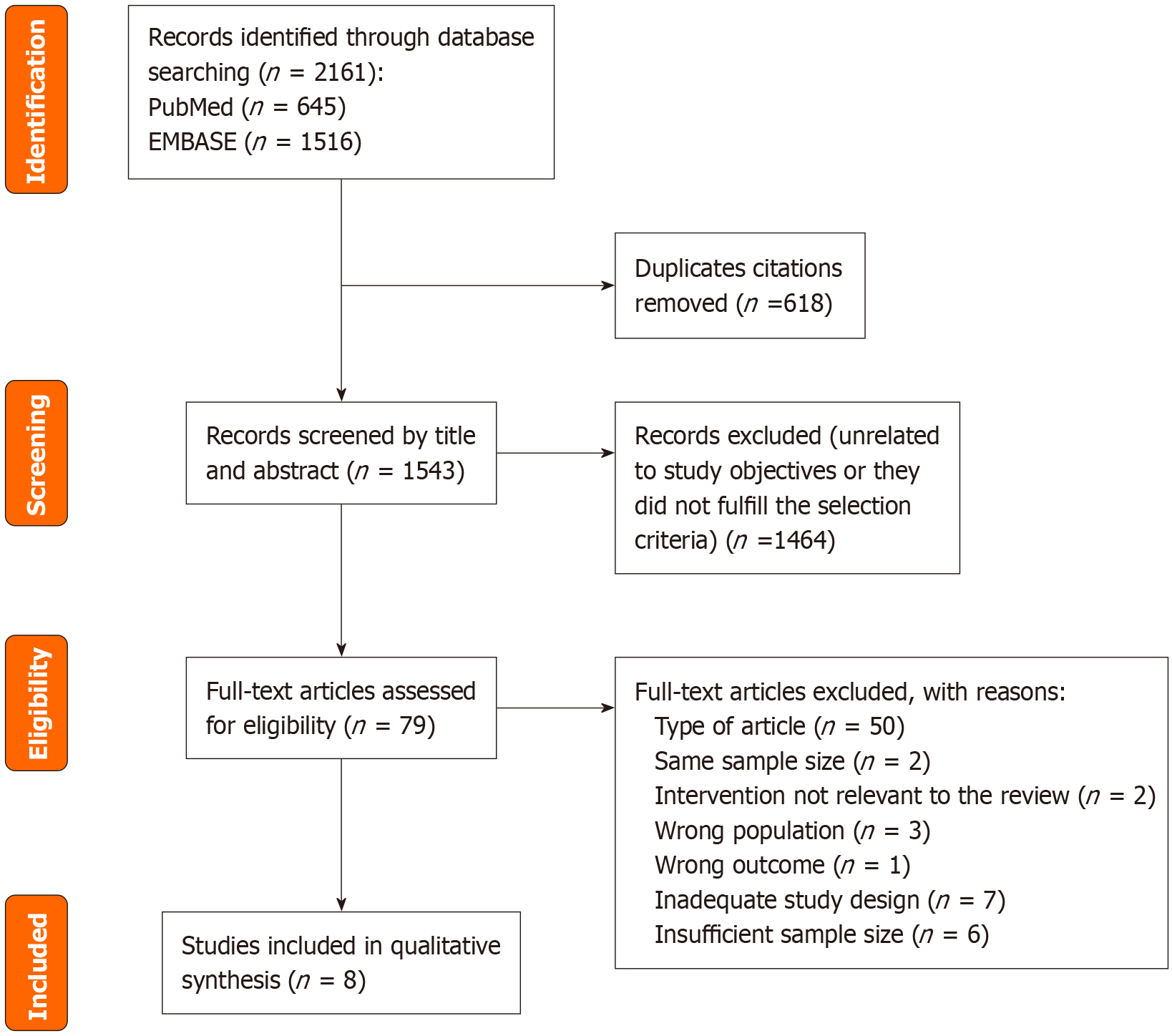
Searches in the databases yielded 645 citations in PubMed and 1516 in EMBASE. Of these 2161 studies, 618 were duplicates and therefore removed. A total of eight studies were included in qualitative synthesis. Among the included publications, six were randomized control trials (RCT) and two were retrospective cohort studies. These studies evaluated 90-day mortality or survival in SAH patients treated with corticosteroids (n = 2), pentoxifylline (n = 1), anakinra plus zinc (n = 2), granulocyte colony-stimulating factor (n = 1), amoxicillin-clavulanate (n = 1), fecal microbiota transplantation (n = 1) or extracorporeal liver assist device (n = 1). While most studies were conducted in Western countries, two had a global scope.
Steroids remain the first-line therapy for SAH despite reports of them not having any 90-day survival benefit. These results highlight the need for multicenter, biomarker-guided RCTs evaluating emerging treatments to improve mid-term survival in SAH.
Core Tip: Severe alcoholic hepatitis (SAH) is associated with high mortality, yet effective mid-term treatments remain limited. This systematic review exclusively examines survival or mortality at 90 days, synthesizing evidence from eight recent studies. While corticosteroids remain the mainstay of current therapy, their survival benefit appears confined to the first 28 days. In contrast, interventions such as Fecal Microbiota Transplantation and granulocyte colony-stimulating factor have emerged as promising approaches for improving 90-day survival, though further validation is needed. This review highlights the urgent need for innovative therapies and high-quality trials to address mid-term survival in SAH, a critical but underexplored therapeutic window.
- Citation: Quiñones-Calvo M, Alvarado-Jara R, García-Renedo P, Stallings E, Grifol-Clar E, Fernández-Rodríguez CM. Beyond corticosteroids: A systematic review of novel therapeutic strategies in severe alcoholic hepatitis and 90-day survival. World J Gastroenterol 2025; 31(35): 109987
- URL: https://www.wjgnet.com/1007-9327/full/v31/i35/109987.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i35.109987
Alcoholic liver disease (ALD) is a leading global cause of liver-related morbidity, mortality and liver transplantation (LT). Among its clinical manifestations, severe alcoholic hepatitis (SAH) represents the most acute and life-threatening form, typically arising in the context of underlying advanced chronic liver disease. SAH is characterized by recent-onset jaundice in patients with alcohol use disorder (AUD), alongside a Maddrey discriminant function (MDF) score ≥ 32 or a Model for End-Stage Liver Disease (MELD) score > 20 at admission[1,2]. The diagnosis of alcoholic hepatitis is based on criteria from the National Institute on Alcohol Abuse and Alcoholism[2].
Three-month mortality rates in SAH remain high, reaching up to 50%[3]. Current standard therapy includes corticosteroids and nutritional support, with early LT considered in carefully selected non-responders[1]. However, treatment options remain limited. Corticosteroid use is contraindicated in the presence of active infection, gastrointestinal bleeding, or renal dysfunction, limiting their applicability in real-world settings. Moreover, corticosteroids have demonstrated benefit only within the first 28 days, with no confirmed impact on longer-term survival[4]. In response to these cha
The objective of this systematic review is to comprehensively evaluate the impact of pharmacological interventions on 90-day mortality and survival in SAH. The inclusion of the 90-day endpoint aims to bridge a critical gap in previous analyses that predominantly focused on short-term (28-30 days) outcomes[4,6,7]. This mid-term timeframe is clinically meaningful, as it captures therapeutic effects during systemic inflammation resolution and early liver regeneration, while reducing confounding factors from alcohol relapse, infections, and comorbidities. Therefore, assessing 90-day mortality or survival provides a more rigorous and clinically relevant measure of treatment efficacy in SAH.
The systematic review was conducted and reported according to the “Preferred Reporting Items for Systematic and Meta-Analyses” (PRISMA) guidelines[8]. The review protocol was registered in the PROSPERO database (CRD420251000149)[9].
PICO Framework: Population (P): Patients with SAH, defined as MDF ≥ 32 or MELD > 20. (1) Intervention (I): Pharmacological treatments or early LT; (2) Comparison (C): Placebo, standard of care (SOC), or other active treatments; and (3) Outcome (O): Survival or mortality at 90-day.
Research question: What is the effect of different treatments for SAH, defined as MDF ≥ 32, MELD score > 20, on 90-day survival or mortality?
A systematic literature search was performed in PubMed (https://pubmed.ncbi.nlm.nih.gov) and EMBASE (https://www.embase.com), initially conducted on December 27, 2024 from the last 10 years. A second search was conducted on March 27, 2025 to capture newly published studies during manuscript preparation. No language restrictions were applied.
Combinations of the following keywords and controlled vocabularies (MeSH, Emtree) were searched with the help of a health librarian (Grifol-Clar E):
PubMed: (“Hepatitis, Alcoholic”[Mesh] OR “Alcohol Hepatitis”[tiab:~4] OR (alcoholic[tiab] AND hepatitis[tiab]) OR “hepatitis alcohol”[tiab:~4]) AND (“Therapeutics”[Mesh] OR therapeutic*[tiab] OR therap*[tiab] OR treatment*[tiab] OR transplant*[tiab]) AND (“survival curve”[tiab:~4] OR “survival probability”[tiab:~4] OR “survival rate”[tiab:~4] OR “life expectancy”[tiab:~4] OR survival[tiab] OR “Survival”[Mesh] OR “Survival Rate”[Mesh] OR “Rate Survival”[tiab:~4] OR “Cumulative Survival Rate”[tiab:~4] OR “Mean Survival Time”[tiab:~4]) Filters: From 2014/12/27-2025/3/27.
EMBASE: ('alcoholic hepatitis'/exp OR ((alcoholic NEAR/4 hepatitis):ti,ab) OR (alcoholic:ti,ab AND hepatitis:ti,ab) OR ((hepatitis NEAR/4 alcohol):ti,ab)) AND (‘therapy’/exp OR therapeutic*:ti,ab OR therap*:ti,ab OR treatment*:ti,ab OR transplant*:ti,ab) AND (((survival NEAR/4 curve*):ti,ab) OR ((survival NEAR/4 probabilit*):ti,ab) OR ((survival NEAR/4 rate*):ti,ab) OR ((life NEAR/4 expectanc*):ti,ab) OR survival:ti,ab OR ‘survival’/exp OR ‘survival rate’/exp OR ((rate NEAR/4 survival):ti,ab) OR ((cumulative NEAR/4 survival NEAR/4 rate):ti,ab) OR ((mean NEAR/4 survival NEAR/4 time):ti,ab)) AND[27-12-2014]/sd NOT[27-03-2025]/sd AND [embase]/Lim NOT ([embase]/Lim AND [medline]/Lim).
No grey literature or trial registries were included to maintain focus on peer-reviewed, indexed studies.
Two reviewers (Quiñones-Calvo M and Alvarado-Jara R) independently screened titles and abstracts. Full texts of eligible studies were reviewed in duplicate. Discrepancies were resolved through discussion with a third reviewer (Fernández-Rodríguez CM). Zotero was used for reference management and deduplication.
Inclusion criteria: (1) Human studies: Randomized control trials (RCT) or prospective/retrospective cohorts; (2) Adult patients with SAH (MDF ≥ 32 or MELD > 20); (3) Interventions involving systemic pharmacologic therapies or early LT; (4) Outcomes reporting survival or mortality at 90 days; and (5) Studies published between 2015 and 2025.
Exclusion criteria: (1) Studies not meeting PICO criteria; and (2) Abstracts, narrative reviews, editorials, expert opinions, case reports, case series, case-controls, guidelines, or other systematic reviews.
Three reviewers (Quiñones-Calvo M, Alvarado-Jara R, García-Renedo P) independently extracted data on study characteristics, treatment and control details, and outcomes [relative risk (RR), odds ratio (OR), or hazard ratio (HR)]. A standardized data extraction form was used[9].
Risk of bias was assessed using the Cochrane RoB 2.0 tool for randomized trials[10] and the Risk-of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool for non-randomized studies[11]. Disagreements in assessments were resolved by consensus discussion among at least two of the three reviewers.
The overall certainty of evidence was evaluated using the GRADE approach[12]. A summary-of-findings table was generated using GRADEpro software (www.gradepro.org). Disagreements in assessments were resolved by consensus discussion among at least two of the three reviewers.
Due to clinical and methodological heterogeneity, a meta-analysis was not feasible. Instead, a qualitative synthesis of findings was performed using narrative descriptions and tabulated summaries[9].
The systematic search retrieved a total of 2161 records: 645 from PubMed and 1516 from EMBASE. After the removal of 618 duplicates, 1543 unique articles remained for initial screening. Two independent reviewers reviewed titles and abstracts, leading to the exclusion of 1464 records that did not meet eligibility criteria or were not relevant to the research question.
A total of 79 full-text articles were assessed for eligibility. Following detailed evaluation, eight studies met the inclusion criteria and were included in the final qualitative synthesis.
A PRISMA flow diagram illustrating the study selection process is provided in Figure 1.
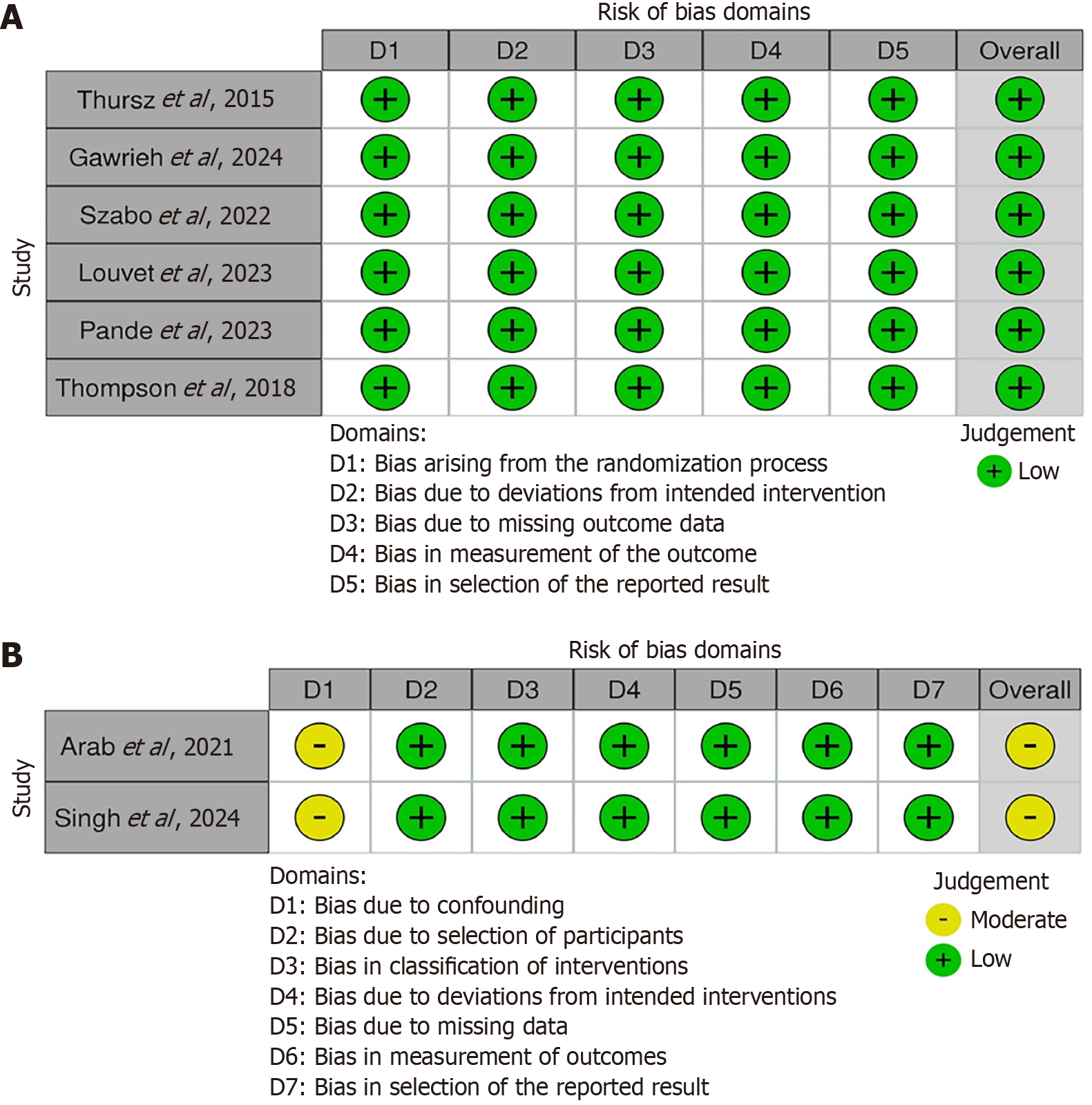
Risk of bias of the included studies was assessed using the Cochrane RoB 2.0 tool for randomized trials[10] (Figure 2A) and the ROBINS-I tool for non-randomized studies[11] (Figure 2B).
Certainty of evidence was assessed using the GRADE approach[12] (Table 1).
| Intervention | Comparator | Outcome | No. of Studies | Study design | Certainty (GRADE) | Effect estimate | Comments |
| Corticosteroids[13,14] | Placebo[13] | 90-day mortality | 1 | RCT | ↑↑↑↑ (high) | OR 1.02 (P = 0.87) | No benefit observed |
| Non-treatment[14] | 1 | Observational study | ↑↑↑ (moderate) | HR 0.92 (P = 0.871) | Downgraded one level due to study design | ||
| Pentoxifylline[13] | Placebo | 90-day mortality | 1 | RCT | ↑↑↑↑ (high) | OR 0.97 (P = 0.81) | No benefit observed |
| Anakinra + zinc[15] | Prednisone | 90-day survival | 1 | RCT | ↑↑↑ (moderate) | HR 0.34 (P = 0.018) | The findings across studies are inconsistent |
| Anakinra + zinc + pentoxifylline[16] | Methylprednisolone | 90-day survival | 1 | RCT | ↑↑ (low) | HR 0.69 (P = 0.28) | HR reported without CI; precision cannot be assessed. Downgraded one level due to serious imprecision |
| G-CSF[17] | Corticosteroids | 90-day survival | 1 | Observational study | ↑↑↑ (moderate) | HR 0.38 (P < 0.01) | Strength: Large cohort with global scope; Weakness: Needs RCT validation |
| Standard medical treatment | ↑↑↑ (moderate) | HR 0.26 (P < 0.01) | |||||
| Amoxicillin-clavulanate + prednisolone[18] | Prednisolone | 90-day mortality | 1 | RCT | ↑↑↑↑ (high) | HR 0.78 (P = 0.30) | No significant effect |
| FMT[19] | Prednisolone | 90-day survival | 1 | Randomized control trial | ↑↑↑↑ (high) | HR 0.528 (P = 0.044) | Promising results; small sample |
| ELAD[20] | Standard of care | 91-day survival | 1 | RCT | ↑↑↑↑ (high) | HR 1.03 (P = 0.90) | No benefit observed |
Characteristics of included studies: A total of eight studies were included in the qualitative synthesis. Among the included publications, six were RCTs and two were retrospective cohort studies. Three of these studies evaluated 90-day mortality and five evaluated 90-day survival in SAH patients treated with corticosteroids (n = 2), pentoxifylline (n = 1), anakinra plus zinc (n = 1) or anakinra plus zinc plus pentoxifylline (PTX) (n = 1), G-CSF (n = 1), Amoxicillin-clavulanate (n = 1), FMT (n = 1) or ELAD (n = 1). While five studies were conducted in Western countries, one was conducted in India and two had a global scope. Study sample sizes and geographic variability are described in Table 2.
| Ref. | Intervention | Study design | n (intervention/control) | Effect estimate (90-day survival) | Effect estimate (90-day mortality) | Statistical significance | Country |
| Thursz et al[13], 2015 | PRED | Multicenter, RCT | 277/276 | - | OR 1.02 (95%CI: 0.77-1.35) | P = 0.87 | United Kingdom |
| PTX | 276/276 | - | OR 0.97 (95%CI: 0.73-1.28) | P = 0.81 | |||
| Arab et al[14], 2021 | Corticosteroids | Multicenter, retrospective | 1225/2155 | - | HR 0.92 (95%CI: 0.33-2.56) | P = 0.871 | Global |
| Gawrieh et al[15], 2024 | Anakinra + zinc | Multicenter, phase IIb RCT | 74/73 | HR 0.34 (95%CI: 0.14-0.83) | - | P = 0.018 | United States |
| Szabo et al[16], 2022 | Anakinra + PTX + zinc | Multicenter, RCT | 53/50 | HR 0.69 | - | P = 0.28 | United States |
| Singh et al[17], 2025 | G-CSF | Multicenter, retrospective | 224/10800 (steroids) | HR 0.38 (95%CI: 0.23-0.62) | - | P < 0.01 | Global |
| 224/8573 (standard medical treatment) | HR 0.26 (95%CI: 0.17-0.41) | - | P < 0.01 | ||||
| Louvet et al[18], 2023 | Amoxicillin-clavulanate + PRED | Multicenter, RCT | 142/142 | - | HR 0.78 (95%CI: 0.47-1.27) | P = 0.30 | France/Belgium |
| Pande et al[19], 2023 | FMT | Single center, RCT | 60/60 | HR 0.528 (95%CI: 0.279-0.998) | - | P = 0.044 | India |
| Thompson et al[20], 2018 | ELAD | Multicenter, open-label RCT | 96/107 | HR 1.03 (95%CI: 0.69-1.53) | - | P = 0.90 | United States/United Kingdom/Australia |
Corticosteroids and traditional agents: Corticosteroids have long served as the standard treatment in SAH, but recent high-quality data suggest that they do not offer any survival benefits beyond 30 days. In the pivotal Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial by Thursz et al[13], neither prednisolone (PRED) (OR 1.02; P = 0.87) nor PTX (OR 0.97; P = 0.81) showed a significant advantage over placebo in 90-day mortality. This was consistent with a study by Arab et al[14] in a large retrospective cohort across 17 countries, which found no statistically significant reduction in 90-day mortality with corticosteroid use (HR 0.92; P = 0.871).
Novel immunomodulatory therapies: Biologic agents such as Anakinra have yielded inconclusive results. Gawrieh et al[15] reported a significant survival benefit with prednisone compared to anakinra plus zinc, which led to early termi
G-CSF therapy demonstrated robust survival benefits in a large international retrospective cohort. Singh et al[17] reported a HR of 0.38 compared to corticosteroids (P < 0.01), reinforcing its potential immune-regenerative effects.
Antibiotics and microbiota-targeting interventions: Modulating gut microbiota has emerged as a key target in SAH. Louvet et al[18] tested prophylactic amoxicillin-clavulanate in conjunction with PRED but failed to find a significant survival benefit (HR 0.78; P = 0.30). Conversely, FMT from healthy donors significantly improved 90-day survival (HR 0.528; P = 0.044) in a single-center trial from India[19].
Device-based therapy: The ELAD system failed to improve survival outcomes in a multicenter open-label RCT (HR 1.03; P = 0.90)[20]. Current data do not support routine use of ELAD in SAH.
Substantial heterogeneity in patient characteristics, intervention protocols and primary outcomes precluded the feasibility of a meta-analysis and limited comparability across studies. The primary factors contributing to the infeasibility of a meta-analysis included the variability in interventions, differences in study design - with two studies being retrospective and six RCTs - and inconsistency in outcome reporting. Specifically, three studies assessed 90-day mortality and five assessed 90-day survival, with this endpoint serving as the primary outcome in four studies and a secondary outcome in the remaining four.
In this systematic review, we chose a 90-day survival or mortality endpoint to capture both short- and mid-term therapeutic effects in SAH. This timeframe is clinically meaningful, as it reflects patient outcomes during the resolution of systemic inflammation and early liver recovery, while minimizing the confounding effects of alcohol relapse, infections, and other long-term comorbidities[21]. Moreover, unlike the commonly used 28-day endpoint, a 3-month survival measure provides a more rigorous assessment of true therapeutic efficacy.
Corticosteroids: Steroids remain the first-line therapy for SAH patients, as they are the only pharmacological treatment shown to improve 28-day survival[4,13,14]. However, their benefit does not extend beyond this period, underscoring the need for alternative therapeutic strategies[4]. Their primary mechanism of action is their potent anti-inflammatory effect, which mitigates the systemic inflammatory response syndrome (SIRS) characteristic of SAH[22]. The standard regimen consists of 40 mg PRED daily, with treatment continuation assessed using the Lille score on day 7. Based on this, therapy is continued for a month in responders or discontinued in non-responders[23]. Despite improvements in Lille score, patients remain at high risk of infection[24].
The STOPAH trial was a multicenter double-blind RCT with a 2-by-2 factorial design that evaluated the efficacy of 40 mg/day PRED and 400 mg PTX three times daily for 28 days (n = 1103)[13].
Aside from its factorial design, one of the key strengths of this trial was the use of stratified randomization based on geographic region and risk category. Patients were allocated into four groups: PTX-matched placebo and PRED-matched placebo group (n = 276), PRED and PTX-matched placebo group (n = 277), PTX and PRED-matched placebo group (n = 276) or PRED and PTX group (n = 274), so as to also investigate the synergistic effect between PRED and PTX. The four groups were matched to their baseline characteristics. Another key strength of the study lies in the intention-to-treat (ITT) analyses between treated and untreated groups with the use of logistic regression with adjustments for risk category and factorial design[13].
The weakness of this study for our systematic review is that the primary endpoint was 28-day mortality, while secondary endpoints included 90-day mortality and the need for LT. Because the trial ended after all patients had completed 28 days of follow-up, 33 patients included in the 28-day mortality analysis were not assessed for 90-day mortality or the need for LT. Furthermore, although 1103 patients were randomized, only 1053 patients were included in the primary endpoint analysis. At 90 days, data for 12% of patients were missing due to loss of follow-up (5%), with
Notably, threatening infections occurred at a significantly higher rate in patients receiving PRED (P = 0.002), although infection-related mortality did not differ between groups[13].
In the study by Arab et al[14], the primary endpoint was 30-day mortality, with 90-day mortality acting as a secondary endpoint. Additionally, there were no differences in survival between different steroid types, including PRED, prednisone and methylprednisolone. Notably, in this study, the most common reason for steroid discontinuation was non-variceal gastrointestinal bleeding rather than infection, and mortality rates due to infection were similar between treatment groups[14], consistent with findings from the STOPAH trial[13].
While the study's large sample size, global scope, and adjustment for variables such as age, sex, ethnicity, cirrhosis, dialysis, and MELD score, particularly in light of higher baseline MELD and MDF scores among patients receiving steroids, are notable strengths, its retrospective design limits the certainty of its conclusions. This is due to the heterogeneity in indications for steroid use, dosage, and patient characteristics[14].
Nutrition support and early LT: Despite its well-established prognostic relevance in liver disease, nutritional support remains understudied in SAH trials. Barriers include heterogeneity in assessment tools and difficulties in blinding nutritional interventions.
One small trial was identified but excluded from synthesis due to insufficient power[25]. Given the role of malnutrition and sarcopenia in adverse outcomes[26], this area warrants further investigation.
Early LT is the only intervention consistently associated with improved long-term survival in carefully selected steroid non-responders[27-29]. However, we were unable to identify any eligible studies assessing 90-day survival or mortality post-LT in SAH, highlighting a significant gap in the literature. The number of liver transplants for SAH continues to rise, despite the ethical, clinical and logistical complexities associated with early LT, underscoring the need for high-quality prospective data. Patient selection should be conducted by a multidisciplinary team, including hepatologists, transplant surgeons, addiction specialists, and social workers[23]. Abstinence from alcohol is essential for favorable long-term outcomes, yet post-LT relapse occurs in 15%-50% of patients[22]. LT raises complex ethical concerns, including equity in organ allocation, the adequacy of psychosocial assessment, and relapse risk. Recent consensus statements call for standardized selection protocols to address these challenges.
Alcohol abstinence: None of the included studies evaluated pharmacologic treatments for AUD such as naltrexone, acamprosate, or baclofen, likely because these agents are not routinely initiated during the acute phase of SAH. Because promising trials are evaluating baclofen and acamprosate in cirrhosis, their role in post-SAH recovery warrants investigation. Likewise, non-pharmacological interventions such as cognitive-behavioral therapy, psychotherapy, or peer-support programs were represented in the evidence base, reflecting their limited application in the critical care context of SAH.
Nonetheless, alcohol abstinence remains the strongest predictor of long-term survival in patients with ALD[22]. In the ELAD RCT, which was included in this qualitative synthesis, alcohol relapse was evaluated as a secondary outcome. Among patients who tested negative for alcohol at discharge based on phosphatidylethanol (PEth) testing, 91-day survival was significantly higher (64.8%, 46/71 patients; P < 0.05)[20]. These data reinforce the prognostic importance of abstinence even in the subacute setting, although they do not establish a causal relationship between abstinence and survival at 3 months.
Importantly, abstinence does not significantly influence mid-term (90-day) survival, which is largely driven by hepatic decompensation, systemic inflammation, and infections. This finding supports the use of a 90-day survival endpoint as a reliable and objective marker for evaluating treatment efficacy in SAH, minimizing the confounding effects of relapse and behavioral interventions that typically influence longer-term outcomes[30].
Anti-inflammatory agents: (1) Pentoxifylline. Pentoxifylline is a non-specific phosphodiesterase inhibitor with anti-
Gawrieh et al[15] performed a double-blind, multicenter, phase IIb RCT in which they compared Anakinra 100 mg subcutaneously for 14 days combined with oral zinc sulfate 220 mg daily for 90-day and a prednisone-matching placebo, to prednisone 40 mg orally once daily for 30 days combined with Anakinra and zinc-matching placebo. The Day-7 Lille score (> 0.45) was used as a stopping rule for prednisone or its placebo. The primary outcome of the trial was an ITT analysis of 90-day survival. Its key strength is stratified randomization by site and MELD score (low MELD < 25 vs high MELD > 25)[15].
Apart from the Prednisone increase of 90-day survival, they reported an increase in transplant-free survival at 90 days (HR 0.30; P = 0.004). There was a lower incidence of acute kidney injury (AKI) (P = 0.001) and no significant difference in infection rates (P = 0.389). These results were confirmed in a prespecified interim analysis, which led to early termination of the trial. Notably, in this study, all drug treatments were discontinued in cases of uncontrolled infection or a > 5-point increase in MELD score. These findings contrast with the earlier literature discussed previously, as they suggest that corticosteroid treatment, when discontinued on Day 7 in patients with a Lille score > 0.45, is associated with improved 90-day survival. In the present study, the 90-day survival rate was 90%, compared to 79% in the Arab et al[14] and 70% in the Thursz et al[13] for patients in the corticosteroid group. This discrepancy may be explained by the inclusion criteria used by Gawrieh et al[15], who enrolled only patients with MELD scores between 20 and 35. In the centers where the study was conducted, patients with MELD scores > 35 are often considered for early LT as the primary treatment. The authors hypothesized that the observed survival benefit may be partially attributable to the use of the Day-7 Lille score as a stopping rule, which could reduce the risk of opportunistic infections. Other contributing factors might include increased infection control measures during the coronavirus disease 2019 pandemic and a younger study population compared to previous trials. Importantly, no fungal infections were reported in the prednisone group in this study[15].
Szabo et al[16] performed a double-blind, multicenter RCT to assess the efficacy of a combination therapy of three agents: Anakinra (100 mg subcutaneous for 14 days), zinc sulfate (220 mg orally for 180 days) plus PTX (400 mg orally 3 times daily for 28 days) in comparison to methylprednisolone (32 mg for 28 days). The association between PTX and protection against hepatorenal syndrome remains controversial[31,32]. Again, survival at 90 days was a secondary outcome of the study, as the primary outcome was 180-day survival. The incidence rates of AKI and infections were comparable between groups, except for a significantly higher fungal infection rate in the methylprednisolone group (P < 0.02)[16] as previously described in the literature[24].
Its key strength is the randomization stratified by MELD score, but the study has multiple weaknesses. Patients in both groups withdrew from the study due to adverse effects or disease progression, including eight in the methylprednisolone group and ten in the intervention group. Notably, the study did not report the confidence interval of the hazard ratio in either the main article or the supplementary materials, which may introduce reporting bias. However, due to non-significant results, the study was included in the qualitative synthesis[16].
Targeting liver tissue regeneration: (1) Pegfilgrastim is a drug that promotes long-term neutrophil production. In this systematic search, only one RCT was identified that compared the combination of pegfilgrastim with SOC, which consisted of either PRED 40 mg daily or pentoxifylline 400 mg three times a day, against SOC alone. This study was excluded due to a high risk of bias and a small sample size[33]; and (2) G-CSF is a glycoprotein that stimulates the bone marrow to produce and release neutrophils and CD34 + stem cells into the bloodstream, thereby promoting hepatic regeneration. In our systematic review, we identified several published RCTs reporting improved 90-day survival when G-CSF was used alone or in combination with PRED. However, these studies were excluded from our qualitative synthesis because they did not meet the selection criteria[34-36].
Singh et al[17] performed a multicenter retrospective cohort study. The primary endpoint was 90-day survival. Patients with more comorbidities, as well as younger and predominantly white individuals, were more frequently treated with G-CSF than with Standard Medical Treatment (SMT) or corticosteroids. After propensity score matching, 90-day overall survival was significantly better in patients treated with G-CSF than with SMT (HR 0.26; P < 0.01) or corticosteroids (HR 0.38; P < 0.01). These findings suggest that G-CSF is not inferior to corticosteroids. Gastrointestinal bleeding was less frequent in the G-CSF group compared to corticosteroids, while the incidence of bacterial sepsis was similar between groups. The G-CSF group included patients treated with filgrastim, pegfilgrastim and lenograstim, whereas the corticosteroid group included those treated with prednisone, PRED, methylprednisolone or dexamethasone. Patients who had received corticosteroids were excluded from the G-CSF group and vice versa. Although the study’s large sample size and the use of (1:1) propensity score matching to control confounding variables (such as age, gender and comorbidities) to reduce the selection bias are its notable strengths, its retrospective design limits the certainty of the conclusions. Treatment indications of G-CSF and corticosteroids, the type of drug within these groups, dosage and patient characteristics were neither homogeneous nor standardized[17]. Furthermore, nearly 98% of patients were from the United States, with only 2% from Europe, Asia or South America, limiting the generalizability of the results compared to other studies such as that by Arab et al[14].
Despite these encouraging findings, larger RCTs are needed to establish G-CSF as a first-line treatment for SAH. Current guidelines do not recommend its use[1,23], as the evidence remains inconclusive[37,38].
Antioxidant agents: N-acetylcysteine (NAC) is a hepatoprotective and antioxidant agent that contributes to the replenishment of reduced glutathione stores. It has been hypothesized that the combination of prednisone and NAC may reduce infection rates[39]. A single retrospective study evaluating 90-day mortality in patients receiving prednisone and NAC was identified, however it was excluded due to high-risk bias[40].
Modification of gut-liver axis: Clinical trials are investigating drugs that may modulate the dysbiosis and dysfunction of the gut-liver axis that occurs in SAH. Changes in the microbiota lead to increased gut permeability, which activates the immune response and ultimately disrupts the liver's metabolic functions[19].
The gut microbiota is composed of bacteria, fungi, and viruses, which interact symbiotically with the host. The micro
(1) Prophylactic antibiotics: The primary role of antibiotics in SAH is intestinal decontamination to reduce microbiota levels and, consequently, the concentration of toxins reaching the portal circulation. Infection is a major contributor to mortality in patients with SAH, and corticosteroid therapy has been associated with an increased risk of bacterial and fungal infections, up to 20% during or following treatment[13,26,42]. However, whether corticosteroids directly increase mortality from bacterial infections remains controversial. Nonetheless, infection is widely recognized as an independent predictor of 90-day mortality in SAH patients treated with corticosteroids. The use of prophylactic antibiotics, although potentially beneficial, remains contentious due to concerns about promoting the emergence of multidrug-resistant bacteria[24,26].
Louvet et al[18] performed a multicenter, double-blind RCT and compared the addition of amoxicillin-clavulanate to PRED vs PRED and placebo. All participants received 40 mg/day of oral PRED for 30 days. Those in the intervention group received amoxicillin (1 g) and clavulanate (125 mg) three times daily for the same period. Notably, the trial protocol did not mandate corticosteroid discontinuation based on Lille score > 0.45 at day 7, which may have impacted outcomes. The ITT analysis of 90-day all-cause mortality was a secondary outcome, (HR 0.78; P = 0.30), indicating no significant benefit[18]. These results align with previous studies in the field[26].
Prophylactic rifaximin has also been investigated in SAH, although we did not identify any studies that met our inclusion criteria. Most published research on rifaximin in this context stems from lower-quality evidence, such as observational or non-randomized designs, and current data do not support a 90-day survival benefit[43];
(2) Bovine colostrum: We identified an ongoing RCT that aims to evaluate the efficacy of bovine colostrum as an adjuvant therapy to enhance the benefits of corticosteroids in SAH. The primary endpoint of this study is 3-month survival[44];
(3) FMT: Healthy donor FMT mitigates systemic inflammation, gut leakiness and liver damage in SAH by reducing IFN-γ and IL-17A production by T cells and Th17cells[45].
Pande et al[19] conducted a single-center, open-label RCT to evaluate 90-day survival between a control group receiving PRED (40 mg/day for 28 days, with continuation based on Lille score) and an intervention group receiving FMT via a nasoduodenal tube for 7 consecutive days. Donors were healthy co-habitants aged 18-60 years with no recent illnesses.
After accounting for patients who did not receive the intervention (three in the PRED arm, five in the FMT arm), 57 control and 55 intervention patients were analyzed. The ITT analysis showed a significant survival benefit in the FMT group (HR 0.528; P = 0.044). There were no significant baseline differences between groups. Infection-related mortality was significantly higher in the PRED group (P = 0.01), suggesting that the survival benefit of FMT may be driven by reduced infection rates via microbiota modulation. In the FMT group, the leading cause of death was progressive liver failure.
Secondary outcomes included survival at 180 and 365 days, which revealed a potential decline in FMT efficacy over time. This may reflect diminishing donor microbiota persistence and suggests the need to evaluate repeated FMT cycles to sustain benefits. However, optimal frequency and duration of FMT for microbiota homeostasis restoration remain unknown[19]. Interestingly, FMT has also been hypothesized to reduce alcohol craving, though this remains to be confirmed in future studies[46].
A major limitation of the trial by Pande et al[19] was the inability to blind participants due to the use of a nasodu
And (4) Probiotics: An observational study was identified in which patients were allowed to choose between receiving high-dose probiotic infusions, FMT or corticosteroids. However, this study was excluded from the qualitative synthesis due to significant selection bias and a small sample size[47]. Despite this, probiotics remain a promising avenue for future research, particularly for their potential role in restoring gut microbiota balance and enhancing mucosal immunity in SAH patients.
ELAD: The use of cellular therapies such as the ELAD in SAH is based on providing temporary hepatic support to counteract hepatocyte degeneration mediated by inflammation and oxidative stress[22]. ELAD functions by circulating the patient’s blood through cartridges containing HepG2/C3A hepatoblastoma cell lines, which secrete anti-inflammatory cytokines and regenerative growth factors[20].
Thompson et al[20] conducted a multicenter, open-label RCT that evaluated ELAD for 3-5 days in combination with SOC (including corticosteroids, pentoxifylline, NAC, and antibiotics) vs SOC alone. The primary outcome was 91-day overall survival. The ITT analysis found no significant difference between groups (HR 1.03; P = 0.90). Notably, ELAD exposure varied: 45 patients received the full 120 hours, 37 received 72-120 hours, 12 received less than 72 hours, and two did not start treatment due to instability[20]. Baseline characteristics were balanced between groups. A prespecified subgroup analysis among patients with MELD scores < 28 (n = 120) showed a non-significant trend toward improved 91-day survival with ELAD (HR 0.58; P = 0.08). Regression analysis indicated that elevated creatinine and international normalized ratio (INR), but not bilirubin, were associated with poorer outcomes among ELAD recipients[20]. A subsequent RCT (VTL308; NCT02612428) targeted younger SAH patients with less coagulopathy and preserved renal function. However, interim analysis showed no survival benefit at 90-day, prompting early study termination[20,48]. The ELAD studies have limitations, including open-label design and the inability to blind due to the use of an endovascular catheter, which may pose unnecessary risks to patients in the control arm without offering therapeutic benefit.
Hypolipidemic agents: Larsucosterol is a DNA methyltransferase inhibitor with anti-inflammatory, antioxidative, and cell-regeneration properties. A recently published phase 2b randomized controlled trial evaluated its efficacy in patients with SAH, randomly assigning participants in a 1:1:1 ratio to receive either 30 mg or 90 mg of larsucosterol, or placebo. A second dose was administered on day 3 if patients remained hospitalized. In the placebo group, patients received 32 mg of methylprednisolone when clinically indicated, whereas intervention groups received matching placebo[49].
The primary endpoint was the composite of 90-day mortality or LT. No statistically significant differences were observed between treatment groups[49]. This study was not included in the qualitative synthesis due to the lack of Cox, logistic, or Poisson regression models, which were required for consistent effect size comparison in this review. Further research using robust statistical methods is warranted to confirm these preliminary findings.
In summary, according to current European Association for the Study of the Liver[1] and American Association for the Study of Liver Diseases[50] guidelines, the treatment of SAH includes corticosteroids for patients with an MDF ≥ 32, using the Day-7 Lille score as a stopping rule, along with nutritional support and early LT in selected cases, and sustained alcohol abstinence. The emerging treatments presented before have been evaluated, but none of them have been incorporated into the current guidelines[1,50], not even the G-CSF or FMT, despite their preliminary benefit[17,19]. G-CSF requires larger RCTs to confirm its efficacy, as the existing evidence remains inconclusive[37,38]. Similarly, FMT has only been evaluated in studies with modest sample sizes, including the one reported in this systematic review[19]. Regulatory hurdles or concerns about potential adverse effects also hinder clinical adoption, underscoring the need for further research through pragmatic RCTs.
This review was conducted using rigorous methodology, but certain limitations must be acknowledged.
Due to the heterogeneity of interventions, study designs and outcomes across studies, a quantitative meta-analysis was not feasible. Although all included studies reported 90-day survival or mortality outcomes, these were not always designated as primary endpoints, which may have limited the statistical power to detect significant differences. Furthermore, while 90-day survival and mortality both pertain to treatment efficacy, they represent inverse measures, thereby complicating direct comparisons and reducing the feasibility of a meta-analysis.
While our search strategy was comprehensive, the exclusion of grey literature and unpublished studies may have introduced some publication bias. Furthermore, in our systematic review, we identified only eight studies that met our predefined inclusion and exclusion criteria. The limited number of included studies may also reflect publication bias, as many of the novel strategies for SAH have not demonstrated clear benefit and may therefore remain unpublished. Moreover, the limited number of studies included in our systematic review, is likely attributable to the narrowly defined time window selected as the outcome measure. The small number of eligible studies also precluded the possibility of conducting a quantitative meta-analysis, an approach we had not planned in our protocol[9] given our anticipation to the challenge of identifying studies meeting our criteria that were sufficiently comparable. Nonetheless, despite including only eight studies, we ensured that those selected were of high methodological quality. Notably, none of the included studies were judged to have a high risk of bias according to the RoB.2[10] or ROBINS-I[11] assessment tools.
Nevertheless, the included studies provide valuable insight into current and emerging therapeutic strategies for SAH, with a focus on a clinically meaningful mid-term outcome.
Despite its clinical relevance, few studies have employed the 90-day survival or mortality timeframe as a primary endpoint. We encourage further research evaluating this outcome, as it represents a meaningful indicator of treatment efficacy in SAH. This period encompasses the resolution of systemic inflammation and early liver recovery, while minimizing confounding from long-term factors such as alcohol relapse[30].
Steroids remain the first-line therapy for patients with SAH despite the lack of demonstrated improvement in overall 90-day mortality and their known association with increased infection risk[13,26], supported by high-certainty evidence according to the GRADE approach[12].
This systematic review highlights a critical gap in evidence regarding the mid-term outcomes of early LT in SAH, which remains the only intervention with established long-term survival benefit in carefully selected patients[28,29]. Although early LT is not universally available, both FMT and G-CSF have emerged as promising therapeutic strategies, supported by high-and moderate- certainty evidence, respectively, according to the GRADE approach[12]. Their preliminary efficacy in improving 90-day survival[17,19] warrants validation through multicenter, prospective RCTs employing standardized 90-day endpoints and rigorous patient phenotyping. Given the complex interplay of hepatic dysfunction, infectious complications, and behavioral factors in SAH, a multidisciplinary approach involving hepatologists, addiction specialists, transplant teams, and nutritionists is essential in further research.
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018;69:154-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 648] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 2. | Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, McClain C, McCullough A, Mitchell MC, Morgan TR, Nagy L, Radaeva S, Sanyal A, Shah V, Szabo G; NIAAA Alcoholic Hepatitis Consortia. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150:785-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 437] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 3. | Kasper P, Lang S, Steffen HM, Demir M. Management of alcoholic hepatitis: A clinical perspective. Liver Int. 2023;43:2078-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 4. | Louvet A, Thursz MR, Kim DJ, Labreuche J, Atkinson SR, Sidhu SS, O'Grady JG, Akriviadis E, Sinakos E, Carithers RL Jr, Ramond MJ, Maddrey WC, Morgan TR, Duhamel A, Mathurin P. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo-a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology. 2018;155:458-468.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 5. | Fung P, Pyrsopoulos N. Emerging concepts in alcoholic hepatitis. World J Hepatol. 2017;9:567-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Islam AH, Alvizuri C, Desalegn H, Stephenson E, Idalsoaga F, Diaz LA, MacDonald JK, Im GY, Singal AK, Jairath V, Khan MQ, Arab JP. Pharmacological Strategies for the Management of Severe Alcohol-associated Hepatitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2025;S1542-3565(25)00493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Singh S, Murad MH, Chandar AK, Bongiorno CM, Singal AK, Atkinson SR, Thursz MR, Loomba R, Shah VH. Comparative Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic Review and Network Meta-analysis. Gastroenterology. 2015;149:958-70.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 1517] [Article Influence: 303.4] [Reference Citation Analysis (1)] |
| 9. | Quiñones-Calvo M, Alvarado-Jara R, García-Renedo P, Stallings E, Grifol-Clar E, Fernández-Rodríguez C. Severe Alcoholic Hepatitis: A Systematic Review of Current Treatments. PROSPERO 2025 CRD420251000149. [cited 3 August 2025]. Available from: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251000149. |
| 10. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18756] [Article Influence: 2679.4] [Reference Citation Analysis (0)] |
| 11. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 12554] [Article Influence: 1255.4] [Reference Citation Analysis (2)] |
| 12. | Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, Brignardello-Petersen R, Carrasco-Labra A, De Beer H, Hultcrantz M, Kuijpers T, Meerpohl J, Morgan R, Mustafa R, Skoetz N, Sultan S, Wiysonge C, Guyatt G, Schünemann HJ; GRADE Working Group. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 804] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 13. | Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A, Hood S, Masson S, McCune A, Mellor J, O'Grady J, Patch D, Ratcliffe I, Roderick P, Stanton L, Vergis N, Wright M, Ryder S, Forrest EH; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 593] [Article Influence: 53.9] [Reference Citation Analysis (1)] |
| 14. | Arab JP, Díaz LA, Baeza N, Idalsoaga F, Fuentes-López E, Arnold J, Ramírez CA, Morales-Arraez D, Ventura-Cots M, Alvarado-Tapias E, Zhang W, Clark V, Simonetto D, Ahn JC, Buryska S, Mehta TI, Stefanescu H, Horhat A, Bumbu A, Dunn W, Attar B, Agrawal R, Haque ZS, Majeed M, Cabezas J, García-Carrera I, Parker R, Cuyàs B, Poca M, Soriano G, Sarin SK, Maiwall R, Jalal PK, Abdulsada S, Higuera-de la Tijera MF, Kulkarni AV, Rao PN, Guerra Salazar P, Skladaný L, Bystrianska N, Prado V, Clemente-Sanchez A, Rincón D, Haider T, Chacko KR, Cairo F, de Sousa Coelho M, Romero GA, Pollarsky FD, Restrepo JC, Castro-Sanchez S, Toro LG, Yaquich P, Mendizabal M, Garrido ML, Narvaez A, Bessone F, Marcelo JS, Piombino D, Dirchwolf M, Arancibia JP, Altamirano J, Kim W, Araujo RC, Duarte-Rojo A, Vargas V, Rautou PE, Issoufaly T, Zamarripa F, Torre A, Lucey MR, Mathurin P, Louvet A, García-Tsao G, González JA, Verna E, Brown RS, Roblero JP, Abraldes JG, Arrese M, Shah VH, Kamath PS, Singal AK, Bataller R. Identification of optimal therapeutic window for steroid use in severe alcohol-associated hepatitis: A worldwide study. J Hepatol. 2021;75:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 15. | Gawrieh S, Dasarathy S, Tu W, Kamath PS, Chalasani NP, McClain CJ, Bataller R, Szabo G, Tang Q, Radaeva S, Barton B, Nagy LE, Shah VH, Sanyal AJ, Mitchell MC; AlcHepNet Investigators. Randomized trial of anakinra plus zinc vs. prednisone for severe alcohol-associated hepatitis. J Hepatol. 2024;80:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 16. | Szabo G, Mitchell M, McClain CJ, Dasarathy S, Barton B, McCullough AJ, Nagy LE, Kroll-Desrosiers A, Tornai D, Min HA, Radaeva S, Holbein MEB, Casey L, Cuthbert J. IL-1 receptor antagonist plus pentoxifylline and zinc for severe alcohol-associated hepatitis. Hepatology. 2022;76:1058-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 17. | Singh RR, Chhabra P, Dhillon S. G-CSF-In Patients With Severe Alcohol-Associated Hepatitis: A Real-World Experience. Liver Int. 2025;45:e16137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Louvet A, Labreuche J, Dao T, Thévenot T, Oberti F, Bureau C, Paupard T, Nguyen-Khac E, Minello A, Bernard-Chabert B, Anty R, Wartel F, Carbonell N, Pageaux GP, Hilleret MN, Moirand R, Nahon P, Potey C, Duhamel A, Mathurin P. Effect of Prophylactic Antibiotics on Mortality in Severe Alcohol-Related Hepatitis: A Randomized Clinical Trial. JAMA. 2023;329:1558-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 19. | Pande A, Sharma S, Khillan V, Rastogi A, Arora V, Shasthry SM, Vijayaraghavan R, Jagdish R, Kumar M, Kumar G, Mondot S, Dore J, Sarin SK. Fecal microbiota transplantation compared with prednisolone in severe alcoholic hepatitis patients: a randomized trial. Hepatol Int. 2023;17:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 20. | Thompson J, Jones N, Al-Khafaji A, Malik S, Reich D, Munoz S, MacNicholas R, Hassanein T, Teperman L, Stein L, Duarte-Rojo A, Malik R, Adhami T, Asrani S, Shah N, Gaglio P, Duddempudi A, Borg B, Jalan R, Brown R, Patton H, Satoskar R, Rossi S, Parikh A, ElSharkawy A, Mantry P, Sher L, Wolf D, Hart M, Landis C, Wigg A, Habib S, McCaughan G, Colquhoun S, Henry A, Bedard P, Landeen L, Millis M, Ashley R, Frank W, Henry A, Stange J, Subramanian R; VTI-208 Study Group. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: A multinational, prospective, controlled, randomized trial. Liver Transpl. 2018;24:380-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 21. | Louvet A, Labreuche J, Artru F, Bouthors A, Rolland B, Saffers P, Lollivier J, Lemaître E, Dharancy S, Lassailly G, Canva-Delcambre V, Duhamel A, Mathurin P. Main drivers of outcome differ between short term and long term in severe alcoholic hepatitis: A prospective study. Hepatology. 2017;66:1464-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 22. | Bataller R, Arab JP, Shah VH. Alcohol-Associated Hepatitis. N Engl J Med. 2022;387:2436-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 23. | Bataller R, Cabezas J, Aller R, Ventura-Cots M, Abad J, Albillos A, Altamirano J, Arias-Loste MT, Bañares R, Caballería J, Caballería L, Carrión JA, Diago M, Fernández Rodríguez C, Gallego R, García-Cortes M, García-Monzón C, Genescà J, Ginés P, Hernandez-Guerra M, Jorquera F, Lligoña A, Molina E, Pareja MJ, Planas R, Tomé S, Salmerón J, Romero-Gómez M. Alcohol-related liver disease. Clinical practice guidelines. Consensus document sponsored by AEEH. Gastroenterol Hepatol. 2019;42:657-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Hmoud BS, Patel K, Bataller R, Singal AK. Corticosteroids and occurrence of and mortality from infections in severe alcoholic hepatitis: a meta-analysis of randomized trials. Liver Int. 2016;36:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Philips CA, Phadke N, Ganesan K, Ranade S, Augustine P. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J Gastroenterol. 2018;37:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 26. | Quek JWE, Loo JH, Jaroenlapnopparat A, Jimenez C, Al-Karaghouli M, Vargas V, Arab JP, Abraldes JG, Wong YJ. Prophylactic antibiotics in patients with alcohol-associated hepatitis receiving steroids: A systematic review and meta-analysis. Liver Int. 2024;44:2469-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, Duhamel A, Pageaux GP, Leroy V, Dharancy S, Louvet A, Boleslawski E, Lucidi V, Gustot T, Francoz C, Letoublon C, Castaing D, Belghiti J, Donckier V, Pruvot FR, Duclos-Vallée JC. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 678] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 28. | Al-Saeedi M, Barout MH, Probst P, Khajeh E, Weiss KH, Diener MK, Mehrabi A. Meta-analysis of patient survival and rate of alcohol relapse in liver-transplanted patients for acute alcoholic hepatitis. Langenbecks Arch Surg. 2018;403:825-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Louvet A, Labreuche J, Moreno C, Vanlemmens C, Moirand R, Féray C, Dumortier J, Pageaux GP, Bureau C, Chermak F, Duvoux C, Thabut D, Leroy V, Carbonell N, Rolland B, Salamé E, Anty R, Gournay J, Delwaide J, Silvain C, Lucidi V, Lassailly G, Dharancy S, Nguyen-Khac E, Samuel D, Duhamel A, Mathurin P; QuickTrans trial study group. Early liver transplantation for severe alcohol-related hepatitis not responding to medical treatment: a prospective controlled study. Lancet Gastroenterol Hepatol. 2022;7:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 30. | Altamirano J, López-Pelayo H, Michelena J, Jones PD, Ortega L, Ginès P, Caballería J, Gual A, Bataller R, Lligoña A. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: Prediction and impact on long-term survival. Hepatology. 2017;66:1842-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 31. | Parker R, Armstrong MJ, Corbett C, Rowe IA, Houlihan DD. Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013;37:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Idalsoaga F, Diaz LA, Dunn W, Mehta H, Caldentey V, Arnold J, Ayares G, Sarin SK, Maiwall R, Zhang W, Qian S, Simonetto D, Singal AK, Elfeki MA, Khan MQ, Mortuza R, Malhi G, Islam AH, Guizzetti L, Ramirez-Cadiz C, Cabezas J, Echavarria V, Poca M, Cuyas B, Soriano G, Ventura Cots M, Higuera-De La Tijera MF, Abraldes JG, Al-Karaghouli M, Skladaný L, Havaj DJ, Rincón D, Shah V, Arrese M, Kamath PS, Bataller R, Arab JP. Pentoxifylline use in alcohol-associated hepatitis with acute kidney injury does not improve survival: a global study. eGastroenterology. 2025;3:e100179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Tayek JA, Stolz AA, Nguyen DV, Fleischman MW, Donovan JA, Alcorn JM, Chao DC, Asghar A, Morgan TR; Southern California Alcoholic Hepatitis (SCAH) Consortium. A phase II, multicenter, open-label, randomized trial of pegfilgrastim for patients with alcohol-associated hepatitis. EClinicalMedicine. 2022;54:101689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Mishra AK, Shasthry SM, Vijayaraghavan R, Kumar G, Sarin SK. Granulocyte Colony-Stimulating Factor Improves Prednisolone Responsiveness and 90-Day Survival in Steroid-Eligible Severe Alcohol-Associated Hepatitis: The GPreAH Study a Randomized Trial. Am J Gastroenterol. 2025;120:1087-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol. 2014;109:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 36. | Shasthry SM, Sharma MK, Shasthry V, Pande A, Sarin SK. Efficacy of Granulocyte Colony-stimulating Factor in the Management of Steroid-Nonresponsive Severe Alcoholic Hepatitis: A Double-Blind Randomized Controlled Trial. Hepatology. 2019;70:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 37. | Marot A, Singal AK, Moreno C, Deltenre P. Granulocyte colony-stimulating factor for alcoholic hepatitis: A systematic review and meta-analysis of randomised controlled trials. JHEP Rep. 2020;2:100139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Baig M, Walayat S, Dhillon S, Puli S. Efficacy of Granulocyte Colony Stimulating Factor in Severe Alcoholic Hepatitis: A Systematic Review and Meta-Analysis. Cureus. 2020;12:e10474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, Tramier B, Dewaele F, Ghrib S, Rudler M, Carbonell N, Tossou H, Bental A, Bernard-Chabert B, Dupas JL; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 309] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 40. | Amjad W, Alukal J, Doycheva I, Zhang T, Maheshwari A, Yoo H, Thuluvath PJ. A Combination of N-Acetylcysteine and Prednisone Has No Benefit Over Prednisone Alone in Severe Alcoholic Hepatitis: A Retrospective Analysis. Dig Dis Sci. 2020;65:3726-3733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Jung JH, Kim SE, Suk KT, Kim DJ. Gut microbiota-modulating agents in alcoholic liver disease: Links between host metabolism and gut microbiota. Front Med (Lausanne). 2022;9:913842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Vergis N, Atkinson SR, Knapp S, Maurice J, Allison M, Austin A, Forrest EH, Masson S, McCune A, Patch D, Richardson P, Gleeson D, Ryder SD, Wright M, Thursz MR. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology. 2017;152:1068-1077.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 43. | Jiménez C, Ventura-Cots M, Sala M, Calafat M, Garcia-Retortillo M, Cirera I, Cañete N, Soriano G, Poca M, Simón-Talero M, Altamirano J, Lucey M, Garcia-Tsao G, Brown RS Jr, Schwabe RF, Verna EC, Schnabl B, Bosques-Padilla F, Mathurin P, Caballería J, Louvet A, Shawcross DL, Abraldes JG, Genescà J, Bataller R, Vargas V. Effect of rifaximin on infections, acute-on-chronic liver failure and mortality in alcoholic hepatitis: A pilot study (RIFA-AH). Liver Int. 2022;42:1109-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 44. | Sidhu SS, Dusseja A, Shalimar, Nijhawan S, Kapoor D, Goyal O, Kishore H. A multicenter double-blind, placebo-controlled randomized trial to evaluate the safety and efficacy of bovine colostrum in the treatment of severe alcoholic hepatitis (SAH). Trials. 2023;24:515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Baweja S. Fecal microbial transplant suppresses hepatic and systemic inflammatory responses and gut leakiness in severe alcoholic hepatitis patients. Oral Abstracts. Hepatology. 2021;74:1-156. |
| 46. | Bajaj JS, Gavis EA, Fagan A, Wade JB, Thacker LR, Fuchs M, Patel S, Davis B, Meador J, Puri P, Sikaroodi M, Gillevet PM. A Randomized Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology. 2021;73:1688-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 47. | Philips CA, Ahamed R, Oommen TT, Nahaz N, Tharakan A, Rajesh S, Augustine P. Clinical outcomes and associated bacterial and fungal microbiota changes after high dose probiotic therapy for severe alcohol-associated hepatitis: An observational study. Medicine (Baltimore). 2024;103:e40429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 48. | Van Melkebeke L, Korf H, Tsochatzis EA, van der Merwe S, Nevens F, Verbeek J. Treatment of severe alcoholic hepatitis: A systematic review. Curr Opin Pharmacol. 2021;60:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Shiffman M, Da B, Goel A, Kwong A, Stein L, Moreno C, Nicoll A, Mehta A, Louvet A, Flamm S, Pyrsopoulos N, Satapathy S, Kuo A, Ganger D, Aloman C, Strasser SI, Tse E, Russo MW, Rockey D, Gray M, Mitchell M, Thursz M, Krebs W, Scott D, Blevins C, Ellis D, Brown J, Sussman N, Lin W. Larsucosterol for the Treatment of Alcohol-Associated Hepatitis. NEJM Evid. 2025;4:EVIDoa2400243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 628] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/